Abstract
Background
Helicobacter pylori related gastric intestinal metaplasia (IM) is considered to be a precancerous lesion.
Aims
To identify the effects of H pylori eradication on K‐ras mutations, cell kinetics in IM and histological changes in patients with and without gastric cancers in a one‐year prospective study.
Methods
Patients included group A (n = 39), chronic gastritis, and group B (n = 53), intestinal‐type early gastric cancer patients who had all undergone endoscopic mucosal resection (n = 25) or surgical resection (n = 28). K‐ras codon 12 mutations in IM were examined, followed by DNA sequencing analysis. Proliferating and apoptotic cells were detected with anti‐Ki‐67 antibody and using the TUNEL method, respectively.
Results
The incidence of K‐ras mutations in the cancer was only 3.8%. The mutant K‐ras in IM was observed more frequently in group A (46.2%) than in group B patients (1.9%) (p<0.005). After eradication, the K‐ras mutations significantly declined to 12.8% in group A (p<0.005). The mutation pattern of K‐ras codon 12 before eradication was that GGT was mainly changed to AGT (50%) in group A. AGT transformation was not affected by treatment. Apoptosis in IM showed an increase after H pylori eradication in both groups (p<0.05 in group A) although no histological improvement in IM was observed. The monocyte score was significantly higher in group A than in group B (p<0.05); the score improved significantly after eradication.
Conclusions
K‐ras mutations in IM do not always play a role in gastric carcinogenesis but cell kinetics, especially apoptosis, in IM may contribute to it. There are early events in K‐ras mutations which are influenced by H pylori infection; some mutations may also be selected by eradication. These unstable K‐ras mutations in IM may be related to lymphocyte infiltration caused by H pylori infection.
Keywords: Helicobacter pylori, eradication, intestinal metaplasia, K‐ras mutation, cell kinetics
One of the main risk factors for the development of gastric cancer is Helicobacter pylori infection.1,2,3,4,5 It has been postulated that H pylori infection causes chronic gastritis, gastric atrophy, usually with gastric intestinal metaplasia (IM) and dysplasia, and gastric cancer. The stepwise fashion of this infection, which usually continues over decades, has been defined as a sequence of histological events that confer an increasing risk of malignant transformation as described in Correa's hypothesis.6 Although it is fairly well accepted that H pylori infection plays a significant role in causing gastric cancer, the exact mechanisms involved in the pathogenesis remain obscure. In general, IM is believed to be a preneoplastic lesion of the stomach7 which increases the risk of gastric adenocarcinoma, especially intestinal type.6,8 It remains unclear, however, as to whether or not IM is a precancerous lesion or a marker for an increased risk of malignancy.9,10
A K‐ras mutation occurs relatively early in human carcinogenesis,11 and is detected in various types of human malignancies.12,13 Mutations of the K‐ras gene are found in ∼10% of intestinal‐type gastric cancer but they are rarely detected in the diffuse type.14,15,16 Furthermore, a K‐ras mutation has been detected in preneoplastic lesions, such as mucous cell hyperplasia of the pancreas, for which the current term is pancreatic intraepithelial neoplasia, grade 1 (PanIN 1), suffering from chronic inflammation and regenerative or dysplastic epithelia of ulcerative colitis.17,18There have, however, been few reports of this oncogene in H pylori‐associated chronic gastritis and IM either with or without gastric cancer.16,19 Moreover, changes in K‐ras mutations in IM after H pylori eradication have not yet been investigated.
In addition to genetic alterations, one of the pathways by which H pylori is linked to gastric carcinogenesis may be related to a disruption in the balance between gastric epithelial cell proliferation and apoptosis as some investigators have reported.20,21,22,23,24 However, such previous studies on the cell kinetics before and after H pylori eradication have yielded conflicting results.
In the present study, we evaluated the changes of K‐ras mutations and cell kinetics including cell proliferation and apoptosis in order to assess the effect of H pylori eradication in IM, which is a recognised precursor of gastric cancer.
Methods
Patients
All patients undergoing upper gastrointestinal endoscopy at Asahikawa Medical College Hospital between January 2002 and April 2004 were invited to participate in the study. Any patients who had undergone a surgical gastric resection and those taking aspirin or other non‐steroidal anti‐inflammatory drugs were excluded from this study. We enrolled 69 patients with successful H pylori treatment who had atrophic gastritis (n = 43) or intestinal‐type mucosal gastric cancer after endoscopic mucosal resection (EMR) (n = 26).
