CD45 is a transmembrane protein tyrosine phosphatase located on most haematopoietic cells. It has several isoforms, and haematopoietic cells express one or more of the isoforms—CD45RO, CD45RA and CD45RB.1,2 CD45 immunoreactivity is recognised to be highly specific for non–Hodgkin's lymphomas.3,4 CD45‐expressing non‐haemopoietic tumours are very rare. This was first noted by McDonnell et al5 in 1987, who reported a primitive sarcoma expressing CD45. Subsequently, Nandedkar et al6 reported three cases of undifferentiated large‐cell, possibly neuroendocrine, carcinomas expressing CD45. Two of them were lymph node metastasis and one was a pulmonary tumour. All the three cases expressed both CD45 and cytokeratin. Two of the cases behaved in an aggressive manner.6
In contrast to CD45, CD5 is expressed in thymic carcinoma and malignant mesothelioma.7,8 To the best of our knowledge, this is the first reported case of a carcinoma expressing both CD45 and CD5.
Case report
We report a patient in their 60s who presented initially with weight loss over 1 month and bilateral leg weakness with deterioration in mobility of 2 weeks' duration. On examination, the patient was found to have bilateral cervical lymphadenopathy. Furthermore, the patient was an ex‐smoker, and had been treated for chest infection 1 month earlier, but was otherwise not taking any regular medication.
On admission, an epigastric mass, ascites and jaundice were noted. Blood tests showed low albumin, abnormal liver function tests, and normal blood counts, serum calcium, total cholesterol and thyroid‐stimulating hormone.
Upper gastrointestinal endoscopy showed a normal oesophagus and stomach. However, there was a polypoidal lesion in the pharynx, just above the epiglottis, which was biopsied. Furthermore, radiological findings suggested that there was a liver lesion with the appearance of metastasis.
The patient died within a few weeks of diagnosis before any treatment could be instituted. Autopsy was not performed.
Materials and methods
The biopsy specimen was processed for routine paraffin embedding, and 4 μm paraffin sections were stained with H&E stain and used for immunohistochemical analysis with appropriate retrieval techniques, antibody dilutions and controls (table 1).
Table 1 Details of the antibodies used.
| Antibody | Dilution | Company |
|---|---|---|
| MNF‐116 | 1:150 | Dako, Cambridgeshire, UK |
| TTF‐1 | 1:50 | Novocastra (Vision Biosystems), Newcastle, UK |
| CD45 | 1:10 | Dako |
| CD5 (Leu‐1) | 1:50 | Novocastra (Vision Biosystems) |
| CAM5.2 | 1:10 | ICRF(CRUK), UK |
| CD15 | 1:10 | Dako |
| Synaptophysin | 1:40 | Dako |
| EMA | 1:100 | Dako |
| Ki‐67 | 1:50 | Novocastra (Vision Biosystems) |
| CD20 | 1:250 | Dako |
| CD79a | 1:10 | Dako |
| Pax‐5 | 1:50 | Santa Cruz, Heidelberg, Germany |
| CD10 | 1:20 | Novocastra (Vision Biosystems) |
| CD2 | 1:50 | Novocastra (Vision Biosystems) |
| CD3 | 1:50 | Novocastra (Vision Biosystems) |
| CD4 | 1:400 | Novocastra (Vision Biosystems) |
| CD7 | 1:50 | Novocastra (Vision Biosystems) |
| CD8 | 1:50 | Novocastra (Vision Biosystems) |
| CD30 | 1:50 | Novocastra (Vision Biosystems) |
| CD56 | 1:100 | Novocastra (Vision Biosystems) |
| Alk | 1:10 | Dako |
| CD138 | 1:100 | Serotec, Oxford, UK |
| CD38 | 1:50 | Novocastra (Vision Biosystems) |
| Myeloperoxidase | 1:2000 | Dako |
| CD34 | 1:200 | Dako |
| CK5/6 | 1:300 | Dako |
| CK20 | 1:100 | Dako |
| CK14 | 1:100 | Dako |
| CK19 | 1:200 | Dako |
| CK10 | 1:100 | Novocastra (Vision Biosystems) |
| CA19.9 | 1:300 | Novocastra (Vision Biosystems) |
| CEA polyclonal | 1:1000 | Dako |
| CA125 | 1:200 | Dako |
| Chromogranin | 1:200 | Biogenex (Via Launch), Kent, UK |
| Calretinin | 1:100 | Zymed (Invitrogen), Paisley, UK |
| Melan‐A | 1:50 | Novocastra (Vision Biosystems) |
EMA, epithelial membrane antigen; TTF‐1, thyroid transcription factor 1.
