Abstract
Total mesorectal excision (TME) refers to the surgical removal of the complete perirectal soft tissue envelope, using sharp instruments under direct vision, and has become the contemporary standard of care for patients with rectal cancer. Pathologists play a key role in the evaluation of these specimens, including the quality assurance of surgical performance, as well as evaluation of the circumferential radial margin (CRM). While the latter is the most significant predictor of local recurrence, the quality of the excised mesorectum is another important factor in assessing the risk of local recurrence in patients with a negative CRM. Since proper pathological assessment of the TME specimen provides important prognostic information, as well as critical feedback to surgeons regarding technical performance, it is important to have adequate guidelines for the macroscopic handling of these specimens. The CLASSICC study of the Medical Research Council in the United Kingdom, as well as the Dutch TME trial have introduced a new standard for the pathological assessment of TME specimens, including an approach that involves assessment in both the fresh and fixed states, at least 48 hours of fixation of an intact specimen, with observations made on both the external appearance and cross‐sectional slices. This article reviews the pathological assessment of the TME specimen, including basic definitions, current international guidelines, an approach to evaluating the mesorectum and a discussion of special issues relating to margins, lymph node retrieval and effects of neoadjuvant therapy.
Keywords: total mesorectal excision, TME, mesorectum, circumferential radial margin, CRM, rectal cancer
Total mesorectal excision (TME) has become the contemporary standard of care for patients with rectal cancer, as TME greatly reduces local recurrence. Pathologists play a key role in the evaluation of these specimens, including the quality assurance of surgical performance, which benefits both the surgeon and the patient. We are presently in a transition period, where there are variable levels of expertise among surgeons who perform the procedure, as well as pathologists who assess the specimens. The proper pathological assessment of the TME specimen provides important prognostic information for oncologists and patients, as well as critical feedback to surgeons regarding technical performance, and will be reviewed in this paper. The main objective of this article is to review the evidence for a specialised approach to the macroscopic handling of TME specimens.
Background, definitions and current international guidelines
The concept of total mesorectal excision (TME) and the notion that this procedure significantly improves outcome, particularly with regard to local recurrence, for patients undergoing surgery for rectal cancer was introduced by Heald and Ryall during the 1980s.1 MacFarlane, who studied with Heald, subsequently published prospective follow‐up data based on Heald's practice, the result of which was greatly increased interest in the TME technique.2 The results of these studies showed that TME was a superior surgical modality for the treatment of rectal cancer, as local recurrence rates were reduced from 30–40% without TME to <5% with TME. Subsequent studies have confirmed this and it is now generally accepted that a local recurrence rate of <10% should be expected if proper TME techniques are employed.3,4
Box 1 Summary of technique for macroscopic examination of TME specimens
Fresh specimen
Assess the quality of the mesorectum (see table 1)
Paint the non‐peritonealised bare areas of the specimen with ink
Open the specimen along the anterior aspect from the top and the bottom, leaving the bowel intact at a level just above and just below the tumour
Place loose, formalin‐soaked gauze wicks into the unopened ends of the bowel
Fix the specimen for at least 48 hours
Fixed specimen
Slice through the unopened rectum at 3–5 mm intervals; lay slices down on the work surface
-
Inspect these slices to note:
-
-
extent of tumour and the closest distance of tumour to the CRM (record this distance)
-
-
any obviously positive nodes and the distance of any positive node to the CRM (record this distance)
-
-
record whether the closest distance of tumour to CRM is anterior, posterior or lateral
-
-
Fat away from the tumour must also be examined to detect lymph nodes
Block selection
Three blocks of tumour showing closest CRM
Two blocks of tumour showing luminal aspect
All lymph nodes (being careful not to double‐count nodes present in more than one slice)
Any polyps
Proximal and distal resection margins (distal margin includes both mucosa and mesorectum; blocks from mucosal margins may be omitted if tumour is greater than 3 cm away)