In order to improve the accuracy regarding the genetic alterations, the number of samples was increased. Twenty‐eight intestinal‐type early gastric cancer cases that had undergone a surgical resection were randomly selected from the histopathology files of Asahikawa Medical College Hospital during the same period and were added to this study.
In all patients, biopsy specimens were taken in order to assess the presence of an H pylori infection, two from the greater curvature of the antrum and two from the greater curvature of the corpus of the stomach. H pylori status was analysed in each patient by two methods: Wartin–Starry staining and H pylori culture. A patient was regarded as positive for H pylori if one or more of these tests were positive. For the eradication of H pylori, patients were treated with lansoprazole (30 mg), amoxicillin (750 mg), and clarithromycin (400 mg), which were all taken twice daily for 1 week. Following successful eradication, all patients were followed up by endoscopy for 1 year. Of the 69 patients, 5 (4 chronic gastritis patients and 1 gastric cancer patient) were excluded because no IM was seen in the biopsy samples taken before or after treatment.
Finally, 64 patients with IM and available gastric biopsy samples both before and after H pylori eradication were investigated. In these patients, the clearance of H pylori was confirmed by negative results from both Wartin–Starry staining and H pylori culture at follow‐up endoscopy. Written informed consent was obtained from all patients, and approval for the study was given by the ethics committee of Asahikawa Medical College.
Patients were classified as follows: group A (n = 39), chronic gastritis; and group B (n = 53), intestinal‐type early gastric cancer, further divided into group B1 (n = 25) (EMR cases diagnosed as mucosal cancer) and group B2 (n = 28) (surgical resection cases: 10 mucosal cancers and 18 submucosal invasive cancers). All patients in group B1 underwent EMR for their mucosal cancer lesions, and thereafter received treatment for H pylori.
DNA preparation and detection of K‐ras mutations
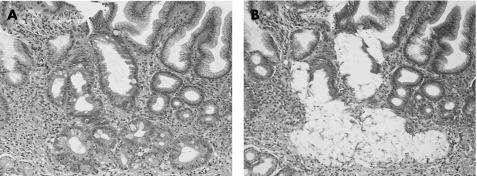
Four tissue sections, each measuring 10 µm in thickness, were serially cut from paraffin embedded tissue blocks. DNA was extracted from IM only (fig 1). In this procedure of DNA extraction, the tissue specimens were precisely microdissected under microscopic visualisation, using a PixCell laser capture microdissection system (Arcturus Engineering, Mountain View, California, USA) to avoid any DNA contamination of inflammatory or stromal cell nuclei. DNA was then extracted from the microdissected tissue specimen by proteinase K treatment, followed by phenol–chloroform extraction.
Figure 1 Metaplastic glands were isolated using a laser capture microdissection system. (A) H&E stained section. (B) The same section, after the removal of metaplastic glands.
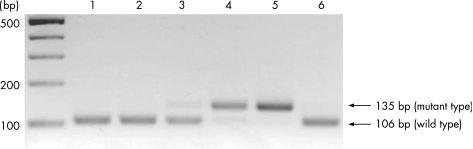
The detection of point mutations in codon 12 of the K‐ras gene was performed by enriched PCR‐restriction fragment length polymorphism as previously described, with minor modifications.25,26 The DNA from the K‐ras sequence of exon 1 was amplified by a first PCR using the mismatched primers described by Levi et al.25 After restriction enzyme digestion using MvaI (Toyobo Company, Tokyo, Japan), PCR was performed in a 20 μl reaction mixture using Ampli‐Taq Gold DNA polymerase (Perkin Elmer Applied Biosystems Division, Foster City, California, USA) according to the manufacturer's manual. In a second PCR and after another round of MvaI digestion, wild‐type fragments were cleaved to yield 29 and 106 base pair (bp) products, whereas mutant fragments yielded 135 bp. Electrophoresis of the digested sample on 3% agarose gel confirmed the mutation band. SW480, a colon cancer cell line, and HT29 were used as a positive and negative control for K‐ras codon 12, respectively (fig 2).
