Abstract
Most neoplastic scrotal masses ultimately prove to be germ cell tumours and are recognisable with routine haematoxylin and eosin‐stained sections. The differential diagnosis may be focused, even before reviewing histological sections, by knowledge of patient age, medical history, tumour site (testicular vs paratesticular) and gross findings. Some cases may prove to be diagnostically challenging, including rare tumours, a common tumour with an unusual pattern, a metastatic tumour, or a neoplasm with features that mimic another tumour. Several morphological patterns are seen with some frequency and these generate recurring sets of differential diagnostic considerations. These common patterns include testicular tumours with a predominant diffuse arrangement of cells with pale to clear cytoplasm, tumours with a glandular/tubular pattern, tumours with a microcystic pattern and tumours composed of oxyphilic cells. Intratubular proliferations of atypical cells, paratesticular glandular and/or papillary tumours, or tumours with spindle cell morphology can also be challenging to diagnose correctly. In some problematic cases, immunohistochemical staining may be useful to resolve these differential diagnoses.
Even before histological sections are reviewed, a few demographic features may focus the differential diagnosis of a testicular neoplasm.
Demographic information and presenting features
Patient age and tumour location (intratesticular vs paratesticular) are two of the most important features. In children the most likely diagnostic possibilities include yolk sac tumour, teratoma and sex cord‐stromal tumours. Juvenile granulosa cell tumour occurs almost exclusively in those less than 6 months of age and commonly presents as a congenital tumour.1 On the other hand, yolk sac tumour—the major differential diagnostic consideration based on morphology—is distinctly rare in boys under 6 months of age, as is teratoma. An intratesticular neoplasm in an infant under 6 months old is therefore highly likely to represent juvenile granulosa cell tumour. Paediatric paratesticular tumours, especially those in the first decade, are most likely to be rhabdomyosarcomas, but some cases will fall into the paratesticular glandular/papillary tumour differential diagnosis or the spindle cell tumour differential diagnosis.
In young men with intratesticular tumours, germ cell tumours and sex cord‐stromal tumours are the most likely diagnoses, with a marked predominance of the former. Those in the second and third decades are most likely to have non‐seminomatous germ cell tumours rather than pure seminomas. Paratesticular tumours occurring in this age group are likely to represent adenomatoid tumours, sarcomas (especially rhabdomyosarcoma and liposarcoma) or, less likely, metastases, although rare entities such as the desmoplastic round cell tumour also merit consideration.
In older patients, spermatocytic seminoma, sex cord‐stromal tumours, metastasis and lymphoma should be placed at the top of the differential diagnosis of an intratesticular mass. For paratesticular masses, as with those occurring in younger men, metastases, adenomatoid tumour and sarcoma should be considered the leading possibilities.
Patient history may provide a clue to the diagnosis. A history of undescended testis or a previous diagnosis of a germ cell tumour or intratubular germ cell neoplasia, unclassified type (IGCNU) makes the possibility of a germ cell tumour very high. A history of previous or current carcinoma, lymphoma or leukaemia may prevent misclassification of a secondary tumour as a primary testicular neoplasm. These considerations make it clear that it is essential that the treating physician provides an adequate history to optimise the possibility of an accurate interpretation.
Production of substances such as lactate dehydrogenase (LDH), α‐fetoprotein (AFP) and human chorionic gonadotropin (hCG) are important because of their use as serum tumour markers. Serum tumour marker data are commonly available before orchiectomy. LDH may be elevated in seminomas and non‐seminomatous germ cell tumours. AFP is produced by yolk sac tumour and correlates with the amount of yolk sac tumour present in mixed germ cell tumours.2 hCG is produced by syncytiotrophoblast cells and serum hCG levels are therefore raised in patients with a tumour which has a choriocarcinoma component. Serum hCG levels may also by increased in seminoma and mixed germ cell tumours as syncytiotrophoblast cells are commonly present in a scattered fashion in these tumours. An increase in serum inhibin levels has been described in patients with Leydig cell tumour, Sertoli cell tumour3 and large cell calcifying Sertoli cell tumours.4
Only a small minority of testicular tumours are endocrinologically active to the extent that they are clinically manifest. The germ cell tumours are especially unlikely to produce clinically significant levels of hormones, but rare cases of androgen excess5 or gynaecomastia6 resulting from hCG production by syncytiotrophoblast cells have been reported. Choriocarcinoma may be associated with gynaecomastia in 10–20% of cases and this phenomenon results from hCG‐mediated stimulation of androgen production by Leydig cells with subsequent conversion of the androgens to oestrogens by aromatase within adipose and other tissues.7,8 hCG also has thyroid stimulating activity, occasionally resulting in hyperthyroidism.7 Hypercalcaemia, probably due to production of a parathyroid hormone‐like substance, has been reported in association with seminoma.9
Endocrinologically active tumours are more likely to be sex cord‐stromal tumours than germ cell tumours. In boys, approximately 10% of cases of precocious pseudopuberty may result from steroid hormone production by Leydig cell tumours.10 Gynaecomastia may be observed at presentation in 15% of adult men with Leydig cell tumours and in occasional patients with Sertoli cell tumours or granulosa cell tumours.10 Large cell calcifying Sertoli cell tumour may be associated with hyperoestrogenaemia and gynaecomastia.11 While primary testicular carcinoid tumours may occur, they are usually not associated with the carcinoid syndrome.12
Some genetic or congenital syndromes may be associated with certain testicular neoplasms. The testicular “tumour” of the adrenogenital syndrome results most commonly from 21‐α‐hydroxylase deficiency which impairs cortisol secretion by the adrenal cortex. The resulting increased adrenocorticotropic hormone production by the pituitary causes hyperplasia of endocrine cells within and adjacent to the testis.13 The mean age of presentation is 23 years and, in most cases, the diagnosis of adrenogenital syndrome is made before detection of a testis mass, although the testicular abnormality may be the presentation in some cases. The patients have a salt‐losing disorder and bilateral involvement is invariable.14 Large cell calcifying Sertoli cell tumours are sometimes associated with the Carney complex and may therefore be bilateral, familial and associated with other lesions such as cardiac myxomas, Cushing's syndrome and cutaneous hyperpigmentation. Intratubular Sertoli cell proliferations may also be seen in Peutz‐Jeghers syndrome15,16 and are commonly associated with gynaecomastia secondary to aromatase production by the lesion, resulting in conversion of androgens to oestrogen. Klinefelter syndrome is associated with an increased frequency of germ cell tumours, many of which are mediastinal, but testicular germ cell tumours may also be seen.17
Aside from the general division of tumours into intratesticular and paratesticular discussed above, careful attention to the gross appearance of a tumour may provide clues to the diagnosis. Seminomas, for example, are commonly composed of one or multiple nodules of homogenous white or tan tissue with scant associated haemorrhage, necrosis or cystic change. Mixed non‐seminomatous germ cell tumours are less homogenous in appearance and frequently have zones of haemorrhage or necrosis. If a teratoma component is present, cystic change is common and cartilage may be grossly apparent. Pure yolk sac tumour, such as may be seen in paediatric patients, may be relatively homogenous and yellow in colour. Sex cord‐stromal tumours are highly variable in appearance but, as they are steroid hormone‐producing tumours, may also be yellow or tan, reflecting the high lipid content.
Histological examination of the testis uninvolved by the neoplasm may also provide useful information. Most importantly, the presence of intratubular germ cell neoplasia may permit initial categorisation of a tumour as a germ cell tumour and its absence is supportive of non‐germ cell type.
Tumours composed of cells with pale to clear cytoplasm in a diffuse arrangement
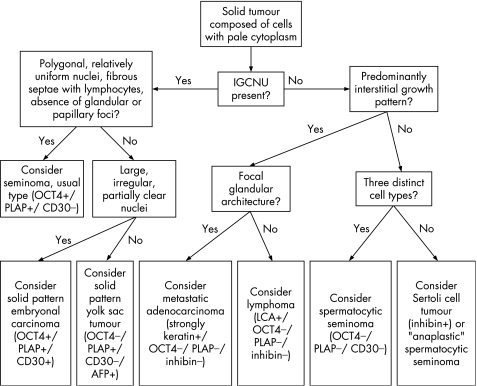
An algorithm showing the usual diagnostic approach for testicular tumours composed of pale/clear cells, including typical (but not invariable) immunohistochemical staining reactions, is shown in fig 1.
Figure 1 Algorithm showing the usual diagnostic approach for testicular tumours composed of pale/clear cells including typical (but not invariable) immunohistochemical staining reactions. IGCNU, intratubular germ cell neoplasia, unclassified type.
Seminoma of usual type is the most common diagnosis for a testicular tumour composed of cells with round or polygonal nuclei and moderately abundant pale cytoplasm in a solid or nested pattern. Several other tumours including spermatocytic seminoma, the solid variant of embryonal carcinoma, solid pattern yolk sac tumour, Sertoli cell tumour and Leydig cell tumour may have a similar histological appearance and must be included in the differential diagnosis. Some metastatic or extrinsic neoplasms such as renal cell carcinoma, lymphoma and melanoma can also present as a testicular mass with a similar histological appearance and, if appropriate patient history is not available, these could be mistaken for seminoma or another primary testicular neoplasm.
Distinguishing seminoma, non‐seminomatous germ cell tumours and extrinsic neoplasms is important because of the differences in prognosis and in the treatment that will be subsequently offered. Following orchiectomy, early stage seminoma is usually treated with adjuvant or therapeutic radiation. Spermatocytic seminoma requires no treatment other than orchiectomy. Chemotherapy and retroperitoneal lymphadenectomy may be indicated for some patients with non‐seminomatous germ cell tumours such as embryonal carcinoma or yolk sac tumour. Treatment for patients with malignant clear cell Sertoli cell tumour or Leydig cell tumour may also include retroperitoneal lymphadenectomy.
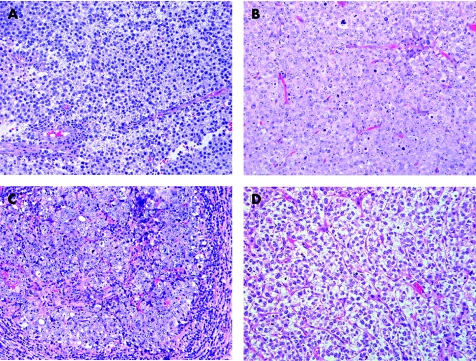
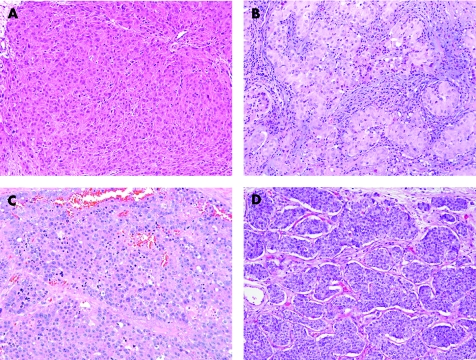
Usual type seminoma can be considered the prototype for this pattern and is characterised histologically by the presence of cells with moderate‐sized polygonal nuclei that may have flat sides. The cytoplasm is moderate in amount and pale to clear (fig 2A). The architecture is generally solid with thin, lymphocyte‐rich, fibrous septae dividing the lesional cells into interconnecting nests. Gland‐like or tubule‐like architecture is absent in most cases but can occur as a variant morphology in rare cases, usually focally. Small granulomas or occasional syncytiotrophoblast cells may be seen. One very important feature is the presence of IGCNU in the testicular tissue adjacent to the tumour in most cases. The presence of IGCNU in a tumour composed of a diffuse arrangement of pale cells essentially limits the differential diagnostic considerations to seminoma, solid embryonal carcinoma and solid yolk sac tumour since spermatocytic seminoma, the sex cord‐stromal tumours, metastatic neoplasms and lymphoma have no association with IGCNU.