The mesorectum refers to a fatty connective tissue layer, measuring 2–3 cm in thickness, with associated vessels, lymphatics and lymph nodes, which surrounds the rectum and is enveloped by fascia. Mesorectal excision refers to the surgical removal of this soft tissue envelope using sharp instruments under direct vision, dissecting between the visceral and parietal pelvic fascia; the potential space between these fasciae has been referred to as the “holy plane”.5 A mesorectal excision can be total (TME) or partial (PME) in extent. The TME refers to complete excision of the mesorectum down to the pelvic floor and is indicated for carcinoma of the middle and lower third of the rectum. In the case of PME, although circumferentially the excision is performed in the same way as in TME, the mesorectum is transected at a right angle to the rectal wall at a distance of 5 cm beyond the gross distal edge of the tumour; PME is sufficient for treatment of carcinomas of the upper third of the rectum.5
In general, a TME specimen with a smooth surface, without incisions or tearing, is an indication of successful surgery. With PME, the plane of transection should be 90° to the rectal wall, without coning. Coning refers to the tendency for the surgeon to cut towards the tubular rectum during distal dissection, rather than staying outside the visceral mesorectal fascia; coning gives the specimen a tapered, conical appearance and is an indication of suboptimal surgical quality.5
The circumferential radial margin (CRM) is a specific term that applies only to rectal tumours, rather than to large intestinal cancers in general. This margin refers to the non‐peritonealised bare area of the rectum located both anteriorly and posteriorly. While the anterior CRM is located only in the most distal aspect of the specimen, below the lowest point of rectal serosa, the posterior CRM has a triangular shape, running up to the start of the sigmoid mesocolon. A positive CRM is defined as direct tumour extension (either continuous or discontinuous) or the presence of a positive lymph node within 1 mm of the radial, non‐peritonealised soft tissue edge.6
British guidelines for the examination of rectal cancer resection specimens include the assessment of contour for bulk, surgical defects, degree of coning in distal portions and the presence or absence of perforation. The recommendation of the United Kingdom Royal College of Pathologists is to leave the bowel intact at the level of the tumour during fixation, in order to allow serial slicing of the fixed specimen and preservation of the CRM, assessment of which would otherwise be compromised by opening the specimen.7
The German Cancer Society and the Working Group of German Cancer Centres require macroscopic assessment of the quality of mesorectal excision as well as documentation of the extent of mesorectal excision (ie TME vs PME), distance between distal tumour edge and distal transection (in cases of PME), coning and specimen surface quality; the latter feature is classified as intact and smooth, circumscribed defect (<5 mm vs >5 mm), extensive defect (muscular layer visible), or incision/tearing of tumour.8 This rigorous approach even includes optional stain marking, which refers to a recently described method to improve sensitivity in detecting small tears in the mesorectum, where the specimen is injected with ink or methylene blue solution postoperatively via the inferior mesenteric or superior rectal artery. While no leakage is seen with optimal TME, leakages indicate defects in the mesorectal fascia.9
Thus far, North American policy regarding the appropriate macroscopic handling of TME specimens has lagged behind that of the Europeans. Current American guidelines, including the Protocol for the Examination of Specimens from Patients with Carcinoma of the Colon and Rectum, published by Compton for the Members of the Cancer Committee of the College of American Pathologists does not yet include macroscopic assessment of the quality of TME.10
Many centres in the United States and Canada continue to examine rectal cancer specimens that have been opened in the region of the tumour, fixed for 24 hours or less, and make little or no attempt to assess the completeness of the mesorectum.
Assessment of the TME specimen
In this section, the methods of assessment of the quality of the mesorectal resection, circumferential resection margin, distal resection margin and lymph nodes are discussed. Box 1 summarises the procedure.
Mesorectum
Quirke and Nagtegaal have both done much to increase awareness of the importance of proper assessment of the mesorectum; both the CLASSICC study of the Medical Research Council in the United Kingdom and the Dutch TME trial have been paramount in defining an adequate protocol.8,11 Table 1 outlines the approach that Quirke developed for the assessment of the TME specimen; this is the protocol that has been followed in a standardised manner in the ongoing Dutch TME trial. Figure 1 shows examples of complete and incomplete mesorecta.
Table 1 Grading of quality and completeness of the mesorectum in a total mesorectal excision specimen.
| Mesorectum | Defects | Coning | CRM | |
|---|---|---|---|---|
| Complete | Intact, smooth | Not deeper than 5 mm | None | Smooth, regular |
| Nearly complete | Moderate bulk, irregular | No visible muscularis propria | Moderate | Irregular |
| Incomplete | Little bulk | Down to muscularis propria | Moderate–marked | Irregular |
Both the specimen as a whole (fresh) and cross‐sectional slices (fixed) are examined in order to make an adequate interpretation.