Figure 2 A K‐ras mutation in codon 12 was detected by enriched PCR‐restriction fragment length polymorphism in gastric intestinal metaplasia (IM) from patients with and without gastric cancer. The arrows indicate the positions of the mutant (135 bp) and wild‐type (106 bp) bands. Lane 1: IM from patient with gastric cancer; lanes 2–4: IM from patients without gastric cancer; lane 5: SW480 as positive control; lane 6: HT29 as negative control.
Sequencing analysis
The mutant fragments identified by enriched PCR‐restriction fragment length polymorphism were confirmed by direct sequencing. The sequence of codon 12 was determined by automated fluorescent DNA sequencing using the dideoxy chain termination method.26 PCR products were used for the cycle sequencing. The products were purified using Centricon‐100 (Amicon Inc, Beverly, Massachusetts, USA) following the manufacturer's protocol and then were sequenced using the Dye Terminator Cycle Sequencing Ready Reaction (Perkin Elmer Applied Biosystems Division, Foster City, California, USA) according to the manufacturer's instructions as reported previously.27 The sequencing reaction products were analysed on ABI PRISM Geluxe (Perkin Elmer) on an ABI PRISM 310 DNA sequencer (Perkin Elmer).
Gastric tissues and histological grading
The specimens were fixed in 10% formalin and embedded in paraffin wax; 4 µm consecutive sections were used for histological examinations by H&E staining and immunohistochemical staining.
All slides (pre‐ and post‐treatment biopsies) were evaluated, and scored for neutrophils, mononuclear cells, and IM according to the updated Sydney system28 by a single pathologist (AT), who was unaware of the patient groups or treatment status. Scores were given numerically as follows: 0 for absence, and 1, 2, 3 for mild, moderate, or severe. IM was classified into complete and incomplete types on the basis of presence or absence of brush border and Paneth cells histologically.29 In some patients, both types of IM were present; these were recorded as being in the group with incomplete IM.
Detection of proliferation and apoptosis
Dewaxed paraffin sections were examined by the avidin–biotin peroxidase complex (ABC, Vector Laboratories, Burlingame, California, USA) method using the primary antibody as follows: MIB‐1 against Ki‐67 antigen of proliferating cells (mouse IgG, Dako, Carpinteria, California, USA). The slides were treated with the antigen‐retrieval technique based on microwave oven heating.
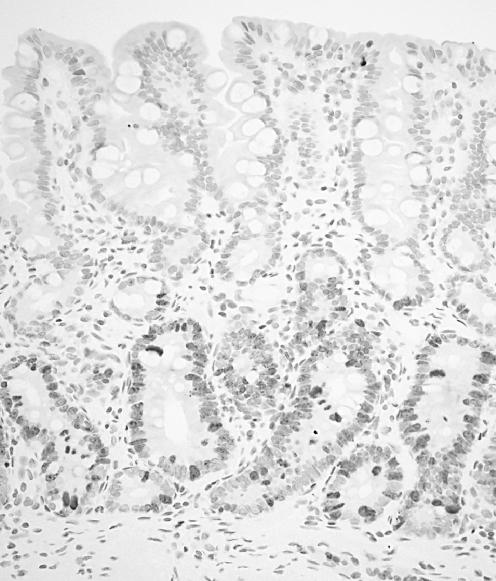
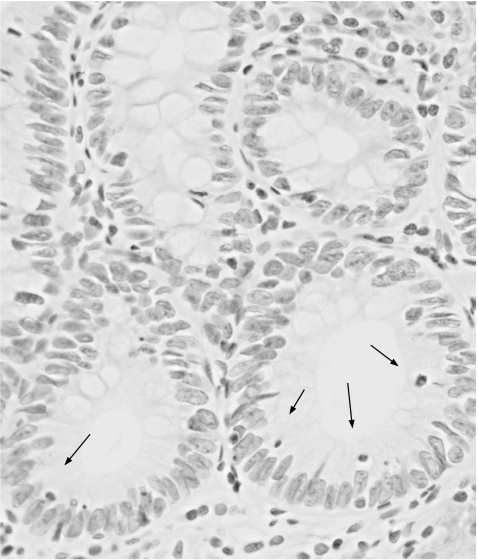
Apoptotic cells in situ were detected by the terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling (TUNEL) method described by Gavrieli et al.30 The slides were dewaxed and rehydrated through a graded alcohol series. The tissue specimens were digested with 20 µg/ml proteinase K (Boehringer, Mannheim, Germany) for 30 min at 37°C. After treating with a 2% H2O2 solution, the sections were preincubated with 100 mM potassium cacodylate, 2 mM cobalt chloride, 0.2 mM dithiothreitol, pH 7.2 for 3 min, and then were incubated with the same buffer containing 0.3 U/µl terminal deoxynucleotidyl transferase (TdT, GIBCO‐BRL Gaithersburg, Maryland, USA) and 0.04 nmol/µl biotinylated dUTP (Boehringer) in a humid chamber at 37°C for 1 h. The slides were rinsed in 30 mM sodium citrate, 300 mM sodium chloride for 30 minutes at room temperature, and washed in phosphate‐buffered saline. After blocking with 10% rabbit serum for 10 min and rinsing briefly in phosphate‐buffered saline, sections were incubated with ABC for 30 min at room temperature. Labelled cells were visualised with diaminobenzidine. The sections were then counterstained with haematoxylin. In IM in each case, a minimum of 300 cells from some fields randomly selected were counted and the fractions (%) of cells that showed positive nuclear staining for Ki‐67 antigen (fig 3) and TUNEL (fig 4) were considered to be the proliferative indices (PI) and the apoptotic indices (AI), respectively. The PI and AI were determined independently by a single physician (JW).