Figure 2 Tumours that have cells with pale to clear cytoplasm. (A) Seminoma has polygonal nuclei and fibrous septation with lymphocytes. (B) “Anaplastic” spermatocytic seminoma lacks the usual polymorphic cellular population. (C) Solid embryonal carcinoma has several seminoma‐like features including a nested appearance and lymphocytic infiltrate. (D) Solid yolk sac tumour resembles seminoma.
Spermatocytic seminoma, similar to usual type seminoma, is composed of generally round cells with pale cytoplasm in a diffuse arrangement. The cells of spermatocytic seminoma, however, are more polymorphic than usual type seminoma and cells of three types are described: small, intermediate‐sized and giant. The intermediate‐sized and giant cells may have characteristic filamentous (“spireme”) chromatin which resembles the chromatin of spermatocytes in meiotic prophase. The associated lymphocytic infiltrate is minimal (if present) and a granulomatous reaction is exceptionally rare, in contrast to its common presence in usual seminoma. Some spermatocytic seminomas, however, have a predominance of intermediate‐sized cells (fig 2B), and this pattern complicates the differential diagnosis with both usual seminoma and solid embryonal carcinoma.18 Foci that have the polymorphic appearance, however, can be identified in such cases with extensive sectioning. IGCNU is absent in spermatocytic seminoma, in contrast to the other germ cell tumours, although intratubular spermatocytic seminoma is common.
Immunohistochemical staining may be helpful to differentiate spermatocytic seminoma from seminoma of the usual type (table 1). Spermatocytic seminoma does not stain with antibodies to placental alkaline phosphatase (PLAP)19,20 and the nuclear transcription factor OCT4 (POU5F1).21,22 In contrast, cell membrane pattern positivity for PLAP is seen in 90–100% of usual seminomas23,24,25,26 and nuclear staining for OCT4 in 100%.21,22 CD117 (c‐kit) immunostaining in a cytoplasmic membrane pattern similar to PLAP is seen in 90–100% of usual type seminomas25,27 and only in about 40% of spermatocytic seminomas.19
Table 1 Immunohistochemical staining reactions in testicular neoplasms19,20,21,22,23,24,26,28,29,33,34,42,44,53,57,62,63,68,73,76,85,89,96,101,102,103,104,105,106.
| AE1/AE3 | Cam5.2 | PLAP | OCT4 | AFP | hCG | c‐kit | CD30 | Inh | Ch | PSA | V | S100 | De | Ca | LCA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seminoma (usual type) | v | v | + | + | – | – | + | – | – | – | – | v | – | N | – | – |
| Spermatocytic seminoma | – | v | – | – | – | – | v | – | N | N | N | – | – | – | N | – |
| Embryonal carcinoma | + | + | + | + | v | – | – | + | – | – | N | – | – | N | – | – |
| Yolk sac tumour | + | + | + | – | v | – | – | – | – | – | N | v | – | N | – | – |
| Choriocarcinoma | + | + | + | – | – | + | N | – | + | – | N | – | – | N | – | N |
| Carcinoid | v | + | – | – | N | N | – | – | – | + | – | v | v | N | v | N |
| IGCNU | – | – | + | + | – | – | + | – | – | – | N | N | – | N | N | – |
| Sertoli cell tumour | v | v | v | – | – | – | – | N | + | – | N | v | v | N | v | – |
| LCCSCT | – | v | – | – | – | – | – | N | + | N | N | + | + | + | N | N |
| Leydig cell tumour | v | v | – | – | – | – | N | N | + | – | N | + | v | – | + | N |
| Adult‐type GCT | v | v | N | – | N | N | N | N | + | N | N | + | v | + | + | N |
| Juvenile GCT | v | + | – | – | – | – | N | N | + | – | N | + | + | v | + | N |
| Adenomatoid tumour | + | + | – | – | – | – | N | N | – | – | N | + | N | N | + | N |
| Mesothelioma | + | + | – | – | – | – | – | v | – | v | – | + | – | – | + | N |
| Rhabdomyosarcoma | – | v | – | – | – | – | – | – | – | – | N | + | – | + | N | N |
| Lymphomas | – | – | – | – | – | – | – | v | – | – | N | v | – | – | – | + |
| Plasmacytoma | – | – | – | N | N | N | v | – | – | N | N | v | – | N | – | v |
| Metastatic prostatic carcinoma | + | + | – | – | N | N | v | N | N | v | + | – | – | N | – | N |
| Metastatic melanoma | – | – | – | – | – | N | v | – | – | – | – | + | + | – | N | – |
The data in this table are derived from the above references and personal observations.
IGCNU, intratubular germ cell neoplasia, unclassified type; LCCSCT, large cell calcifying Sertoli cell tumour; GCT, granulasa cell tumour; PLAP, placental alkaline phosphatase; AFP, α‐fetoprotein; hCG, human chorionic gonadotropin; Inh, inhibin‐α; Ch, chromogranin; PSA, prostate specific antigen; V, vimentin; De, desmin; Ca, calretinin; LCA, leucocyte common antigen; +, usually positive (>80% of cases); v, variable expression (20–80% of cases); −, usually negative (<20% of cases); N, no data available.
Embryonal carcinoma is composed of polygonal cells with moderately abundant, amphophilic or clear cytoplasm. The architecture may be glandular, papillary, solid or a mixture of these patterns. Embryonal carcinomas with solid architecture and clear cytoplasm are most likely to be mistaken for seminoma (fig 2C). A review of multiple sections of tumour may be helpful because predominantly solid pattern embryonal carcinomas may have foci of glandular or papillary architecture that may help to exclude a diagnosis of seminoma. The nuclei of embryonal carcinoma are typically irregular with clumped chromatin and a partially clear vesicular appearance. They are typically more pleomorphic than those of seminoma, with a greater degree of nuclear crowding, and the cellular borders are generally not as well‐defined as in usual seminoma.
In difficult cases, a panel of immunohistochemical stains including CD30, CD117 (c‐kit) and keratin AE1/AE3 may be useful to distinguish seminoma from solid pattern embryonal carcinoma (table 1). CD30 produces membranous staining in 84% of embryonal carcinomas.28 Seminomas are negative or, occasionally, show only focal positivity.23 CD117 expression is expected in seminoma but not in embryonal carcinoma or other non‐seminomatous germ cell tumours.25 Cytokeratin AE1/AE3 typically shows diffuse strong staining in embryonal carcinoma while, at most, focal staining is typical of seminoma.24,29 More recently, podoplanin staining has been identified in seminoma but not in embryonal carcinoma.30
Solid yolk sac tumour may also mimic seminoma but is usually associated with other patterns of yolk sac tumour which permit the correct diagnosis. Solid pattern yolk sac tumour is composed of cells with a more variable nuclear morphology, including frequently smaller nuclei, than those of seminoma; they lack the boxy nuclear configuration of seminoma cells (fig 2D). In addition, thick fibrous septa are lacking and hyaline globules and intercellular basement membrane may be seen.
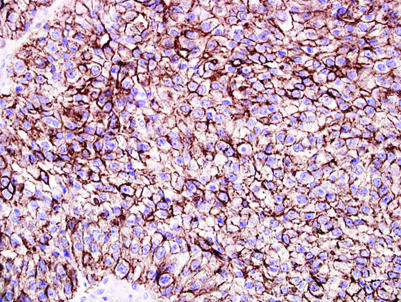
In the differential diagnosis between solid pattern yolk sac tumour and seminoma, AFP, OCT4 and keratin AE1/AE3 may be useful (table 1). Yolk sac tumour may be identified if AFP staining is positive,31 but as AFP staining is seen in only 55–75% of yolk sac tumours and is often patchy, a negative stain is not helpful, especially in a small sample.24,31 OCT4 consistently stains seminoma and does not stain yolk sac tumour.22 Keratin AE1/AE3 shows stronger and more diffuse reactivity in solid yolk sac tumour and focal or negative staining in seminoma (fig 3). A recent study suggested that glypican‐3 may also be helpful since it is positive in yolk sac tumour but negative in seminoma, although the current experience with this marker is limited.32
Figure 3 Strong AE1/AE3 cytokeratin reactivity in a solid yolk sac tumour.
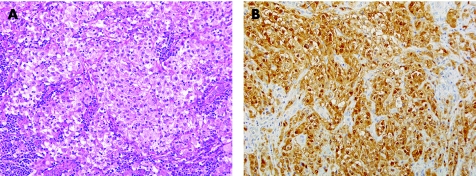
Sertoli cell tumours have a wide range of morphological appearances. They are usually at least partially tubular in architecture, but many tumours also have solid foci. Diffuse solid architecture is less common. The cytoplasm varies from eosinophilic to pale. Some cases may even have an associated lymphocytic infiltrate.33 The combination of a predominant diffuse arrangement, pale cytoplasm and lymphocytic infiltrate may cause a striking resemblance to seminoma (fig 4A).33 Malignant Sertoli cell tumours, which commonly have a diffuse growth pattern, are most likely to resemble seminoma.33 In this differential, the presence of IGCNU supports the diagnosis of seminoma. In addition, Sertoli cell tumours have smaller, often more irregular, nuclei with less prominent nucleoli and show less frequent mitotic figures than seminoma. Immunohistochemical staining for inhibin‐α, OCT4 and PLAP may resolve this important differential diagnosis (table 1). Seminoma is consistently negative for inhibin‐α34 and Sertoli cell tumours are positive in 30–91% of cases.34,35 Calretinin is also useful and, in our experience, more sensitive for Sertoli cell tumour than inhibin (fig 4B) while negative in seminoma. Conversely, OCT4 is negative in Sertoli cell tumour and uniformly reactive in seminoma.21,22,36 PLAP staining, also expected in seminoma, is somewhat less helpful as staining may occur in as few as 80% of seminomas.23
Figure 4 (A) Sertoli cell tumour with a diffuse arrangement of pale cells and an associated lymphocytic infiltrate resembling seminoma. (B) Strong nuclear and cytoplasmic reactivity for calretinin in a Sertoli cell tumour that resembled seminoma.