CRM, circumferential radial margin.
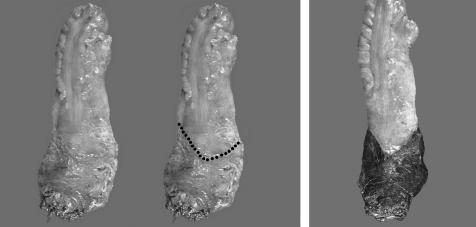
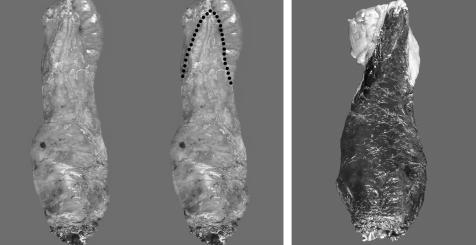
Figure 1 (A, B) Examples of complete mesorecta. The external surface appears smooth, without defects. Wispy fibres on the surface are a clue that dissection occurred within a fascial plane. There is adequate bulk, without coning. A midline cleft or groove, the “rectal buttocks”, seen posteriorly in B, is a normal anatomical landmark and a characteristic of a complete mesorectum. (C) Example of an incomplete mesorectum. The external surface is ragged and the bulk is minimal. There are deep defects, exposing the muscularis propria (arrow).
This assessment is performed by direct visual inspection of the fresh specimen; photodocumentation is desirable, especially in cases where the mesorectum is incomplete. The mesorectal fat is inked about its CRM, including all non‐peritonealised bare areas anteriorly and posteriorly (figs 2 and 3). Care should be taken not to ink the serosal surfaces of the specimen, especially anteriorly, where the serosa extends lower down, as this may produce artefact and lead to difficulty in interpreting serosal involvement by upper rectal tumours that are either circumferential or anterior in their location.12 The rectum may then be opened anteriorly, apart from the segment 2 cm above to 2 cm below the tumour, where the specimen is left intact (fig 4). While leaving this segment of the specimen intact may make intraluminal tumour observations and size measurements more challenging, tumour size should be recorded, although this variable is not related to outcome.13 Pinning the specimen on a corkboard is helpful to prevent shrinkage artefact, and placement of a gauze wick within the lumen of the intact segment is necessary to optimise fixation.14 The duration of specimen fixation should be at least 48 hours; while such a long fixation period is different from the usual protocol used in many laboratories, this is an important step, which facilitates serial cross‐sectional slicing of the specimen.
Figure 2 The anterior CRM consists of a narrow band of non‐peritonealised surface, below the peritoneal reflection, which occurs more inferiorly compared to the posterior side; this bare area is inked prior to fixation.
Figure 3 The posterior CRM consists of a long, triangular, non‐peritonealised surface, typically protected by a greater degree of soft tissue compared to the anterior CRM; this bare area is inked prior to fixation.

Figure 4 After inking the entire CRM (non‐peritonealised bare area) both anteriorly and posteriorly, the proximal and distal segments are opened anteriorly to allow fixation, while the tumour‐containing segment is left intact.
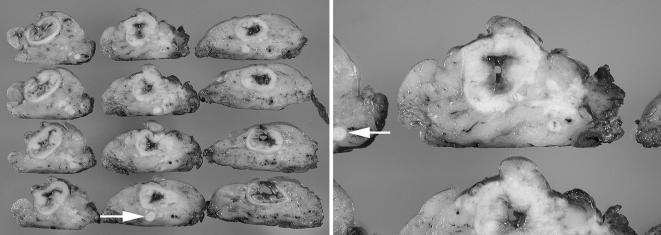
The unopened portion of the fixed specimen is then sliced into thin transverse sections (3–5 mm in thickness). All of the cross‐sectional rings should be laid out to further assess the quality of the mesorectum and the relationship of the tumour to the CRM (fig 5). Photodocumentation may be used, especially in cases of a poor TME or positive CRM. Finally, while two sections each from the superficial and deep parts of tumour appear to be sufficient for grading purposes, additional sections showing the closest relationship of tumour or a positive node to the CRM should be taken, as this permits microscopic refinements of gross observations at the area of greatest concern.15 Thus, the orientation of grossly suspicious nodes that are closely related to the CRM should be preserved in sections, while the remainder of the lymph nodes can be harvested in the usual manner, taking care not to over count nodes that happen to appear in more than one slice due to serial transverse slicing.