Figure 3 Ki‐67 staining in a representative case of gastric intestinal metaplasia (IM). Proliferating cells were found predominantly in the lower portion of IM.
Figure 4 Apoptotic cells (arrows), detected by the TUNEL method in a representative case of gastric intestinal metaplasia.
Statistical analysis
Statistical differences were assessed by the Mann‐Whitney U test between two independent groups and by the chi‐square test or Fisher's exact test between two proportions. The Wilcoxon signed‐rank test was used in the comparison of parameters before and after treatment in the same patient. Statistical significance was defined as p<0.05.
Results
K‐ras codon 12 mutations in IM
The mutant K‐ras in codon 12 was detected in 18 (46.2%) of 39 IM in group A and 1 (1.9%) of 53 IM areas away from cancer in group B before eradication, and 2 (3.8%) of 53 cancers, respectively. One case who had a K‐ras mutation in group B belonged to group B1. One of the 2 cancers which were positive for K‐ras mutations was also positive in parallel with the IM areas. Group A showed a significantly more frequent incidence of K‐ras codon 12 point mutations than group B (p<0.005). Moreover, the incidence (46.2%) of K‐ras gene alterations decreased significantly to 12.8% (5 of 39) after treatment in group A but not in group B. Of the lesions with K‐ras mutations in group A before treatment, GGT (Gly) showed AGT (Ser) transition in 9 (50.0%), GAT (Asp) transition in 6 (33.3%) and TGT (Cys) transversion in 3 (16.7%). Following treatment, 4 (75.0%) showed a transition to AGT (Ser) and 1 (25.0%) showed a transition to GAT (Asp). Hence, mutation patterns showed convergence to Ser transformation by H pylori treatment. The mutation pattern in the patient from group B1 was AGT (Ser). After eradication, the patterns of AGT (Ser) disappeared and one subject who had previously been negative for K‐ras mutation showed a positive finding for the K‐ras mutation of GAT (Asp). The cancer showed two patterns: GCT (Ala) in 1 and GTT (Val) in 1 (table 1).
Table 1 Patterns of K‐ras mutation in gastric intestinal metaplasia.
| Helicobacter pylori eradication | ||||
|---|---|---|---|---|
| Before | n | After | n | |
| Group A | AGT (Ser) | 9 | AGT (Ser) | 4 |
| GAT (Asp) | 6 | GAT (Asp) | 1 | |
| TGT (Cys) | 3 | |||
| Group B1 | AGT (Ser) | 1 | GAT (Asp) | 1 |
| Cancer areas | GCT (Ala) | 1 | ||
| GTT (Val) | 1 | |||
Cell kinetics of IM
PI and AI in IM before H pylori eradication were 36.71% and 0.27% in group A, and 45.69% and 0.52% in group B, respectively. PI in group B was significantly higher than that in group A (p<0.05). AI showed a similar tendency, but the difference did not reach statistical significance. Regarding the cell kinetics after treatment in each group, PI and AI were 40.43% and 0.47% in group A, and 42.32% and 0.83% in group B, respectively. Although there were no significant changes in the PI before and after H pylori eradication, the level of AI increased after H pylori eradication in both groups (p<0.05 in group A) (table 2).