Most patients with metastases to the testis have a known history of malignancy. Renal cell carcinoma, particularly clear cell type, and prostatic adenocarcinoma, especially the “hypernephroid” variant, are potential simulators of seminoma in that both may have rounded nuclei, prominent nucleoli and pale to clear cytoplasm. Tubular or glandular architecture is expected in these tumours but may be absent in individual cases; furthermore, seminomas may display tubule‐like and gland‐like structures. Obtaining adequate patient history may be critical in avoiding an erroneous diagnosis of a seminoma or other primary neoplasm. In most cases, metastases are primarily interstitial in growth with scant seminiferous tubule involvement, but occasionally intratubular growth may be seen and we have seen examples where it has been especially conspicuous, especially in cases of metastatic prostatic carcinoma (fig 5). Patients with testicular metastases have a higher mean age than patients with testicular germ cell tumours. IGCNU is absent in the setting of metastatic disease and prominent lymphatic involvement is commonly seen. Bilateral disease is also more common with metastatic tumours.
Figure 5 Metastatic prostatic adenocarcinoma to the testis showing prominent intratubular as well as intertubular growth.
Strong and diffuse staining of a testicular clear cell tumour with various keratin antibodies excludes a diagnosis of seminoma. In the case of clear cell renal cell carcinoma, immunostaining for the renal cell carcinoma associated antigen (RCC) may be helpful as RCC is positive in about 85% of clear cell renal cell carcinomas37,38,39 but not in seminoma.40 OCT4 is strongly reactive in seminoma and negative in almost all metastatic candidates, although rare renal cell carcinomas and non‐small carcinomas of the lung may be positive.21
Prostatic carcinoma is one of the more commonly encountered metastases in the testis.41,42 In an older patient with a history of prostatic carcinoma or a testicular tumour which is morphologically suggestive of prostatic carcinoma, immunohistochemical staining for prostate specific antigen (PSA) and prostatic acid phosphatase (PAP) staining may be used to confirm the diagnosis of metastatic prostate carcinoma (table 1).42,43
Melanomas usually have solid architecture and may have cells with rounded nuclei, prominent nucleoli and a moderate amount of pale‐staining cytoplasm. Melanin pigment, nuclear cytoplasmic inclusions or prominent binucleation can be useful clues to the diagnosis of melanoma, but all of these features may be lacking in a given case. S‐100 protein immunoreactivity is not seen in germ cell tumours and therefore supports the diagnosis of melanoma,24 although S‐100 positivity can be seen in primary tumours of sex cord‐stromal lineage and melan‐A, another commonly employed melanoma marker, also stains tumours with steroid biosynthetic capacity such as the testicular “tumour” of the adrenogenital syndrome and Leydig cell tumour.44
Non‐Hodgkin's lymphoma may be seen in the testis; it is more likely to occur in older patients and is also more likely to be bilateral than seminoma. If a history of extratesticular lymphoma is not available, lymphoma could potentially be confused with seminoma. Lymphoma typically has a prominent intertubular pattern of growth, the degree of which is unusual for seminoma. In some cases intratubular growth is seen. Lymphoma cells generally have more angulated nuclei than those of seminoma and the cytoplasm of lymphoma is usually not as clear or as abundant. The absence of IGCNU is helpful. Staining for leucocyte common antigen (CD45) supports the diagnosis of lymphoma as such staining is not seen in germ cell tumours (table 1).24 As most testicular lymphomas are of B cell type, staining for CD20 may also be useful.45
Tumours with a glandular/tubular pattern
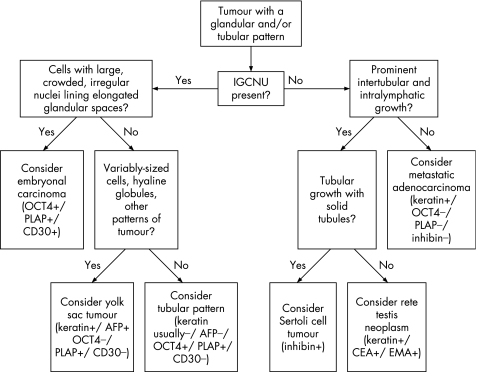
Several tumours of the testis may have a prominent glandular or tubular pattern. Among the germ cell tumours, embryonal carcinoma and yolk sac tumour most commonly display this pattern. Occasional cases of seminoma may also have a tubular pattern.46 Sertoli cell tumours usually have tubular architecture in at least some foci of the tumour. Among the less common tumours, lesions of the rete testis and metastatic adenocarcinomas commonly display glandular architecture. An algorithm showing the usual diagnostic approach for testicular tumours with a glandular/tubular pattern, including typical (but not invariable) immunohistochemical staining reactions is shown in fig 6.
Figure 6 Algorithm showing the usual diagnostic approach for testicular tumours with a glandular/tubular pattern, including typical (but not invariable) immunohistochemical staining reactions. IGCNU, intratubular germ cell neoplasia, unclassified type.
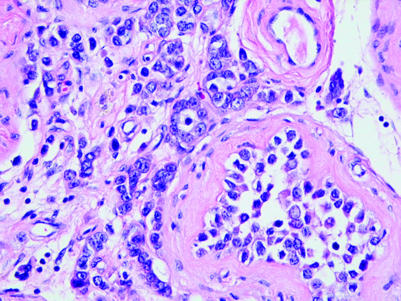
Embryonal carcinomas, as discussed above, commonly display elongated glandular spaces lined by cuboidal or columnar epithelium, although solid areas are commonly also admixed with the glandular areas. The individual cells have nuclei which appear crowded and have large nucleoli and pale chromatin. The cytoplasm is moderate in volume and variable in staining. Amphophilic, eosinophilic or nearly clear cytoplasm may be seen.
In a glandular pattern tumour compatible with embryonal carcinoma, immunohistochemical staining for OCT4, PLAP and CD30 can be used to support the diagnosis, with negative results for CD117 and inhibin‐α helping to further exclude diagnostic alternatives (table 1). OCT4 positivity narrows the differential diagnosis to seminoma and embryonal carcinoma while the absence of staining excludes both seminoma and embryonal carcinoma.21,22,36 Similarly, positive staining for PLAP may be useful to support a diagnosis of a germ cell tumour, but some seminomas, embryonal carcinomas and yolk sac tumours will not stain, limiting the usefulness of a negative stain.47 Furthermore, PLAP immunopositivity may also be seen in some somatic carcinomas, especially colon and lung adenocarcinomas, so it should not be regarded as a specific germ cell tumour marker. CD30 staining is observed in about 90% of embryonal carcinomas but is negative in the sex cord‐stromal tumours and, at most, is identified only focally in seminomas.23,29 CD117 staining is absent in embryonal carcinoma and yolk sac tumour and is present in seminomas.25 Inhibin‐α is negative in germ cell tumours, except in associated syncytiotrophoblast cells; the absence of staining with inhibin therefore supports germ cell origin over sex cord‐stromal origin but, since a portion of sex cord‐stromal tumours are inhibin negative, lack of staining is not diagnostic.34,48
In some cases it may be difficult to distinguish yolk sac tumour from embryonal carcinoma. Yolk sac tumour has smaller and less irregularly shaped nuclei than embryonal carcinoma. Compared with embryonal carcinoma, yolk sac tumour has a greater diversity of patterns. Hyaline globules and basement membrane deposits are much more likely in yolk sac tumour.
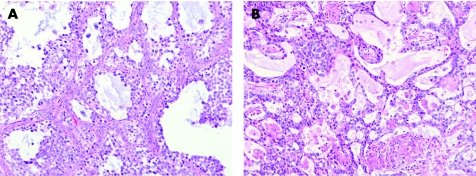
A solid tubular, hollow tubular or even microcystic pattern may be seen in occasional cases of seminoma and suggest a diagnosis of yolk sac tumour (fig 7A).46 Granulomatous inflammation and lymphocytic infiltration may be inconspicuous in these tumours.46 The amount of the tumour with tubular or cystic spaces is variable but, in most cases, the spaces involve less than half of the tumour.46 A prominent solid tubular pattern may also be seen, suggesting Sertoli cell tumour,33,46 but OCT4 (fig 7B) and inhibin immunostaining should clarify this situation. Extension of seminoma into the rete testis may also mimic a glandular neoplasm. The immunohistochemical differential diagnosis of embryonal carcinoma and seminoma has been discussed above.
Figure 7 (A) Tubular pattern seminoma with (B) strong nuclear reactivity with OCT4.
AFP and OCT4 may be useful in distinguishing either embryonal carcinoma or tubular pattern seminoma from yolk sac tumour. AFP reactivity is seen in most cases of yolk sac tumour, is unusual in embryonal carcinoma49 and is not seen in seminoma. OCT4 staining is uniformly positive in embryonal carcinoma and seminoma and negative in yolk sac tumour. CD30 positivity is of additional value in the differential diagnosis of embryonal carcinoma (positive) from yolk sac tumour (negative).
Tubular growth is typical of Sertoli cell tumours and is at least focally present in the majority of tumours. Individual cases may have hollow tubules, solid tubules or a mixture of these patterns, and the tubules may interconnect in a retiform pattern. The absence of IGCNU is a helpful clue to a Sertoli cell tumour as opposed to germ cell tumour. Sertoli cell tumours often express inhibin‐α34 and staining is negative in germ cell tumours. The immunohistochemical markers that may assist have been mentioned in the section on tumours with a diffuse arrangement of cells.
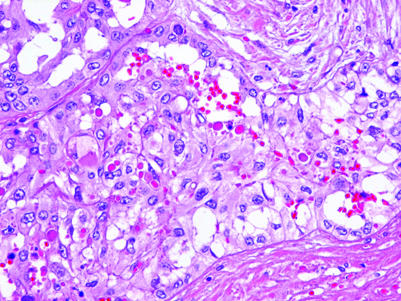
Epithelial lesions of the rete testis are rarely encountered and both adenomas and carcinomas have been described. Hyperplasia of the rete testis may also be seen, usually in association with a testicular tumour that invades the rete (fig 8).50 The benign lesions may be solid or cystic, the cystadenomas having a non‐stratified bland epithelial cell lining. The adenofibromas are solid with an admixture of cords and tubules of rete epithelium with a fibromyomatous stroma. Carcinomas may have a mixture of tubular, papillary and solid patterns with nuclear stratification, nuclear pleomorphism and conspicuous mitotic activity. The diagnosis of adenocarcinoma of the rete testis requires the exclusion of metastatic adenocarcinoma and other primary testicular or paratesticular tumours such as mesothelioma or a mullerian‐type serous carcinoma.51
Figure 8 Epithelial hyperplasia of the rete testis which expands the rete lumen and creates multiple lumen‐like spaces. Note the numerous hyaline globules.