Pathological findings
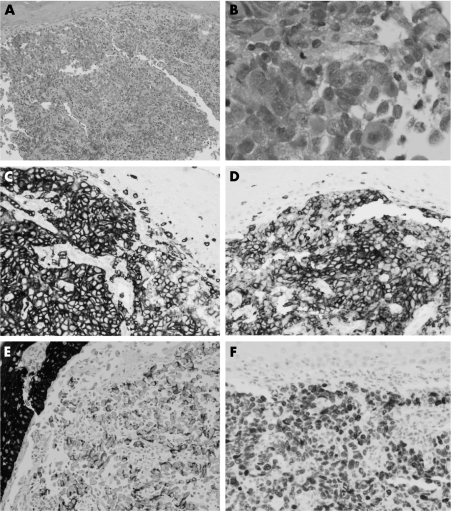
Biopsy showed squamous mucosa with a poorly differentiated malignant tumour. The cells were large, with vesicular nuclei, prominent nucleoli and moderate amounts of eosinophilic cytoplasm, which in some cells was more opaque and fibrillar. Cellular cohesion and pleomorphism were observed. However, there was no discernible pattern. The overlying epithelium did not show dysplasia. Small lymphoid cells, plasma cells and histiocytic cells were seen in the background (fig 1).
Figure 1 (A) Biopsy showing uninvolved squamous mucosa with a poorly differentiated malignant tumour beneath (H&E ×100). (B) The tumour cells are large, with vesicular nuclei, prominent nucleoli and moderate amounts of eosinophilic cytoplasm. The cells are cohesive (H&E ×400). Immunostaining shows expression of (C) CD45 (×200), (D) CD5 (×200), (E) MNF‐116 (pan‐keratin; ×200) and (F) thyroid transcription factor 1 (×200).
The tumour cells expressed MNF‐116, thyroid transcription factor 1, CD45 and CD5. Focal staining for CAM5.2 was observed. Extreme focal staining with CD15, synaptophysin and EMA (epithelial membrane antigen) was also noted. Ki‐67 expression was seen in >80% of cells (fig 1). They were negative for CD20, CD79a, Pax‐5, CD10, CD2, CD3, CD4, CD7, CD8, CD30, CD56, Alk, CD138, CD38, myeloperoxidase, CD34, CK5/6, CK20, CK14, CK19, CK10, CA19.9, CEA polyclonal antibody, CA125, chromogranin, calretinin and melan‐A.
The features suggested an undifferentiated carcinoma with an aberrant immunophenotype, and possibly having its origin in the lung. The expression of CD45 and CD5 seemed to be aberrant.
Discussion
CD45‐positive carcinomas are extremely rare.6 In this report, we document the first reported case of an undifferentiated carcinoma expressing both CD45 and CD5. In addition to expression of keratins, the tumour cells also expressed thyroid transcription factor 1, raising the possibility of an origin in the lung.
CD45 is uniformly distributed on the cytoplasmic membrane of most haematopoietic cells, and is thought to enrich regions of T cell and B cell contact.2 CD5 is predominantly a T cell marker, but is also expressed in a few B cell lymphoproliferative disorders, such as chronic lymphocytic leukaemia and mantle cell lymphoma. Furthermore, as the CD5 antibody used in our laboratory is the 4C7 clone, apart from thymic carcinoma and malignant mesothelioma, it is also known to show immunoreactivity in gastric adenocarcinomas, endometrial carcinomas, small bowel carcinoids, adenocarcinomas of the salivary glands, papillary carcinoma of the thyroid gland, chordomas and uterine leiomyomas.9
With respect to the current case, what are the plausible reasons for the aberrant expression of CD45 and CD5? False positivity of CD45 can be excluded, as the biopsy specimen was immunostained in two separate laboratories. There is a very small possibility that if immunohistochemistry was performed on fresh frozen tissues, the results may have differed. But it seems doubtful that fixation and processing could have resulted in the aberrant phenotype, as both CD45 and CD5 antibodies used in our laboratory have been extensively evaluated in other studies using paraffin sections. Passive acquisition of CD45 and CD5 antigens from the surrounding tumour‐infiltrating T cells and their expression on the cell membrane of the neoplastic epithelial cells could be one explanation. Experimental studies have shown that epithelial cells are able to passively acquire leucocyte antigens and major histocompatibility complex class II molecules.10,11 In the current case, however, the volume of reactive lymphoid infiltrate was small. Furthermore, the tumour cells did not express other T cell/lymphoid cell antigens. Hence, the latter possibility is unlikely.