Table 2 Cell kinetics before and after Helicobacter pylori eradication.
| PI | AI | AI:PI ratio | ||||
|---|---|---|---|---|---|---|
| H pylori eradication | H pylori eradication | H pylori eradication | ||||
| Before | After | Before | After | Before | After | |
| Group A | 36.71 (2.71)* | 40.43 (2.71) | 0.27 (0.07) | 0.47 (0.10) | 0.020 (0.012) | 0.013 (0.004) |
| Group B | 45.69 (2.65)* | 42.32 (3.20) | 0.52 (0.16) | 0.83 (0.30) | 0.010 (0.003) | 0.021 (0.001) |
*p<0.05.
Numbers are mean (SEM).
PI, proliferative index; AI, apoptotic index.
Inflammation and IM scores
Table 3 shows the grade of inflammation and IM before and after treatment. All IMs investigated in the current study were diagnosed as incomplete type without dysplastic glands. At 1 year after successful H pylori eradication, the median score of inflammation such as neutrophils and mononuclear cells significantly improved in group A (p<0.0001 and p<0.005, respectively), but it did not change in group B. The score of mononuclear cells was significantly higher in group A than in group B (p<0.05), although no significant difference was observed in the neutrophils between the two groups. The IM scores were also significantly higher in group B than in group A before H pylori therapy (p<0.05). After 1 year of follow‐up, however, the IM score remained unchanged in comparison to their pretreatment values in both groups A and B.
Table 3 Comparison of scores for histological findings before and after Helicobacter pylori eradication.
| Neutrophils | Mononuclear cells | Intestinal metaplasia | ||||
|---|---|---|---|---|---|---|
| H pylori eradication | H pylori eradication | H pylori eradication | ||||
| Before | After | Before | After | Before | After | |
| Group A | 0.65 (0.10)* | 0.03 (0.03)* | 1.54 (0.11)†,‡ | 1.03 (0.10)† | 1.4 (0.6)§ | 1.3 (0.8) |
| Group B | 0.40 (0.13) | 0.20 (0.09) | 1.15 (0.17)‡ | 1.00 (0.10) | 2.1 (0.9)§ | 1.9 (0.9) |
*p<0.0001, †p<0.005, ‡, ¶p<0.05.
Numbers are mean (SEM).
Discussion
Although the phenomenon in which gastric cancer risk declined after cure of H pylori infection is defined through retrospective31 and prospective32 studies, the changes of the mechanism underlying H pylori‐associated gastric carcinogenesis following the eradication remain unclear. This is the first study to examine the alterations of K‐ras oncogene and cell kinetics in H pylori‐related IM in patients with and without gastric cancer, both before and after H pylori treatment.
The mutant K‐ras was detected in only 3.8% of the cancer; this finding is consistent with the findings of previous reports.14,15,16 However, we found a significantly higher frequency of K‐ras mutations in IM (46.2%) in patients with chronic gastritis in comparison to those with gastric cancer (1.9%) and H pylori infection. We also observed that K‐ras mutations significantly decreased to 12.8% after H pylori eradication. Regarding the K‐ras mutation types in group A, various patterns such as G to A (Ser and Asp) transitions and G to T (Cys) transversion were seen before treatment. It is interesting to note that after H pylori eradication, most individuals (80.0%, 4 of 5) showed transition to AGT (Ser) while other mutation patterns such as TGT (Cys) and GAT (Asp) disappeared. In our data, since the number of cases that could be investigated based on the changes in the K‐ras mutation patterns was small, statistical significance could therefore not be applied. These results indicate, however, that K‐ras mutations in IM with GAT and TGT types may thus be early and unstable in gastric carcinogenesis because they disappeared after H pylori treatment. AGT (Ser) remained in most cases treated with H pylori in our study. Gong et al reported the G to A transition (Ser) to be important for the progression of gastric mucosal cells to a more advanced premalignant stage.19 Lee et al also showed a similar result, so that frequent G to A transversions were detected in gastric cancers.33 Taking both our results and other data into consideration, mutations with AGT (Ser) were thus considered more likely to be advantageous in K‐ras gene alterations. Hiyama et al reported interesting data in which K‐ras mutations were detected in 3.0% in the background mucosa without cancer and in 10.9% in those with cancer, thus indicating a significant difference. In addition, most (70%) of the patterns of K‐ras mutations detected in chronic gastritis patients were G to C transitions (GCT, Ala).16 However, their data do not show K‐ras gene alterations in only IM, and thus their findings are different from those of our investigation. In the current study, we used a laser capture microdissection system to extract DNA from IM. This method allows the procurement of relatively pure metaplastic cell populations from the complex heterogeneous cell mixtures.34 Therefore, it is considered that the specificity of genetic alterations in DNA extracted selectively from IM is higher than that in the hand‐microdissected samples.35 Our results may suggest that the mutations with AGT (Ser) are important in gastric tumourigenesis, but not in others. We investigated K‐ras oncogene alterations in IM using only one biopsy sample obtained from the antrum. It will be necessary to study whether the patients with mutations have the same mutation in multiple IM foci in each patient in order to confirm more clearly the role of K‐ras mutation in IM.