Adenocarcinomas of the rete testis are usually reactive for cytokeratin (Cam 5.2 and keratin 7), epithelial membrane antigen, and carcinoembryonic antigen. OCT4 staining is not seen. Staining with CA‐125 (a mullerian marker) or staining with mesothelial markers such as calretinin, cytokeratin 5/6 or WT‐1 favour a diagnosis of a mullerian‐type tumour or mesothelioma.51 Metastatic adenocarcinomas, as discussed in the previous section, may mimic a primary testicular neoplasm such as embryonal carcinoma or glandular pattern yolk sac tumour. Again, obtaining adequate history, examination of the surrounding testis for evidence of IGCNU and immunohistochemical staining may help to resolve this differential diagnosis. OCT4 and EMA are useful in this situation. In contrast to seminoma and embryonal carcinoma, somatic adenocarcinomas are almost always OCT4 negative. The majority of somatic carcinomas show EMA reactivity, which is not seen in embryonal carcinoma and seminoma, and yolk sac tumours are only occasionally EMA positive.24 Specific antigens may be useful if a particular site of origin is suspected. Prostatic carcinoma is one of the most common metastatic carcinomas encountered in the testis41 and staining for PSA and PAP may confirm prostatic origin. Thyroid transcription factor‐1 may similarly be useful if a pulmonary adenocarcinoma is suspected52 and cytokeratin 20 and CDX‐2 may be useful if colorectal adenocarcinoma is suspected.
Tumours with a microcystic pattern
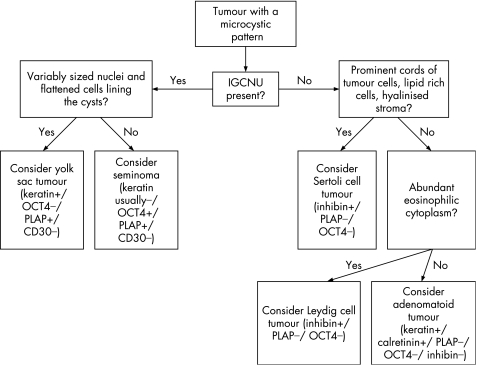
An algorithm showing the usual diagnostic approach for tumours with a microcystic pattern, including typical (but not invariable) immunohistochemical staining reactions, is shown in fig 9.
Figure 9 Algorithm showing the usual diagnostic approach for tumours with a microcystic pattern, including typical (but not invariable) immunohistochemical staining reactions. IGCNU, intratubular germ cell neoplasia, unclassified type.
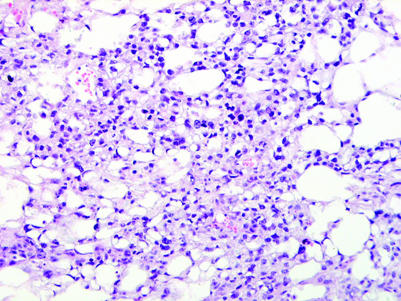
A microcystic or reticular pattern, seen as thin cords of neoplastic cells or cell processes which surround small clear spaces of varying size, is the most common pattern of yolk sac tumour (fig 10). A similar arrangement of cells may also be seen in seminomas,46 Leydig cell tumours,53 Sertoli cell tumours54 and in paratesticular adenomatoid tumours. Association of a microcystic pattern with other distinctive patterns of yolk sac tumour is clearly helpful in this differential, as is the usual presence of more characteristic morphologies for the other considered entities in this differential diagnosis. The usual paratesticular epicentre of adenomatoid tumour contrasts with the intratesticular location of the other microcystic neoplasms. The presence of IGCNU limits the consideration to seminoma and yolk sac tumour. In this circumstance, the more variable nuclear morphology of yolk sac tumour and the frequently flattened cellular profiles of the cells lining the cysts contrast with the more uniform appearance and polygonal cells in microcystic seminomas. Microcystic Leydig cell tumours typically have abundant eosinophilic cytoplasm and infrequent mitotic figures; Reinke crystals are pathognomonic. Cords of tumour cells, lipid‐rich cells, hyalinised stroma, thick‐walled vessels, distinct tubules, retiform patterns and low mitotic rates are characteristic of Sertoli cell tumour.
Figure 10 Yolk sac tumour with a microcystic pattern.
Immunohistochemical stains useful in resolving this differential include OCT4, inhibin‐α, calretinin, melan‐A, AFP and PLAP (table 1). AFP is negative in Leydig cell tumours53 so immunohistochemical staining for AFP supports the diagnosis of yolk sac tumour, but sensitivity is only moderate with 55–75% of yolk sac tumours staining.24,31 PLAP similarly may aid in the distinction of microcystic Leydig cell tumour from yolk sac tumour, if positive, because approximately half of yolk sac tumours are positive for PLAP and the sex cord‐stromal tumours are negative, with the exception of occasional Sertoli cell tumours.34,53,55 Negative immunoreactivity with either AFP or PLAP is not helpful in this differential. Inhibin‐α, melan‐A and calretinin may be used as positive markers for Leydig cell tumours; yolk sac tumours are negative with these stains.34,55,56,57,58 The usefulness of OCT4 in the differential of seminoma and yolk sac tumour has been commented on previously.
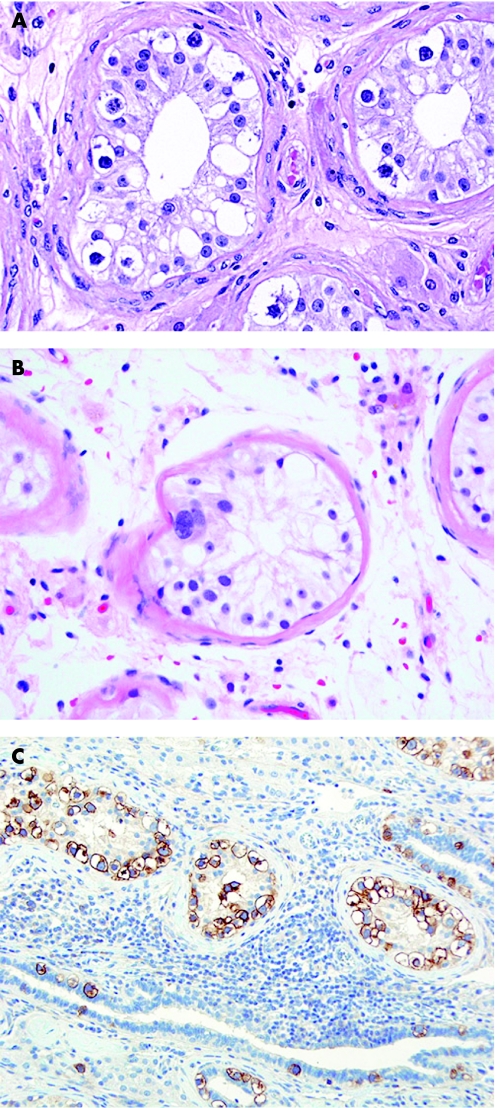
Adenomatoid tumours are of mesothelial derivation and therefore express mesothelial markers including calretinin and the Wilms' tumour suppressor gene product (WT1).59 Adenomatoid tumours may occasionally have an intratesticular component and, in this situation, they are more prone to be mistaken for a germ cell tumour or sex cord‐stromal tumour. A sex cord‐stromal tumour may be especially suggested if an adenomatoid tumour has a more cellular solid arrangement (fig 11) than the usual vacuolated appearance. Immunohistochemical staining for inhibin‐α is useful in this situation as staining is not seen in adenomatoid tumour.34 Calretinin is not helpful because of its positivity in both adenomatoid tumour and sex cord‐stromal tumours.
Figure 11 Adenomatoid tumour with only focal vacuolated cells.
Oxyphilic tumours
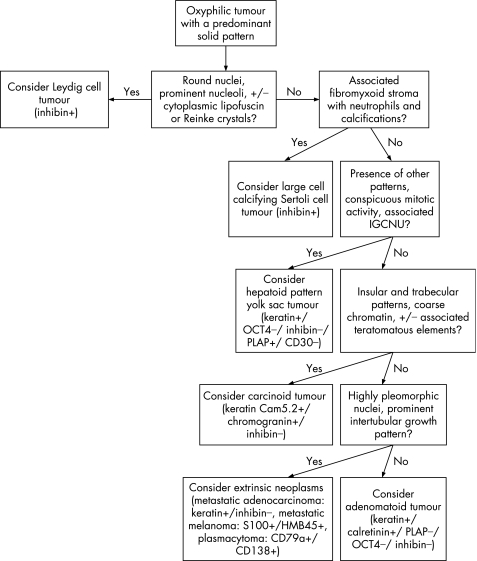
An algorithm showing the usual diagnostic approach for tumours with oxyphilic cytoplasm, including typical (but not invariable) immunohistochemical staining reactions, is shown in fig 12.
Figure 12 Algorithm showing the usual diagnostic approach for tumours having oxyphilic cytoplasm, including typical (but not invariable) immunohistochemical staining reactions. IGCNU, intratubular germ cell neoplasia, unclassified type.
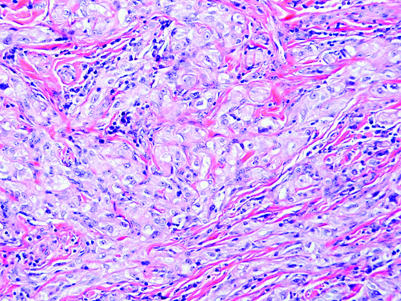
Leydig cells are seen in the interstitium of the normal testis as clusters of cells with round‐to‐polygonal cell outlines, abundant eosinophilic cytoplasm, round nuclei and prominent nucleoli. Cytoplasmic lipofuscin pigment or Reinke crystals (rod‐shaped cytoplasmic inclusions of refractile eosinophilic material) may be seen. Leydig cell tumours are usually composed of similar cells typically arranged in a solid pattern (fig 13A) but sometimes have cord‐like, tubular or, rarely, microcystic arrangements. Leydig cell tumours can be considered the prototype for the tumours composed of oxyphilic cells, even though some cases have prominent clear cells secondary to abundant cytoplasmic lipid.
Figure 13 Tumours composed of oxyphilic cells. (A) Leydig cell tumour with a diffuse arrangement of polygonal to slightly spindled cells having abundant eosinophilic cytoplasm. (B) Large cell calcifying Sertoli cell tumour with a nested arrangement of cells with eosinophilic cytoplasm in a fibromyxoid stroma. Note neutrophilic infiltrate (calcification is not present in this field). (C) Hepatoid yolk sac tumour. (D) Carcinoid tumour with insular pattern.
