We report a case of prostatic ductal adenocarcinoma which expressed thyroid transcription factor‐1 (TTF‐1) on immunohistochemical examination, and highlight the fact that this aberrant staining is a potential pitfall in the evaluation of this lesion. Prostatic core biopsy specimens from a 65‐year‐old man revealed an adenocarcinoma with large‐calibre glands lined by stratified hyperchromatic nuclei. There was no accompanying conventional acinar adenocarcinoma and TTF‐1 was distinctly positive in the nuclei of the malignant cells. This led to a search for possible lung and other primaries. When a thorough systemic investigation disclosed no evidence of other primary tumours, a radical prostatectomy was performed which revealed predominant ductal adenocarcinoma with a minor component of acinar adenoarcinoma. TTF‐1 measurement was repeated which showed staining confined to the ductal adenocarcinoma. Prostate specific antigen (PSA), PSAP and racemase were also found. Our findings underscore the increasing spectrum of lesions that may express TTF‐1 on immunohistochemical examination.
TTF‐1 is part of a family of homeodomain transcription factors with restricted expression in the thyroid and lung.1,2,3 Its immunohistochemical detection has been used in diagnostic surgical pathology to distinguish thyroid and lung tumours from those of other organs.4,5 Specifically, antibodies to TTF‐1 have been reported to be useful in distinguishing pulmonary adenocarcinoma from other primary carcinomas.6 TTF‐1 is expressed by differentiated thyroid neoplasms.7 It can assist in the differential diagnostic investigation of mesothelioma from pulmonary adenocarcinoma, small cell lung carcinoma from Merkel cell carcinoma,2,3 and neuroendocrine tumours of the lung from well‐differentiated neuro‐endocrine tumours at other sites.1,8 TTF‐1 mRNA has been discovered in orbital tissues,9 C cells and parathyroid cells.10
More recently, nuclear TTF‐1 protein expression was reported in primary and metastatic colonic adenocarcinoma,11,12 primary ovarian epithelial neoplasms,13 thyroid‐like nasopharyngeal papillary adenocarcinoma,7 gastric atrophic gastritis and ciliated metaplasia,14 and brain ependymomas.15 Cytoplasmic TTF‐1 reactivity has also been described in hepatocellular carcinoma and normal hepatocytes.16,17,18 We recently encountered a case of prostatic ductal type adenocarcinoma on core biopsy which reacted with the TTF‐1 antibody leading to a search to exclude a possible primary lung adenocarcinoma.
Case report
A 65‐year‐old Chinese man attended for a follow‐up examination for episodic gross haematuria. Flexible urethrocystoscopy was unremarkable, but he was found to have a raised serum PSA level of 4.42 μg/l. Digital rectal examination revealed a 1 cm nodule over the left lateral prostate lobe. A transrectal ultrasound‐guided 12‐core prostate biopsy showed left lobe cores featuring adenocarcinoma with papillary and cribriform architecture lined by cells with hyperchromatic stratified nuclei (fig 1A, B). Some extracellular mucin was found. There was no conventional acinar adenocarcinoma or high‐grade prostatic intraepithelial neoplasia. Although ductal prostatic adenocarcinoma was considered, immunohistochemistry was performed to confirm a prostatic origin of the malignant glands, as well as to rule out possible metastases from the lung and colon. Immunohistochemical examination showed nuclear reactivity for TTF‐1 (Novocastra, dilution 1:50, clone: SPT24, antigen retrieval: pressure cook in citrate buffer at pH 6.0) with focal positivity for CK7, 34βE12 and CK5/6 (fig 1C). No staining was found for CK20, thyroglobulin, PSA (DAKO M075001, clone ER‐PR8, IgG1, dilution 1:400, antigen retrieval: pressure cook in microwave oven, in Tris EDTA at pH 9.0) and PSAP (DAKO M0792, clone PASE/4LJ, IgG1, dilution 1:4000, no antigen retrieval). A comment was made to rule out the possibility of lung metastases to the prostate gland.