H pylori eradication dramatically improved the inflammation scores, that is, neutrophils and mononuclear cells, but not the degree of IM, thus confirming the findings of previous studies in which the effect of H pylori eradication therapy led to an improvement in the severity of inflammation.36,37 However, no significant improvement in inflammation scores was found in patients with gastric cancer (group B). Although the reason cannot be clearly explained, one possibility for this finding may be the small number of patients investigated in comparison to those in group A. Interestingly, the score of mononuclear cells in group A showed a significantly higher level than that in group B at the pretreatment assessment, although no significant differences in the neutrophil scores were observed between groups A and B. Recently, Brembeck et al reported that they have generated a novel mouse model in which the cytokeratin 19 promotor, specifically active in pancreatic ductal cells, is fused to mutant K‐ras.38 These results showed dramatic evidence of lymphocytic infiltration around the periductal area, in both the interlobular and intralobular pancreas of transgenic mice, which may thus act as an adaptive immune response to activated ras‐mediated signalling. Taking both our findings and previous results into consideration, a frequent K‐ras mutation in IM may therefore be associated with evidence of lymphocyte infiltration but not with gastric carcinogenesis, even though the K‐ras gene is considered to be an oncogene.
In the present study, we first found the cellular proliferation of IM to be significantly up‐regulated in patients with gastric cancer more than in those without gastric cancer. With regard to apoptosis, there was a similar tendency even though no significant difference was seen. This finding supports the report of Shiotani et al that IM results in proliferation‐dominant cell kinetics may be one of the components of gastric carcinogenesis.39 Commonly, an imbalance of cell proliferation and apoptosis is known to play a role in cancer development, and tumour progression should thus be considered in the context of both proliferative activity and cell loss.40 One of the pathways by which H pylori is linked to gastric carcinogenesis may be related to the disruption of cell kinetics. Uemura et al reported that H pylori eradication inhibited the development of new cancers in patients who underwent EMR for 2 years after EMR, while 9% of the patients who did not receive this treatment developed new early stage gastric cancers after 3 years of follow‐up.31 Taking into account this clinical report and our results, the increase of apoptosis in IM by the clearance of H pylori may be associated with the prevention of cancer development. To our knowledge, there has only been one study that has assessed the cell kinetics in H pylori‐associated IM in patients with gastritis both before and after H pylori eradication, although the sample size investigated in that study was small (14 cases).24 A remarkable reduction in proliferation was shown, whereas the degree of apoptosis remained unaltered in IM by treatment, by which the clearance of H pylori may retard gastric carcinogenesis. However, we failed to detect such an association in the current study. This discrepancy seems to be a result of the difference in the sample number and the sampling error. As mentioned in the results, the severity of IM was significantly higher in patients with cancer (group B1) than in those with chronic gastritis (group A) prior to treatment. Hence, an evaluation of the cell kinetics in IM from the group A cases was therefore particularly difficult.
Take‐home messages
H pylori‐related gastric IM is considered to be a precancerous lesion.
The prevalence of K‐ras mutation in patients with IM without cancer was significantly higher than in patients with intestinal‐type gastric cancer.
After eradication, apoptosis in IM increased, and K‐ras mutations significantly declined in patients with chronic gastritis. AGT transformation in mutation pattern was not affected by the treatment.
The K‐ras mutations in IM were related to lymphocyte infiltration caused by H pylori infection.
There are early events in K‐ras mutations, which are influenced by H pylori infection; some mutations may also be selected by eradication. H pylori eradication before the development of stable mutations will likely halt the risk of gastric cancer.