Some other tumours may have cells with eosinophilic cytoplasm in a solid pattern and may resemble Leydig cell tumour. Among these are large cell calcifying Sertoli cell tumour (fig 13B), hepatoid yolk sac tumour (fig 13C), carcinoid tumour (fig 13D), intratesticular adenomatoid tumour (fig 11) and metastatic tumours (especially melanoma and carcinomas of colonic, prostatic, urothelial and pulmonary origin). These often can be recognised based on conventional microscopy. The large cell calcifying Sertoli cell tumour is frequently arranged in small solid nests and cords with a conspicuous fibromyxoid stroma containing a neutrophilic infiltrate and, by definition, has associated calcifications. These vary from small psammoma body‐like structures to large amorphous masses. Intratubular growth, unlike Leydig cell tumour, may be identified at the tumour periphery. Hepatoid yolk sac tumour is usually a focal pattern in a yolk sac tumour with conventional features. Unlike those of Leydig cell tumour, the cells have conspicuous mitotic activity and there is usually associated IGCNU. Carcinoid tumours of the testis usually have well‐defined insular and trabecular patterns with the characteristic “salt and pepper” chromatin and, in some cases, distinct cytoplasmic granules. There may be associated teratomatous elements. The intratesticular adenomatoid tumour is often eccentrically located near the hilum of the testis and usually shows a tubular arrangement of cells with conspicuous cytoplasmic vacuolisation. The anaplasia and mitotic activity of metastatic tumours to the testis, their frequent involvement of lymphatics and features specific to their origin contrast with those of Leydig cell tumour.
Inhibin‐α is the most useful immunohistochemical stain for identifying tumours of the sex cord‐stromal category and is almost always positive in Leydig cell tumour. Expression of calretinin and vimentin are also common. PLAP immunoreactivity is unusual for Leydig cell tumours55 but 27% of Sertoli cell tumours express PLAP, so PLAP immunopositivity is not by itself diagnostic of germ cell neoplasia.34 While expression of inhibin‐α is consistent in Leydig cell tumours,34,35,44 it is variable in Sertoli cell tumours with reported rates as low as 30%34 and as high as 91%.35 Therefore, in the differential diagnosis of Leydig cell tumour and Sertoli cell tumour, the absence of inhibin‐α immunoreactivity is supportive of Sertoli cell tumour.34 Expression of CD99, common in Leydig cell tumours, is also less frequent in Sertoli cell tumours.34 Conversely, S‐100 protein immunoreactivity is seen in 64% of Sertoli cell tumours35,53 in but only about one‐quarter of Leydig cell tumours.35,44,55,60 The large cell calcifying Sertoli cell tumour, however, has different immunoreactivity properties from conventional Sertoli cell tumour.61,62 Similar to Leydig cell tumour, it is immunoreactive for inhibin‐α, S‐100 and vimentin,61,63,64,65 and immunohistochemistry is not useful in the distinction of Leydig cell tumour from large cell calcifying Sertoli cell tumour.66 Primary testicular carcinoid tumours are rare, comprising 0.23% of testis tumours.12,67 They stain with keratin, chromogranin and synaptophysin,68,69 but these stains are not specific as occasional Sertoli cell tumours may also be immunoreactive for each.35 Addition of inhibin‐α to this panel for a suspected carcinoid tumour might help to avoid confusion.
Adenomatoid tumours differ immunohistochemically from Leydig cell tumours by virtue of strong and diffuse reactivity for cytokeratin (AE1/AE3) and negativity for inhibin. Leydig cell tumours are typically only focally reactive with AE1/AE3 in a minority of cases.35
A variety of somatic tumours could potentially be seen as oxyphilic‐type tumours in the testis. Expression of inhibin by metastatic carcinoma is unusual, in contrast to Leydig cell tumour.70 Metastatic melanoma may be seen in the testis.41 S‐100 protein staining is commonly used to confirm a diagnosis of metastatic melanoma, but should be used with caution in this setting. Some Leydig cell tumours and most Sertoli cell tumours are S‐100 positive.24,35,53 Similarly, melan‐A is frequently reactive in both Leydig cell tumour and melanoma. HMB‐45 is more useful. Inhibin‐α and calretinin are more commonly immunoreactive in Sertoli and Leydig cell tumours than in melanoma.
Plasmacytoma may rarely present in the testis71,72 and should be considered if a round cell neoplasm with eosinophilic cytoplasm does not stain with either keratin or inhibin. Plasmacytoma may be identified by immunoreactivity for CD79a or CD138. CD20 and CD45 immunostaining, seen in B cell lymphomas, is commonly negative in plasmacytoma.72
Tumours in children
Yolk sac tumour and juvenile granulosa cell tumour may be morphologically similar in some cases (fig 14). Several patterns of each of these tumours may resemble the other. As mentioned earlier, patient age may be very helpful in this differential. Microcystic foci may be identified in both tumours and both tumours show solid growth. Increased mitotic rates and cellular apoptosis are common in juvenile granulosa cell tumour as well as yolk sac tumour. Identification of other characteristic patterns of yolk sac tumour is a very important differentiating feature. The characteristic follicles of juvenile granulosa cell tumour, which have a well‐defined mantle of stratified tumour cells as their lining (fig 14A), is distinctive and does not occur in yolk sac tumour, which usually has spaces lined by a limited number of cell layers or formed in a background of diffuse rather than lobular growth (fig 14B). The follicles of the juvenile granulosa cell tumour often contain mucicarminophilic material. Unfortunately, not every juvenile granulosa cell tumour shows nicely developed follicles. Many juvenile granulosa cell tumours have a lobular arrangement of cellular tumour foci with an intervening spindle cell stroma, unlike the less organised architecture of yolk sac tumour.
Figure 14 (A) Juvenile granulosa cell tumour showing well developed follicles lined by multiple layers of tumour cells and containing lightly staining secretion. Note intervening fibromuscular stroma. (B) Yolk sac tumour with multiple cystic spaces.
Immunohistochemical staining for AFP, PLAP and inhibin‐α may be helpful. AFP, if positive, is supportive of yolk sac tumour but sensitivity is only moderate.31 PLAP staining, seen in yolk sac tumour but not in juvenile granulosa cell tumour, may also be helpful.73 Inhibin‐α staining is seen in approximately 80% of juvenile granulosa cell tumours but not in yolk sac tumour.34
Intratubular atypical cells
Intratubular atypical cells may be observed in the testicular tubules adjacent to a testicular neoplasm, adjacent to a fibrous scar remaining from a regressed germ cell tumour or, less frequently, in a testicular biopsy performed during orchiopexy for an undescended testis or in the evaluation of infertility. Distinguishing IGCNU from atypical germ cells of unknown significance is important because of the high incidence of current or subsequent germ cell neoplasia in patients with IGCNU.74,75 IGCNU cells resemble those of seminoma, with the same nuclear and cytoplasmic qualities (fig 15A). Atypical germ cells of unknown significance have enlarged nuclei, are sometimes multinucleated, and have a uniformly dense chromatin and round nuclear configuration, in contrast to the flat‐sided nuclei of IGCNU (fig 15B). They lack the prominent nucleoli of IGCNU.
Figure 15 Intratubular atypical germ cells. (A) Intratubular germ cell neoplasia, unclassified type, showing seminoma‐like cells at the base of seminiferous tubules. (B) Intratubular atypical germ cell of unknown significance with an enlarged, ellipsoid, hyperchromatic nucleus and inconspicuous nucleoli. (C) Immunostain for placental alkaline phosphatase highlighting the cytoplasmic membranes of intratubular germ cell neoplasia, unclassified type.
PLAP staining is helpful in identifying IGCNU.76,77,78,79,80 Staining in a cytoplasmic membrane pattern is seen in IGCNU (fig 15C) but not in normal spermatogonia, non‐IGNCU atypical germ cells or in Sertoli cells.76,77,78,80 The sensitivity of PLAP for this purpose varies from 83% to 100%.47,76,79,81 OCT4, a nuclear stain, can also be used to identify IGCNU,22,36 with 100% sensitivity in our experience. Furthermore, it is specific for IGCNU when scattered atypical intratubular cells are under consideration, although intratubular embryonal carcinoma will also be highlighted. c‐kit is another IGCNU marker with sensitivity of 83–96%,27,82 but it is less specific as normal early spermatogenic cells and Leydig cells may also be stained.83
Intratubular syncytiotrophoblastic cells, associated with IGCNU, are present in the tubules adjacent to a substantial fraction of germ cell tumours and their presence can be confirmed with hCG staining.84
Intratubular Sertoli cell proliferations, sometimes associated with Peutz‐Jeghers syndrome, may be seen.15 Progression to invasive Sertoli cell tumour is seen in a minority of cases associated with Peutz‐Jeghers syndrome.15
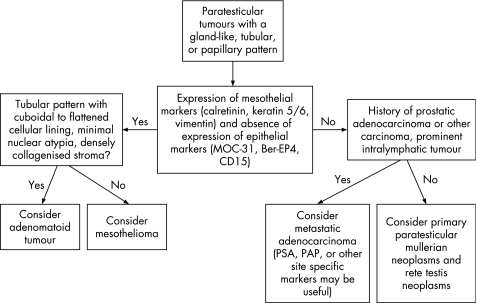
Paratesticular tumours with a glandular‐like, tubular or papillary pattern
An algorithm showing the usual diagnostic approach for paratesticular tumours with a gland‐like, tubular or papillary pattern, including typical (but not invariable) immunohistochemical staining reactions, is shown in fig 16.
Figure 16 Algorithm showing the usual diagnostic approach for paratesticular tumours with a gland‐like, tubular or papillary pattern, including typical (but not invariable) immunohistochemical staining reactions. PSA, prostate specific antigen; PAP, prostatic acid phosphatase.
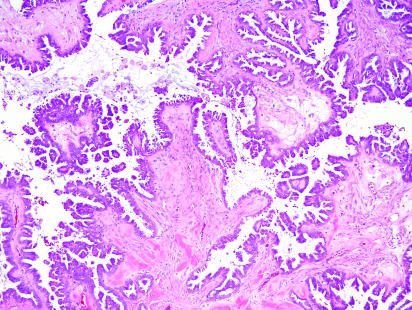
Paratesticular tumours with a glandular, tubular or papillary histological appearance include metastatic adenocarcinoma, mullerian‐type epithelial tumours (fig 17), adenomatoid tumour and mesothelioma. Rare tumours of the rete testis such as rete adenocarcinoma and Sertoliform cystadenoma of the rete testis may also occur. Occasional germ cell tumours or sex‐cord stromal tumours may simulate a paratesticular neoplasm in a few cases.
Figure 17 Papillary serous borderline tumour growing on the external aspect of the testis. Note the branching fibrovascular cores and the detached buds of epithelium.
Classification of a paratesticular tumour into the broad categories of epithelial tumours, mesothelial tumours, sex cord‐stromal tumours or germ cell tumours can be facilitated with a panel of immunohistochemical stains. An initial useful panel is one similar to that used for differentiating epithelial and mesothelial neoplasms of other sites, possibly including MOC‐31 (ERA), BerEP4 and CD15 as epithelial markers and calretinin, keratin 5/6 and vimentin as mesothelial markers. Inhibin‐α could also be included to account for the possibility of a sex cord‐stromal tumour extending into the paratesticular region.