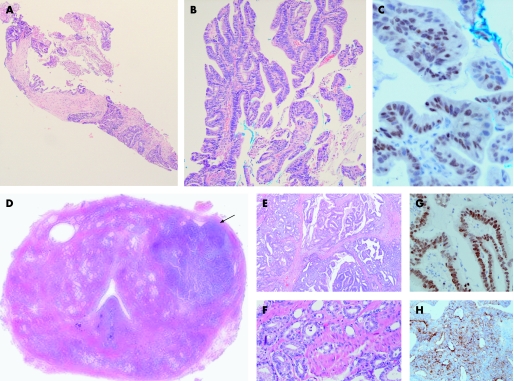
Figure 1 (A) Prostatic core biopsy specimen showing malignant large cribriform glands occupying the entire core length. (B) Papillary structures with fibrovascular cores covered by columnar cells with stratified hyperchromatic nuclei. (C) Thyroid transcription factor‐1 (TTF‐1) expressed in the nuclei of the malignant cells. (D) Whole mount of radical prostatectomy specimen with a dominant nodule in the left lobe anteriorly (arrow). (E) Papillary and cribriform structures with columnar cells resembling features seen in the core biopsies. (F) Acinar adenocarcinoma component with malignant acini permeating in between benign prostatic glands in the radical prostatectomy. (G) TTF‐1 nuclear positivity in ductal adenocarcinoma. (H) Cytoplasmic granular reactivity for prostate specific antigen (PSA) in ductal adenocarcinoma cells of radical prostatectomy.
Chest radiography and gastrointestinal endoscopy showed no remarkable findings. CT scans of the thorax, abdomen and pelvis revealed a 2.1×1.7 cm enhancing nodule in the left prostate lobe without extraprostatic extension and no enlarged pelvic lymph nodes.
The patient sought a second opinion. Repeat measurement of the serum PSA level was 5.2 μg/l. He opted for a radical prostatectomy (robot‐assisted) in view of the negative search for an alternative primary tumour. The radical prostatectomy specimen showed a vague 2 cm ill‐defined nodular lesion macroscopically discernible in the left lobe. Histologically, adenocarcinoma with both ductal and conventional acinar appearances was present, predominantly affecting the left lobe. Ductal type adenocarcinoma formed approximately 80% of the tumour, while acinar adenocarcinoma Gleason 4+3 constituted the rest (fig 1D, E, F). There was perineural invasion and a single lymphovascular embolus. High‐grade prostatic intraepithelial neoplasia was found in the peripheral zones of both lobes posteriorly. There was focal extraprostatic extension, pT3a. Both right (×8) and left (×12) iliac lymph nodes were without metastases. Immunohistochemistry for TTF‐1 (Novocastra, dilution 1:10, clone: SPT24, antigen retrieval: microwave in microwave oven, milestone T/T Mega at 98°C, 12 min in Tris‐EDTA, pH 9.0) was repeated, revealing distinct nuclear positivity in the ductal adenocarcinoma cells (fig 1F). No TTF‐1 reactivity was observed in the acinar adenocarcinoma. PSA (polyclonal antibody, Ventana (catalogue number 760‐2506), 1:5 dilution, pre‐treatment with 10 min chymotrypsin at 37°C), PSAP (PSAE/4LJ clone, Neomarkers (catalogue MS‐321‐P1), 1:3000 dilution, pre‐treatment with 25 min microwave at power 4 in buffer tris‐EDTA pH 8.7) and racemase (antibody clone 13H4,Ra DAKO dilution 1:50, microwave in microwave oven, milestone T/T Mega, at 98°C for 12 min in Ventana CC1 Solution, code No. 950‐124) were expressed in both ductal and acinar adenocarcinoma elements (fig 1H).
Following this unusual observation, we subjected prostate tissue microarrays constructed from radical prostatectomies19 to TTF‐1 immunohistochemical examination, all of which were negative. However, all these tissue cores were derived from acinar adenocarcinomas and no ductal patterns were included.
Postoperative recovery was uneventful. A repeat MRI/MRS scan in December 2006 revealed a lymphocele in the right pelvic wall, while a 1.1×1.6×1.3 cm nodule was discovered in the left hemipelvis between the bladder and rectum, probably representing a haematoma or postoperative collection, although a metastatic deposit could not be radiologically excluded. Serum PSA levels in November and December 2006 were 0.96 μg/l and 1.4 μg/l, respectively, indicating a rising trend postoperatively.
Take‐home messages
TTF‐1 may be expressed in prostatic ductal adenocarcinoma.
On core biopsies, PSA and PSAP may not always decorate malignant cells of prostatic ductal adenocarcinoma.
The coexistence of acinar adenocarcinoma favours a papilloglandular malignancy observed in the prostate to be of likely prostatic origin.
The SPT24 antibody appears to possess greater affinity for TTF‐1 protein that can potentially lead to a positive reaction in a small proportion of colorectal adenocarcinomas.