We recently found that microsatellite instability as a genetic alteration in H pylori‐related IM, plays a role in the early events leading to gastric carcinogenesis. H pylori eradication reduced microsatellite instability during the one‐year post‐treatment period.41 In this prospective study, however, our current results suggest that K‐ras codon 12 mutations in IM may not be associated with gastric carcinogenesis. There are early events in K‐ras mutations which are influenced by inflammation‐related H pylori infection, and some mutations such as AGT (Ser) may thus be selected by eradication. Furthermore, these unstable K‐ras mutations in IM may be related to the lymphocyte infiltration caused by H pylori infection. If H pylori is eradicated before the development of stable mutations, the risk of gastric cancer will likely be prevented. Furthermore, apoptosis in IM showed the increase based on the treatment. Our 1‐year prospective study may indicate that the prevention of gastric cancer by H pylori therapy is not associated with an improvement of K‐ras mutations and the degree of IM, but up‐regulation in apoptosis control in IM contributes to retard gastric carcinogenesis. In the current study, the number of samples analysed may be small, particularly considering that K‐ras alterations in IM were investigated. However, we believe that the data presented here, based on a pilot study, are very interesting and will generate interest to extend the studies. Further investigations are thus required using a larger series of samples with a longer‐term follow‐up to determine the possible role of H pylori‐associated IM as a precancerous lesion.
Acknowledgements
The authors would like to thank the endoscopists of their department, Drs N Ueno, Y Konno, C Ishikawa and Y Inaba, and Ms H Suzuki for providing the tissue specimens.
Abbreviations
AI - apoptotic index
EMR - endoscopic mucosal resection
IM - intestinal metaplasia
PI - proliferative index
Footnotes
Competing interests: None declared.
References
- 1.Correa P, Fox J, Fontham E.et al Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer 1990662569–2574. [DOI] [PubMed] [Google Scholar]
- 2.Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol 1993196(Suppl)3–6. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer IARC monographs on the evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. Lyon: IARC, 199461177–240. [PMC free article] [PubMed] [Google Scholar]
- 4.Graham D Y. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterology 200035(Suppl 12)90–97. [PubMed] [Google Scholar]
- 5.Uemura N, Okamoto S, Yamamoto S.et al Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001345784–789. [DOI] [PubMed] [Google Scholar]
- 6.Correa P, Shiao Y H. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res 1994541941s–3s. [PubMed] [Google Scholar]
- 7.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 199519S37–S43. [PubMed] [Google Scholar]
- 8.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988483554–3560. [PubMed] [Google Scholar]
- 9.Filipe M I, Munoz N, Matko I.et al Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer 199457324–329. [DOI] [PubMed] [Google Scholar]
- 10.Miehlke S, Hackelsberger A, Meining A.et al Severe expression of corpus gastritis is characteristic in gastric cancer patients infected with Helicobacter pylori. Br J Cancer 199878263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogelstein B, Fearon E R, Hamilton S R.et al Genetic alterations during colorectal‐tumor development. N Engl J Med 1988319525–532. [DOI] [PubMed] [Google Scholar]
- 12.Soh K, Yanagisawa A, Hiratsuka H.et al Variation in K‐ras codon 12 point mutation rate with histological atypia within individual colorectal tumors. Jpn J Cancer Res 199384388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper G M. Guanine nucleotide binding proteins. In: Cooper GM, ed. Oncogenes. 2nd edn. Sudbury, MA: Jones and Bartlett Publishers, 1995222–242.