Metastatic adenocarcinomas are more common than paratesticular mullerian neoplasms. Glandular or papillary architecture may be seen in metastatic prostatic adenocarcinoma which not uncommonly may have a prominent intrarete pattern of growth. PSA and PAP immunostaining may be useful if this is a diagnostic consideration.42
Paratesticular mullerian neoplasms consistently stain with antibodies for keratin AE1/AE3 and BerEP4,85,86,87 but may also be immunoreactive for PLAP.88 Expression of cytokeratin 7, MOC‐31 and CD15 is also common and immunoreactivity for oestrogen receptor protein or progesterone receptor protein is supportive of a mullerian‐type tumour.85
Similar to mesothelioma of the pleura or peritoneal cavity, scrotal mesothelioma may have glandular or biphasic morphology. The glandular component may have a tubular or papillary pattern. The cells are cuboidal and the cytoplasm is usually eosinophilic. Confirmation of the diagnosis by immunohistochemical staining is usually advisable.
Paratesticular adenomatoid tumours are also of mesothelial origin, but are benign. They are typically composed of small tubules lined by cuboidal to flattened cells with minimal nuclear atypia, often in a densely collagenised stroma. Usually the cells have conspicuous cytoplasmic vacuoles, but these may be minimal in some cases. These tumours may resemble some of the sex cord‐stromal tumours, germ cell tumours or metastatic adenocarcinomas that have a tubular pattern. In such cases, confirmation of the mesothelial differentiation of the tubule lining cells may be helpful.
Most paratesticular adenomatoid tumours and malignant mesotheliomas, in contrast to many adenocarcinomas, stain for calretinin, keratin 5/6 and vimentin, but not with MOC‐31, Ber‐EP4 and CD15.51,85,89,90,91,92 Calretinin is not specific for mesothelioma and is also commonly seen in sex cord‐stromal tumours93 and urothelial carcinomas.57
Tumours with spindle cell morphology
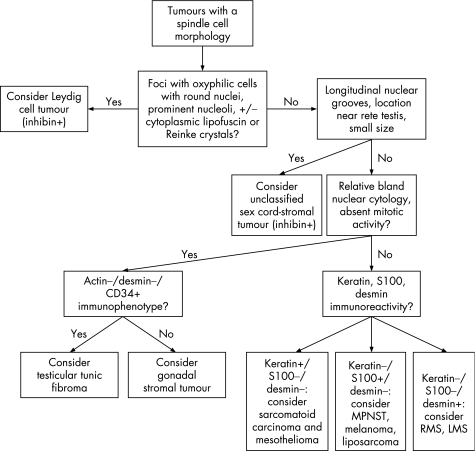
A variety of primary testicular, paratesticular and secondary neoplasms may have a partially or diffusely spindle cell appearance. Unclassified sex cord‐stromal tumours may be composed of spindle cells, some of which may have longitudinal nuclear grooves. In our experience and that of others,60 lesions with this appearance are often small, typically <1 cm, with a tendency to occur near the rete testis. S‐100 protein and smooth muscle actin immunoreactivity may be seen.60 An algorithm showing the usual diagnostic approach for tumours with a spindle cell morphology, including typical (but not invariable) immunohistochemical staining reactions, is shown in fig 18.
Figure 18 Algorithm showing the usual diagnostic approach for tumours with a spindle cell morphology, including typical (but not invariable) immunohistochemical staining reactions. MPNST, malignant peripheral nerve sheath tumour; RMS, rhabdomyosarcoma; LMS, leiomyosarcoma.
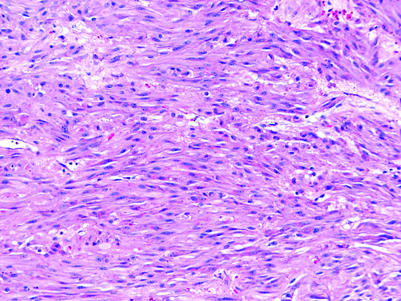
Leydig cell tumours may be composed partially of spindle cells (fig 19).44 The presence of typical morphology in other foci is of crucial help in the diagnosis of these cases. Inhibin‐α, calretinin and melan‐A expression is usually retained but can be lost in the sarcomatoid component of a malignant Leydig cell tumour.44
Figure 19 Leydig cell tumour with spindle cell pattern.
Benign fibrous tumours of the testis and paratestis exist. Gonadal stromal fibromas usually demonstrate an actin+/desmin+/CD34− immunophenotype while testicular tunic fibromas demonstrate an actin−/desmin−/CD34+ immunophenotype.94
Malignant mesotheliomas of the tunica vaginalis have a sarcomatous or biphasic morphology in about half of cases.90,95 In the case of a suspected mesothelioma, calretinin, vimentin, and keratin 5/6 staining may be useful supportive evidence.90,95,96 Sarcomatoid carcinoma must be excluded by the absence of immunoreactivity with epithelial markers such as B72.3, Leu‐M1 and Ber‐EP4.90 S‐100 protein is negative, which helps to exclude the possibilities of metastatic melanoma and malignant peripheral nerve sheath tumour, as does significant cytokeratin reactivity.96
In children and adolescents, rhabdomyosarcoma is a significant consideration for a paratesticular neoplasm. Most are of the embryonal subtype, although approximately 6% are of the alveolar subtype,97 consisting mostly of relatively uniform cells with densely chromatic nuclei, often arranged in nests with central discohesion and sometimes having occasional tumour giant cells. The spindle cell variant of embryonal rhabdomyosarcoma, which is prognostically favourable, tends to be confused with other spindle cell tumours, notably leiomyosarcoma or nerve sheath tumours. Myogenin and Myo‐D1 reactivity, however, are confirmatory. Other considerations include leiomyosarcoma, which may develop within the testis98 or paratesticular region,99 and has the expected smooth muscle actin and desmin staining. Liposarcomas, including sclerosing liposarcomas with areas of spindle cell morphology, may also occur in the spermatic cord.
Testicular scar‐like lesions
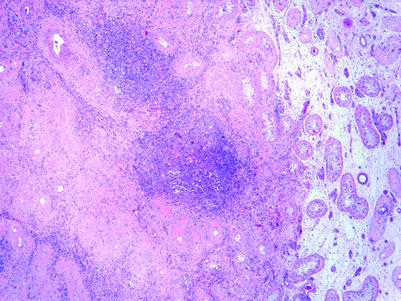
Patients without treatment prior to orchiectomy may occasionally present with a testicular mass that, upon histological evaluation, is composed of densely collagenised tissue without readily identifiable neoplastic tissue. More commonly, patients may present with metastatic germ cell tumour to the retroperitoneum or other sites, and a small testis mass is discovered on ultrasonographic evaluation. These testicular scars may be nodular or stellate. The surrounding testis is typically atrophic. Lymphoplasmacytic infiltration, hyalinised “ghost” tubules and vascular prominence are commonly seen in the scars (fig 20) and IGCNU and Leydig cell prominence are commonly seen in the surrounding testis.100 These features may be useful in recognising the significance of these lesions as regressed germ cell tumours.100
Figure 20 Mostly regressed germ cell tumour showing fibrous scar with lymphocytic infiltrate, hyalinised tubules within the scar and atrophic tubules peripheral to it. Small foci of embryonal carcinoma (not visible at this magnification) remain amid the lymphocytes.
Abbreviations
AFP - α‐fetoprotein
EMA - epithelial membrane antigen
hCG - human chorionic gonadotropin
IGCNU - intratubular germ cell neoplasia, unclassified type
LDH - lactate dehydrogenase
PAP - prostatic acid phosphatase
PLAP - placental alkaline phosphatase
PSA - prostate specific antigen
RCC - renal cell carcinoma associated antigen
Footnotes
Competing interests: None declared.