Discussion
When TTF‐1 was first integrated into the immunohistochemical armamentarium of diagnostic surgical pathology, it was regarded as a tissue‐specific transcription factor with almost exclusive expression in the normal thyroid and lung and carcinomas derived from these tissues.3 As with several other antibodies that began their use in a similarly restrictive fashion, an increasing spectrum of lesions and tumours has been discovered to have occasional expression of TTF‐1, leading to the need to be aware of exceptions and pitfalls in its interpretation. Some instances of aberrant TTF‐1 expression are thought to be related to gastric bronchopulmonary transdetermination, as in TTF‐1 nuclear reactivity in ciliated cell metaplasia of the stomach.14 Others may be associated with its presence during fetal differentiation and its persistence in site‐specific locations such as ependymomas of the third ventricle.15 More frequently, lung metastases of colorectal origin have been shown to express nuclear TTF‐1, resulting in confusion over a primary versus metastatic origin of the tumour. Comperat et al11 found that 10% of lung metastases of colorectal origin showed TTF‐1 reactivity when the SPT24 Novocastra antibody clone was used, while the lesions were not indicated when the 8G7G3/1 Dako antibody was used. When tested against the primary colorectal adenocarcinoma, 5% of cases were also positive with the SPT24 antibody. It was therefore inferred that the SPT24 antibody possessed greater affinity for TTF‐1 protein that can potentially lead to a positive reaction in a small proportion of colorectal adenocarcinomas.11 A similar conclusion was reached by Penman et al12 who found that three of six colonic adenocarcinomas showed focal nuclear positivity for TTF‐1 using the SPT24 antibody but were negative with the 8G7G3/1 clone. In other instances of aberrant expression of TTF‐1, the Dako 8G7G3/1 clone highlighted a case of mixed serous and endometrioid carcinoma and a case of serous carcinoma of the ovary, with staining confined to more solid, poorly differentiated parts of the tumour.13 The ependymomas that reacted with TTF‐1 used the clone 8G7G3/1 from Neomarkers,15 while the report of TTF‐1 positivity in gastric ciliated metaplasia used an antibody from Zymed without details of the clone14 and the report of TTF‐1 expression in thyroid‐like nasopharyngeal papillary adenocarcinoma did not provide information on the antibody.7
In our case, immunohistochemistry for TTF‐1 on biopsy cores and subsequent radical prostatectomy used the SPT24 antibody at different dilutions in two separate laboratories, with a similar finding of TTF‐1 nuclear reactivity in ductal adenocarcinoma. The radical prostatectomy showed concomitant positivity for PSA and PSAP which was absent in the biopsy cores and may represent sampling issues in the latter. Apart from the fact that the coexistence of conventional acinar adenocarcinoma points towards a ductal adenocarcinoma of prostate origin when malignant papilloglandular structures lined by stratified columnar cells are found in core biopsies of the prostate, the presence of PSA, PSAP and racemase reactivity in malignant cells can corroborate a prostatic primary tumour, potentially obviating exhaustive and costly investigations for an alternative primary origin. We did not repeat the staining with the Dako 8G7G3/1 clone. The SPT24 clone is believed to be more sensitive than 8G7G3/1, and a balance between enhanced sensitivity/lowered specificity and vice versa has to be accepted when antibodies are used in diagnostic immunohistochemistry.
In the prostate, TTF‐1 positivity has been reported only in small cell carcinoma and advocated as a possible distinguishing marker from Gleason pattern 5 adenocarcinoma which is negative.20 To our knowledge, ours is the first reported case of TTF‐1 reactivity in prostatic ductal adenocarcinoma. It is important to be aware of this pitfall, as the histological appearance of prostatic ductal adenocarcinoma may mimic a glandular malignancy from other organ sites such as the lung, colon or rectum, and TTF‐1 reactivity in this tumour may lead to a prolonged, expensive and futile hunt. Cytoplasmic TTF‐1 reactivity is sometimes found in tumours, especially non‐neoplastic liver tissue, but is considered to be a non‐specific finding.16
The underlying mechanism of TTF‐1 expression in prostatic ductal adenocarcinoma is unclear. TTF‐1 is a 38 kDa homeodomain‐containing nuclear protein belonging to the Nkx2 gene family which is involved in activating transcription during embryogenesis. It is possible that molecular pathways to prostate carcinogenesis along ductal adenocarcinoma lines may involve dedifferentiation or transdifferentiation.20 More such cases need to be studied to determine whether there is consistent TTF‐1 expression in these tumours and its significance.