- 14.Tahara E. Molecular mechanism of stomach carcinogenesis. Cancer Res Clin Oncol 1993119265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arber N, Shapira I, Ratan J.et al Activation of c‐K‐ras mutations in human gastrointestinal tumors. Gastroenterology 20001181045–1050. [DOI] [PubMed] [Google Scholar]
- 16.Hiyama T, Haruma K, Kitadai Y.et al K‐ras mutation in Helicobacter pylori‐associated chronic gastritis in patients with and without gastric cancer. Int J Cancer 200297562–566. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa A, Ohtake K, Ohashi K.et al Frequent c‐Ki‐ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res 199353953–956. [PubMed] [Google Scholar]
- 18.Chaubert P, Benhattar J, Saraga E.et al K‐ras mutations and p53 alterations in neoplastic and nonneoplastic lesions associated with longstanding ulcerative colitis. Am J Pathol 1994144767–775. [PMC free article] [PubMed] [Google Scholar]
- 19.Gong C, Mera R, Bravo J C.et al KRAS mutations predict progression of preneoplastic gastric lesions. Cancer Epidemiol Biomark Prev 19998167–171. [PubMed] [Google Scholar]
- 20.Moss S F, Calam J, Agarwal B.et al Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 199638498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner S, Beil W, Westermann J.et al Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 19971131836–1847. [DOI] [PubMed] [Google Scholar]
- 22.Jones N L, Shannon P T, Cutz E.et al Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol 19971511695–1703. [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshi T, Sasano H, Kato K.et al Cell damage and proliferation in human gastric mucosa infected by Helicobacter pylori—a comparison before and after H pylori eradication in eradication in non‐atrophic gastritis. Hum Pathol 1999301412–1417. [DOI] [PubMed] [Google Scholar]
- 24.Leung W K, Yu J, To K F.et al Apoptosis and proliferation in Helicobacter pylori‐associated gastric intestinal metaplasia. Aliment Pharmacol Ther 2001151467–1472. [DOI] [PubMed] [Google Scholar]
- 25.Levi S, Urbano‐Ispizua A, Gill R.et al Multiple K‐ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res 1991513497–3502. [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain‐terminating inhibitors. Proc Natl Acad Sci USA 1997745463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watari J, Saitoh Y, Obara T.et al Natural history of colorectal nonpolypoid adenomas: a prospective colonoscopic study and relation with cell kinetics and K‐ras mutations. Am J Gastroenterol 2002972109–2115. [DOI] [PubMed] [Google Scholar]
- 28.Dixon M F, Genta R M, Yardley J H.et al Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996201161–1181. [DOI] [PubMed] [Google Scholar]
- 29.Kawachi T, Kogure K, Tanaka N.et al Studies of intestinal metaplasia in the gastric mucosa by detection of disaccharidases with “Tes‐Tape”. J Natl Cancer Inst 19745319–30. [PubMed] [Google Scholar]
- 30.Gavrieli Y, Sherman Y, Ben‐Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992119493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uemura N, Mukai T, Okamoto S.et al Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarker Prev 19976639–642. [PubMed] [Google Scholar]
- 32.Wong B C‐Y, Lam S K, Wong W M.et al Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China: a randomized controlled trial. JAMA 2004291187–194. [DOI] [PubMed] [Google Scholar]
- 33.Lee K H, Lee J S, Suh C.et al Clinicopathologic significance of the K‐ras gene codon 12 point mutation in stomach cancer. An analysis of 140 cases. Cancer 1995752794–2801. [DOI] [PubMed] [Google Scholar]
- 34.Bonner R F, Emmert‐Buck M, Cole K.et al Laser capture microdissection: molecular analysis of tissue. Science 19972781481–1483. [DOI] [PubMed] [Google Scholar]
- 35.Dillon D, Zheng K, Costa J. Rapid, efficient genotyping of clinical tumor samples by laser‐capture microdissection/PCR/SSCP. Exp Mol Pathol 200170195–200. [DOI] [PubMed] [Google Scholar]
- 36.van der Hulst R W, van der Ende A, Dekker F W.et al Effect of Helicobacter pylori eradication on gastritis in relation to cagA: A prospective 1‐year follow‐up study. Gastroenterology 199711325–30. [DOI] [PubMed] [Google Scholar]
- 37.Witteman E M, Mravunac M, Becx M J.et al Improvement of gastric inflammation and resolution of epithelial damage one year after eradication of Helicobacter pylori. J Clin Pathol 199548250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brembeck F H, Schreiber F S, Deramaudt T B.et al The mutant K‐ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res 2003632005–2009. [PubMed] [Google Scholar]
- 39.Shiotani A, Iishi H, Ishiguro S.et al Epithelial cell turnover in relation to ongoing damage of the gastric mucosa in patients with early gastric cancer: increase of cell proliferation in paramalignant lesions. J Gastroenterol 200540337–344. [DOI] [PubMed] [Google Scholar]
- 40.Wyllie A H. The biology of cell death in tumors. Anticancer Res 19965131–136. [PubMed] [Google Scholar]
- 41.Tanaka A, Watari J, Tanabe H.et al Effect of eradication of Helicobacter pylori on genetic instabilities in gastric intestinal metaplasia. Aliment Pharmacol Ther symp ser 20062194–202. [Google Scholar]