References
- 1.Lawrence W D, Young R H, Scully R E. Juvenile granulosa cell tumor of the infantile testis. A report of 14 cases. Am J Surg Pathol 1985987–94. [DOI] [PubMed] [Google Scholar]
- 2.Talerman A. Endodermal sinus (yolk sac) tumor elements in testicular germ‐cell tumors in adults: comparison of prospective and retrospective studies. Cancer 1980461213–1217. [DOI] [PubMed] [Google Scholar]
- 3.Bergada I, Del Toro K, Katz O.et al Serum inhibin B concentration in a prepubertal boy with gynecomastia and Peutz‐Jeghers syndrome. J Pediatr Endocrinol Metab 200013101–103. [DOI] [PubMed] [Google Scholar]
- 4.Toppari J, Kaipia A, Kaleva M.et al Inhibin gene expression in a large cell calcifying Sertoli cell tumour and serum inhibin and activin levels. APMIS 1998106101–113. [DOI] [PubMed] [Google Scholar]
- 5.Fung L, Honey R, Gardiner G. Testicular seminoma presenting with features of androgen excess. Urology 199444927–929. [DOI] [PubMed] [Google Scholar]
- 6.Nagi D K, Jones W G, Belchetz P E. Gynaecomastia caused by a primary mediastinal seminoma. Clin Endocrinol (Oxf) 199440545–549. [DOI] [PubMed] [Google Scholar]
- 7.Fox H, Reeve N L. Endocrine effects of testicular neoplasms. Invest Cell Pathol 1979263–73. [PubMed] [Google Scholar]
- 8.Morrish D W, Venner P M, Siy O.et al Mechanisms of endocrine dysfunction in patients with testicular cancer. J Natl Cancer Inst 199082412–418. [DOI] [PubMed] [Google Scholar]
- 9.da Silva M A, Edmondson J W, Eby C.et al Humoral hypercalcemia in seminomas. Med Pediatr Oncol 19922038–41. [DOI] [PubMed] [Google Scholar]
- 10.Kim I, Young R H, Scully R E. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol 19859177–192. [DOI] [PubMed] [Google Scholar]
- 11.Waxman M, Damjanov I, Khapra A.et al Large cell calcifying Sertoli tumor of the testis. Light microscopic and ultrastructural study. Cancer 1984541574–1581. [DOI] [PubMed] [Google Scholar]
- 12.Berdjis C C, Mostofi F K. Carcinoid tumors of the testis. J Urol 1977118777–782. [DOI] [PubMed] [Google Scholar]
- 13.Kirkland R T, Kirkland J L, Keenan B S.et al Bilateral testicular tumors in congenital adrenal hyperplasia. J Clin Endocrinol Metab 197744369–378. [DOI] [PubMed] [Google Scholar]
- 14.Rutgers J L, Young R H, Scully R E. The testicular “tumor” of the adrenogenital syndrome. A report of six cases and review of the literature on testicular masses in patients with adrenocortical disorders. Am J Surg Pathol 198812503–513. [PubMed] [Google Scholar]
- 15.Venara M, Rey R, Bergada I.et al Sertoli cell proliferations of the infantile testis: an intratubular form of Sertoli cell tumor? Am J Surg Pathol 2001251237–1244. [DOI] [PubMed] [Google Scholar]
- 16.Ulbright T M, Amin M B, Young R H. Intratubular large cell hyalinizing Sertoli cell neoplasia of the testis: a report of 8 cases of a distinctive lesion of the Peutz‐Jeghers syndrome. Am J Surg Pathol 200731827–835. [DOI] [PubMed] [Google Scholar]
- 17.Caroll P, Morse M, Koduru P.et al Testicular cerm cell tumor in patient with Klinefelter syndrome. Urology 19883172–74. [DOI] [PubMed] [Google Scholar]
- 18.Albores‐Saavedra J, Huffman H, Alvarado‐Cabrero I.et al Anaplastic variant of spermatocytic seminoma. Hum Pathol 199627650–655. [DOI] [PubMed] [Google Scholar]
- 19.Kraggerud S M, Berner A, Bryne M.et al Spermatocytic seminoma as compared to classical seminoma: an immunohistochemical and DNA flow cytometric study. APMIS 1999107297–302. [DOI] [PubMed] [Google Scholar]
- 20.Cummings O W, Ulbright T M, Eble J N.et al Spermatocytic seminoma: an immunohistochemical study. Hum Pathol 19942554–59. [DOI] [PubMed] [Google Scholar]
- 21.Looijenga L H, Stoop H, de Leeuw H P.et al POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 2003632244–2250. [PubMed] [Google Scholar]
- 22.Jones T D, Ulbright T M, Eble J N.et al OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 200428935–940. [DOI] [PubMed] [Google Scholar]
- 23.Cheville J C, Rao S, Iczkowski K A.et al Cytokeratin expression in seminoma of the human testis. Am J Clin Pathol 2000113583–588. [DOI] [PubMed] [Google Scholar]
- 24.Niehans G A, Manivel J C, Copland G T.et al Immunohistochemistry of germ cell and trophoblastic neoplasms. Cancer 1988621113–1123. [DOI] [PubMed] [Google Scholar]
- 25.Leroy X, Augusto D, Leteurtre E.et al CD30 and CD117 (c‐kit) used in combination are useful for distinguishing embryonal carcinoma from seminoma. J Histochem Cytochem 200250283–285. [DOI] [PubMed] [Google Scholar]
- 26.Bentley A J, Parkinson M C, Harding B N.et al A comparative morphological and immunohistochemical study of testicular seminomas and intracranial germinomas. Histopathology 199017443–449. [DOI] [PubMed] [Google Scholar]
- 27.Izquierdo M A, Van der Valk P, Van Ark‐Otte J.et al Differential expression of the c‐kit proto‐oncogene in germ cell tumours. J Pathol 1995177253–258. [DOI] [PubMed] [Google Scholar]
- 28.Ferreiro J A. Ber‐H2 expression in testicular germ cell tumors. Hum Pathol 199425522–524. [DOI] [PubMed] [Google Scholar]
- 29.Suster S, Moran C A, Dominguez‐Malagon H.et al Germ cell tumors of the mediastinum and testis: a comparative immunohistochemical study of 120 cases. Hum Pathol 199829737–742. [DOI] [PubMed] [Google Scholar]
- 30.Badve S, Morimiya A, Agarwal B.et al Podoplanin: a histological marker for seminoma. Mod Pathol 20061988129A [Google Scholar]
- 31.Eglen D E, Ulbright T M. The differential diagnosis of yolk sac tumor and seminoma. Usefulness of cytokeratin, alpha‐fetoprotein, and alpha‐1‐antitrypsin immunoperoxidase reactions. Am J Clin Pathol 198788328–332. [DOI] [PubMed] [Google Scholar]
- 32.Zynger D L, Dimov N D, Luan C.et al Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol 2006301570–1575. [DOI] [PubMed] [Google Scholar]
- 33.Henley J D, Young R H, Ulbright T M. Malignant Sertoli cell tumors of the testis: a study of 13 examples of a neoplasm frequently misinterpreted as seminoma. Am J Surg Pathol 200226541–550. [DOI] [PubMed] [Google Scholar]
- 34.Kommoss F, Oliva E, Bittinger F.et al Inhibin‐alpha CD99, HEA125, PLAP, and chromogranin immunoreactivity in testicular neoplasms and the androgen insensitivity syndrome. Hum Pathol 2000311055–1061. [DOI] [PubMed] [Google Scholar]
- 35.Iczkowski K A, Bostwick D G, Roche P C.et al Inhibin A is a sensitive and specific marker for testicular sex cord‐stromal tumors. Mod Pathol 199811774–779. [PubMed] [Google Scholar]
- 36.de Jong J, Stoop H, Dohle G R.et al Diagnostic value of OCT3/4 for pre‐invasive and invasive testicular germ cell tumours. J Pathol 2005206242–249. [DOI] [PubMed] [Google Scholar]
- 37.Shen S S, Krishna B, Chirala R.et al Kidney‐specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod Pathol 200518933–940. [DOI] [PubMed] [Google Scholar]
- 38.Avery A K, Beckstead J, Renshaw A A.et al Use of antibodies to RCC and CD10 in the differential diagnosis of renal neoplasms. Am J Surg Pathol 200024203–210. [DOI] [PubMed] [Google Scholar]
- 39.Wang H Y, Mills S E. KIT and RCC are useful in distinguishing chromophobe renal cell carcinoma from the granular variant of clear cell renal cell carcinoma. Am J Surg Pathol 200529640–646. [DOI] [PubMed] [Google Scholar]
- 40.McGregor D K, Khurana K K, Cao C.et al Diagnosing primary and metastatic renal cell carcinoma: the use of the monoclonal antibody ‘Renal Cell Carcinoma Marker'. Am J Surg Pathol 2001251485–1492. [DOI] [PubMed] [Google Scholar]
- 41.Patel S R, Richardson R L, Kvols L. Metastatic cancer to the testes: a report of 20 cases and review of the literature. J Urol 19891421003–1005. [DOI] [PubMed] [Google Scholar]
- 42.Tu S M, Reyes A, Maa A.et al Prostate carcinoma with testicular or penile metastases. Clinical, pathologic, and immunohistochemical features. Cancer 2002942610–2617. [DOI] [PubMed] [Google Scholar]
- 43.Baykal K, Yildirim S, Inal H.et al Metastasis of prostate adenocarcinoma to testis. Int J Urol 19974104–105. [DOI] [PubMed] [Google Scholar]
- 44.Ulbright T M, Srigley J R, Hatzianastassiou D K.et al Leydig cell tumors of the testis with unusual features: adipose differentiation, calcification with ossification, and spindle‐shaped tumor cells. Am J Surg Pathol 2002261424–1433. [DOI] [PubMed] [Google Scholar]
- 45.Ferry J A, Harris N L, Young R H.et al Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol 199418376–390. [DOI] [PubMed] [Google Scholar]
- 46.Ulbright T M, Young R H. Seminoma with tubular, microcystic, and related patterns: a study of 28 cases of unusual morphologic variants that often cause confusion with yolk sac tumor. Am J Surg Pathol 200529500–505. [DOI] [PubMed] [Google Scholar]
- 47.Manivel J C, Jessurun J, Wick M R.et al Placental alkaline phosphatase immunoreactivity in testicular germ‐cell neoplasms. Am J Surg Pathol 19871121–29. [DOI] [PubMed] [Google Scholar]
- 48.McCluggage W G, Ashe P, McBride H.et al Localization of the cellular expression of inhibin in trophoblastic tissue. Histopathology 199832252–256. [DOI] [PubMed] [Google Scholar]
- 49.Mostofi F K, Sesterhenn I A, Davis C J., Jr Immunopathology of germ cell tumors of the testis. Semin Diagn Pathol 19874320–341. [PubMed] [Google Scholar]
- 50.Ulbright T M, Gersell D J. Rete testis hyperplasia with hyaline globule formation. A lesion simulating yolk sac tumor. Am J Surg Pathol 19911566–74. [DOI] [PubMed] [Google Scholar]
- 51.Amin M B. Selected other problematic testicular and paratesticular lesions: rete testis neoplasms and pseudotumors, mesothelial lesions and secondary tumors. Mod Pathol 200518(Suppl 2)S131–S145. [DOI] [PubMed] [Google Scholar]
- 52.Weirich G, Nahrig J, Treiber U.et al Immunohistochemical assessment of a testicular tumor in a 63‐year‐old patient: proposal for an integrated clinicopathologic approach. Appl Immunohistochem Mol Morphol 20031196–100. [DOI] [PubMed] [Google Scholar]
- 53.Billings S D, Roth L M, Ulbright T M. Microcystic Leydig cell tumors mimicking yolk sac tumor: a report of four cases. Am J Surg Pathol 199923546–551. [DOI] [PubMed] [Google Scholar]
- 54.Young R H, Koelliker D D, Scully R E. Sertoli cell tumors of the testis, not otherwise specified: a clinicopathologic analysis of 60 cases. Am J Surg Pathol 199822709–721. [DOI] [PubMed] [Google Scholar]
- 55.McCluggage W G, Shanks J H, Whiteside C.et al Immunohistochemical study of testicular sex cord‐stromal tumors, including staining with anti‐inhibin antibody. Am J Surg Pathol 199822615–619. [DOI] [PubMed] [Google Scholar]
- 56.Busam K J, Iversen K, Coplan K A.et al Immunoreactivity for A103, an antibody to melan‐A (Mart‐1), in adrenocortical and other steroid tumors. Am J Surg Pathol 19982257–63. [DOI] [PubMed] [Google Scholar]