Therefore, like the CD5 expression in epithelial tumours, CD45 expression, in the current case, is likely to be a true aberrant antigen expression.
The use of PCR‐based clonality analysis for T cell receptor genes and immunoglobulin heavy‐chain gene could be helpful in establishing the correct diagnosis in such cases.12 In fact, PCR was attempted in this case. However, as tissue was depleted in the paraffin block, we could not obtain DNA of adequate quality and concentration to perform the analysis.
While interpreting immunohistochemistry in an undifferentiated malignant tumour, two aspects need to be emphasised. (1) Immunostains should be interpreted in the morphological context. Attention to detail is essential and subtle morphological clues should not be overlooked. (2) It is important to use a wide panel of immunohistochemical analyses to include various lineage‐specific leucocyte and epithelial antibodies so as to avoid misdiagnosis.
References
- 1.Dawes R, Petrova S, Liu Z.et al Combinations of CD45 isoforms are crucial for immune function and disease. J Immunol 20061763417–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furukawa T, Itoh M, Krueger N X.et al Specific interaction of the CD45 protein‐tyrosine phosphatase with tyrosine‐phosphorylated CD3 zeta chain. Proc Natl Acad Sci USA 19949110928–10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtin P J, Pinkus G S. Leukocyte common antigen—a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Hum Pathol 198516353–365. [DOI] [PubMed] [Google Scholar]
- 4.Michels S, Swanson P E, Frizzera G.et al Immunostaining for leukocyte common antigen using an amplified avidin‐biotin‐peroxidase complex method and paraffin sections. A study of 735 hematopoietic and nonhematopoietic human neoplasms. Arch Pathol Lab Med 19871111035–1039. [PubMed] [Google Scholar]
- 5.McDonnell J M, Beschorner W E, Kuhajda F P.et al Common leukocyte antigen staining of a primitive sarcoma. Cancer 1987591438–1441. [DOI] [PubMed] [Google Scholar]
- 6.Nandedkar M A, Palazzo J, Abbondanzo S L.et al CD45 (leukocyte common antigen) immunoreactivity in metastatic undifferentiated and neuroendocrine carcinoma: a potential diagnostic pitfall. Mod Pathol 1998111204–1210. [PubMed] [Google Scholar]
- 7.Pomplun S, Wotherspoon A C, Shah G.et al Immunohistochemical markers in the differentiation of thymic and pulmonary neoplasms. Histopathology 200240152–158. [DOI] [PubMed] [Google Scholar]
- 8.Tateyama H, Eimoto T, Tada T.et al Immunoreactivity of a new CD5 antibody with normal epithelium and malignant tumors including thymic carcinoma. Am J Clin Pathol 1999111235–240. [DOI] [PubMed] [Google Scholar]
- 9.Kornstein M J, Rosai J. CD5 labelling of thymic carcinomas and other nonlymphoid neoplasms. Am J Clin Pathol 1998109722–6.5. [DOI] [PubMed] [Google Scholar]
- 10.Tabibzadeh S S, Kong Q F, Kapur S. Passive acquisition of leukocyte proteins is associated with changes in phosphorylation of cellular proteins and cell‐cell adhesion properties. Am J Pathol 1994145930–940. [PMC free article] [PubMed] [Google Scholar]
- 11.Waldburger J M, Rossi S, Hollander G A.et al Promoter IV of the class II transactivator gene is essential for positive selection of CD4+ T cells. Blood 20031013550–3559. [DOI] [PubMed] [Google Scholar]
- 12.Lasota J, Hyjek E, Koo C H.et al Cytokeratin‐positive large‐cell lymphomas of B‐cell lineage. A study of five phenotypically unusual cases verified by polymerase chain reaction. Am J Surg Pathol 199620346–354. [DOI] [PubMed] [Google Scholar]