Footnotes
Competing interests: None.
References
- 1.Agoff S N, Lamps L W, Philip A T.et al Thyroid transcription factor‐1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumours. Mod Pathol 200013238–242. [DOI] [PubMed] [Google Scholar]
- 2.Cheuk W, Kwan M Y, Suster S.et al Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med 2001125228–231. [DOI] [PubMed] [Google Scholar]
- 3.Ordonez N G. Value of thyroid transcription factor‐1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 2000241217–1223. [DOI] [PubMed] [Google Scholar]
- 4.Bejarano P A, Baughman R P, Biddinger P W.et al Surfactant proteins and thyroid transcription factor‐1 in pulmonary and breast carcinomas. Mod Pathol 19969445–452. [PubMed] [Google Scholar]
- 5.Jerome Marson V, Mazieres J, Groussard O.et al Expression of TTF‐1 and cytokeratins in primary and secondary epithelial lung tumours: correlation with histological type and grade. Histopathology 200445125–134. [DOI] [PubMed] [Google Scholar]
- 6.Zamecnik J, Kodet R. Value of thyroid transcription factor‐1 and surfactant apoprotein A in the differential diagnosis of pulmonary carcinomas: a study of 109 cases. Virchows Arch 2002440353–361. [DOI] [PubMed] [Google Scholar]
- 7.Carrizo F, Luna M A. Thyroid transcription factor‐1 expression in thyroid‐like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol 20054189–192. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira A M, Tazelaar H D, Myers J L.et al Thyroid transcription factor‐1 distinguishes metastatic pulmonary from well‐differentiated neuroendocrine tumours of other sites. Am J Surg Pathol 200125815–819. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya K K, Coenen M J, Bahn R S. Thyroid transcription factor‐1 in orbital adipose tissues: potential role in orbital thyrotropin receptor expression. Thyroid 200515422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K, Kobayashi Y, Katoh R.et al Identification of thyroid transcription factor‐1 in C cells and parathyroid cells. Endocrinology 19981393014–3017. [DOI] [PubMed] [Google Scholar]
- 11.Comperat E, Zhang F, Perrotin C.et al Variable sensitivity and specificity of TTF‐1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod Pathol 2005181371–1376. [DOI] [PubMed] [Google Scholar]
- 12.Penman D, Downie I, Roberts F. Positive immunostaining for thyroid transcription factor‐1 in primary and metastatic colonic adenocarcinoma: a note of caution. J Clin Pathol 200659663–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham A D, Williams A R, Salter D M. TTF‐1 expression in primary ovarian epithelial neoplasia. Histopathology 200648764–765. [DOI] [PubMed] [Google Scholar]
- 14.Rau T, Dimmler A, Hafner M.et al Aberrant expression of TTF‐1 and forkhead factor HFH‐4 in atrophic gastritis and ciliated metaplasia suggests gastric broncho‐pulmonary transdetermination. J Pathol 2005206383–387. [DOI] [PubMed] [Google Scholar]
- 15.Zamecnik J, Chanova M, Kodet R. Expression of thyroid transcription factor 1 in primary brain tumours. J Clin Pathol 2004571111–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bejarano P A, Mousavi F. Incidence and significance of cytoplasmic thyroid transcription factor‐1 immunoreactivity. Arch Pathol Lab Med 2003127193–195. [DOI] [PubMed] [Google Scholar]
- 17.Lei J Y, Bourne P A, diSant'Agnese P A.et al Cytoplasmic staining of TTF‐1 in the differential diagnosis of hepatocellular carcinoma vs cholangiocarcinoma and metastatic carcinoma of the liver. Am J Clin Pathol 2006125519–525. [DOI] [PubMed] [Google Scholar]
- 18.Pan C C, Chen P C, Tsay S H.et al Cytoplasmic immunoreactivity for thyroid transcription factor‐1 in hepatocellular carcinoma: a comparative immunohistochemical analysis of four commercial antibodies using a tissue array technique. Am J Clin Pathol 2004121343–349. [DOI] [PubMed] [Google Scholar]
- 19.Ng V W, Koh M, Tan S Y.et al Is triple immunostaining with 34betaE12, p63, and racemase in prostate cancer advantageous? A tissue microarray study. Am J Clin Pathol 2007127248–253. [DOI] [PubMed] [Google Scholar]
- 20.Yao J L, Madeb R, Bourne P.et al Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol 200630705–712. [DOI] [PubMed] [Google Scholar]