- 57.Lugli A, Forster Y, Haas P.et al Calretinin expression in human normal and neoplastic tissues: a tissue microarray analysis on 5233 tissue samples. Hum Pathol 200334994–1000. [DOI] [PubMed] [Google Scholar]
- 58.Movahedi‐Lankarani S, Kurman R J. Calretinin, a more sensitive but less specific marker than alpha‐inhibin for ovarian sex cord‐stromal neoplasms: an immunohistochemical study of 215 cases. Am J Surg Pathol 2002261477–1483. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz E J, Longacre T A. Adenomatoid tumors of the female and male genital tracts express WT1. Int J Gynecol Pathol 200423123–128. [DOI] [PubMed] [Google Scholar]
- 60.Renshaw A A, Gordon M, Corless C L. Immunohistochemistry of unclassified sex cord‐stromal tumors of the testis with a predominance of spindle cells. Mod Pathol 199710693–700. [PubMed] [Google Scholar]
- 61.Plata C, Algaba F, Andujar M.et al Large cell calcifying Sertoli cell tumour of the testis. Histopathology 199526255–259. [DOI] [PubMed] [Google Scholar]
- 62.Kratzer S S, Ulbright T M, Talerman A.et al Large cell calcifying Sertoli cell tumor of the testis: contrasting features of six malignant and six benign tumors and a review of the literature. Am J Surg Pathol 1997211271–1280. [DOI] [PubMed] [Google Scholar]
- 63.De Raeve H, Schoonooghe P, Wibowo R.et al Malignant large cell calcifying Sertoli cell tumor of the testis. Pathol Res Pract 2003199113–117. [DOI] [PubMed] [Google Scholar]
- 64.Cano‐Valdez A M, Chanona‐Vilchis J, Dominguez‐Malagon H. Large cell calcifying Sertoli cell tumor of the testis: a clinicopathological, immunohistochemical, and ultrastructural study of two cases. Ultrastruct Pathol 199923259–265. [DOI] [PubMed] [Google Scholar]
- 65.Bufo P, Pennella A, Serio G, Mastropasqua M G.et al Malignant large cell calcifying Sertoli cell tumor of the testis (LCCSCTT). Report of a case in an elderly man and review of the literature. Pathologica 199991107–114. [PubMed] [Google Scholar]
- 66.Bennett A, Ulbright T M, Ramnani D.et al Immunohistochemical expression of calretinin, CD99, and alpha‐inhibin in Sertoli and Leydig cells and their lesions, emphasizing large cell calcifying Sertoli cell tumor. Mod Pathol 200518128A [Google Scholar]
- 67.Talerman A, Roth L M.Pathology of the testis and its adnexa. New York: Churchill Livingstone, 1986
- 68.Reyes A, Moran C A, Suster S.et al Neuroendocrine carcinomas (carcinoid tumor) of the testis. A clinicopathologic and immunohistochemical study of ten cases. Am J Clin Pathol 2003120182–187. [DOI] [PubMed] [Google Scholar]
- 69.Zavala‐Pompa A, Ro J Y, el‐Naggar A.et al Primary carcinoid tumor of testis. Immunohistochemical, ultrastructural, and DNA flow cytometric study of three cases with a review of the literature. Cancer 1993721726–1732. [DOI] [PubMed] [Google Scholar]
- 70.Comperat E, Tissier F, Boye K.et al Non‐Leydig sex‐cord tumors of the testis. The place of immunohistochemistry in diagnosis and prognosis. A study of twenty cases. VirchowsArch2004444567–571. [DOI] [PubMed] [Google Scholar]
- 71.Kremer M, Ott G, Nathrath M.et al Primary extramedullary plasmacytoma and multiple myeloma: phenotypic differences revealed by immunohistochemical analysis. J Pathol 200520592–101. [DOI] [PubMed] [Google Scholar]
- 72.Ferry J A, Young R H, Scully R E. Testicular and epididymal plasmacytoma: a report of 7 cases, including three that were the initial manifestation of plasma cell myeloma. Am J Surg Pathol 199721590–598. [DOI] [PubMed] [Google Scholar]
- 73.Goswitz J J, Pettinato G, Manivel J C. Testicular sex cord‐stromal tumors in children: clinicopathologic study of sixteen children with review of the literature. Pediatr Pathol Lab Med 199616451–470. [DOI] [PubMed] [Google Scholar]
- 74.Skakkebaek N E. Carcinoma in situ of the testis: frequency and relationship to invasive germ cell tumours in infertile men. Histopathology 19782157–170. [DOI] [PubMed] [Google Scholar]
- 75.Skakkebaek N E, Berthelsen J, Visfeld J. Clinical aspects of testicular carcinoma‐in‐situ. Int J Androl 19814(Suppl)153–162. [DOI] [PubMed] [Google Scholar]
- 76.Burke A P, Mostofi F K. Intratubular malignant germ cells in testicular biopsies: clinical course and identification by staining for placental alkaline phosphatase. Mod Pathol 19881475–479. [PubMed] [Google Scholar]
- 77.Kuczyk M A, Serth J, Bokemeyer C.et al Overexpression of the p53 oncoprotein in carcinoma in situ of the testis. Pathol Res Pract 1994190993–998. [DOI] [PubMed] [Google Scholar]
- 78.Ramani P, Yeung C K, Habeebu S S. Testicular intratubular germ cell neoplasia in children and adolescents with intersex. Am J Surg Pathol 1993171124–1133. [DOI] [PubMed] [Google Scholar]
- 79.Loftus B M, Gilmartin L G, O'Brien M J.et al Intratubular germ cell neoplasia of the testis: identification by placental alkaline phosphatase immunostaining and argyrophilic nucleolar organizer region quantification. Hum Pathol 199021941–948. [DOI] [PubMed] [Google Scholar]
- 80.Giwercman A, Cantell L, Marks A. Placental‐like alkaline phosphatase as a marker of carcinoma‐in‐situ of the testis. Comparison with monoclonal antibodies M2A and 43‐9F. APMIS 199199586–594. [DOI] [PubMed] [Google Scholar]
- 81.Hustin J, Collette J, Franchimont P. Immunohistochemical demonstration of placental alkaline phosphatase in various states of testicular development and in germ cell tumours. Int J Androl 19871029–35. [DOI] [PubMed] [Google Scholar]
- 82.Rajpert‐De Meyts E, Skakkebaek N E. Expression of the c‐kit protein product in carcinoma‐in‐situ and invasive testicular germ cell tumours. Int J Androl 19941785–92. [DOI] [PubMed] [Google Scholar]
- 83.Sandlow J I, Feng H L, Cohen M B.et al Expression of c‐KIT and its ligand, stem cell factor, in normal and subfertile human testicular tissue. J Androl 199617403–408. [PubMed] [Google Scholar]
- 84.Berney D M, Lee A, Shamash J.et al The frequency and distribution of intratubular trophoblast in association with germ cell tumors of the testis. Am J Surg Pathol 2005291300–1303. [DOI] [PubMed] [Google Scholar]
- 85.McClure R F, Keeney G L, Sebo T J.et al Serous borderline tumor of the paratestis: a report of seven cases. Am J Surg Pathol 200125373–378. [DOI] [PubMed] [Google Scholar]
- 86.Ulbright T M, Young R H. Primary mucinous tumors of the testis and paratestis: a report of nine cases. Am J Surg Pathol 2003271221–1228. [DOI] [PubMed] [Google Scholar]
- 87.Young R H, Scully R E. Testicular and paratesticular tumors and tumor‐like lesions of ovarian common epithelial and mullerian types. A report of four cases and review of the literature. Am J Clin Pathol 198686146–152. [DOI] [PubMed] [Google Scholar]
- 88.Jones M A, Young R H, Srigley J R.et al Paratesticular serous papillary carcinoma. A report of six cases. Am J Surg Pathol 1995191359–1365. [DOI] [PubMed] [Google Scholar]
- 89.Delahunt B, Eble J N, King D.et al Immunohistochemical evidence for mesothelial origin of paratesticular adenomatoid tumour. Histopathology 200036109–115. [DOI] [PubMed] [Google Scholar]
- 90.Jones M A, Young R H, Scully R E. Malignant mesothelioma of the tunica vaginalis. A clinicopathologic analysis of 11 cases with review of the literature. Am J Surg Pathol 199519815–825. [DOI] [PubMed] [Google Scholar]
- 91.Iczkowski K A, Katz G, Zander D S.et al Malignant mesothelioma of tunica vaginalis testis: a fatal case with liver metastasis. J Urol 2002167645–646. [DOI] [PubMed] [Google Scholar]
- 92.Winstanley A M, Landon G, Berney D.et al The immunohistochemical profile of malignant mesotheliomas of the tunica vaginalis: a study of 20 cases. Am J Surg Pathol 2006301–6. [DOI] [PubMed] [Google Scholar]
- 93.Cao Q J, Jones J G, Li M. Expression of calretinin in human ovary, testis, and ovarian sex cord‐stromal tumors. Int J Gynecol Pathol 200120346–352. [DOI] [PubMed] [Google Scholar]
- 94.Jones M A, Young R H, Scully R E. Benign fibromatous tumors of the testis and paratesticular region: a report of 9 cases with a proposed classification of fibromatous tumors and tumor‐like lesions. Am J Surg Pathol 199721296–305. [DOI] [PubMed] [Google Scholar]
- 95.Shimada S, Ono K, Suzuki Y.et al Malignant mesothelioma of the tunica vaginalis testis: a case with a predominant sarcomatous component. Pathol Int 200454930–934. [DOI] [PubMed] [Google Scholar]
- 96.Agapitos E, Pavlopoulos P M, Marinos E.et al Malignant mesothelioma of the tunica vaginalis testis: an immunohistochemical and ultrastructural study of two cases. Br J Urol 199780345–346. [DOI] [PubMed] [Google Scholar]
- 97.Leuschner I, Newton W A, Jr, Schmidt D.et al Spindle cell variants of embryonal rhabdomyosarcoma in the paratesticular region. A report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol 199317221–230. [DOI] [PubMed] [Google Scholar]
- 98.Washecka R M, Mariani A J, Zuna R E.et al Primary intratesticular sarcoma. Immunohistochemical ultrastructural and DNA flow cytometric study of three cases with a review of the literature. Cancer 1996771524–1528. [DOI] [PubMed] [Google Scholar]
- 99.Fisher C, Goldblum J R, Epstein J I.et al Leiomyosarcoma of the paratesticular region: a clinicopathologic study. Am J Surg Pathol 2001251143–1149. [DOI] [PubMed] [Google Scholar]
- 100.Balzer B L, Ulbright T M. Spontaneous regression of testicular germ cell tumors: an analysis of 42 cases. Am J Surg Pathol 200630858–865. [DOI] [PubMed] [Google Scholar]
- 101.Hawkins E, Heifetz S A, Giller R.et al The prepubertal testis (prenatal and postnatal): its relationship to intratubular germ cell neoplasia: a combined Pediatric Oncology Group and Children's Cancer Study Group. Hum Pathol 199728404–410. [DOI] [PubMed] [Google Scholar]
- 102.Kaufmann O, Koch S, Burghardt J.et al Tyrosinase, melan‐A, and KBA62 as markers for the immunohistochemical identification of metastatic amelanotic melanomas on paraffin sections. Mod Pathol 199811740–746. [PubMed] [Google Scholar]
- 103.Leroy X, Zini L, Leteurtre E.et al Morphologic subtyping of papillary renal cell carcinoma: correlation with prognosis and differential expression of MUC1 between the two subtypes. Mod Pathol 2002151126–1130. [DOI] [PubMed] [Google Scholar]
- 104.Polski J M, Janney C G. Ber‐H2 (CD30) immunohistochemical staining in malignant melanoma. Mod Pathol 199912903–906. [PubMed] [Google Scholar]
- 105.Wick M R, Swanson P E, Manivel J C. Placental‐like alkaline phosphatase reactivity in human tumors: an immunohistochemical study of 520 cases. Hum Pathol 198718946–954. [DOI] [PubMed] [Google Scholar]
- 106.Hittmair A, Rogatsch H, Hobisch A.et al CD30 expression in seminoma. Hum Pathol 1996271166–1171. [DOI] [PubMed] [Google Scholar]