Figure 5 Two examples of transversely sectioned slices laid out for inspection and blocking. Cancer may involve the mesorectum as either continuous or discontinuous tumour deposits. It is important to measure the closest distance of these deposits to the CRM, as well as the closest distance of any grossly positive lymph nodes to the CRM (arrow), as a distance of <1 or 2 mm in either case, constitutes a positive CRM.
While patients with an incomplete mesorectum have a significantly higher risk of local recurrence compared to patients with a complete mesorectum (36% vs 20%, p = 0.02), there is no prognostic difference between patients with a complete mesorectum compared to those with a nearly complete mesorectum.16 Although part of the recurrences may be due to an increased frequency of having a positive CRM, the prognostic value of evaluating the TME quality is especially important for patients with negative margins. In fact, for patients with a negative CRM, the overall recurrence rate is increased when the mesorectum is incomplete compared to when it is complete (28.6% vs 14.9%, p = 0.03).16 However, for patients with a positive CRM, there is no added value to the assessment of surgical quality in predicting prognosis.16
Recent studies have mapped out where in the mesorectum discontinuous tumour deposits and positive lymph nodes are likely to occur. When Wang et al studied 18 TME specimens by microscopic examination of whole‐mount sections of mesorectums that were serially sliced and embedded entirely, they found discontinuous mesorectal tumour deposits in nearly 60% of the specimens, with nearly one third of these occurring in the outermost aspect of the mesorectum.17 While nearly half of the discontinuous tumour deposits occurred in the posterior mesorectum, deposits occurred ipsilaterally more frequently than contralaterally in relation to laterally located tumours.
Circumferential resection margin
Accurate determination of CRM status is essential, since this is the single most important factor for predicting the risk of local recurrence in patients with rectal cancer. However, although both direct tumour extension and the presence of positive lymph nodes within 1 mm of the CRM are considered to be a positive margin, there is evidence to suggest that not all positive CRMs are equal with respect to impact on recurrence risk. Nagtegaal et al showed that patients with a positive CRM due to direct tumour extension developed local recurrence more frequently than those with a positive CRM due to positive nodes (22.1% vs 12.4%, p = 0.06); in fact, in their study there was no difference in the rate of local recurrence between patients with a positive CRM due to positive nodes compared to those with a negative CRM.16 It also appears that the risk of having a positive CRM is related to the quality of the mesorectum, since patients with a positive CRM due to tumour extension more frequently had an incomplete mesorectum compared to those with a negative CRM (44% vs 24%, p<0.05); interestingly, there was no difference in the quality of the mesorectum among patients with and without a positive CRM when the CRM positivity was due to the presence of positive nodes.16
As previously mentioned, a radial margin of ⩽1 mm is regarded as positive and there is adequate evidence that tumour within 1 mm of the CRM is associated with an increased risk of local recurrence. Using data from the Norwegian Cancer Registry (n = 686), Wibe et al showed a 22% local recurrence rate among patients with a positive CRM (<1 mm margin) compared to a 5% local recurrence rate among patients with a negative CRM.18 Further, radial margins <1 mm are predictive of an increased risk of distant metastases (37% vs 15% for patients with radial margins >1 mm) and shorter survival (70% vs 90% at 2 years for patients with radial margins >1 mm).8 However, there is now evidence that tumour extension to within even 2 mm of the radial margin is predictive of a worse outcome; patients who met these more conservative criteria for a positive CRM had an increased rate of local recurrence in a study by Nagtegaal et al (16% vs 6% for patients with radial margins >2 mm).6
The location of tumour within the mesorectum has important prognostic implications, since the amount of soft tissue between the tubular rectum and the CRM varies circumferentially. Lee et al retrospectively analysed 401 patients with rectal cancer who underwent TME procedures to determine if tumour location was related to outcome. They found that in stage matched males, anterior tumours were associated with an increased rate of local recurrence and death.19 This result is likely related to the relatively lesser amount of soft tissue located between the anterior rectal wall and the CRM; the fact that this result was limited to males may be explained by the greater frequency of complete pelvic exenterations performed on females in this study.
Furthermore, the lower the cancer is in the rectum, the higher the risk of local recurrence. Whether this is related to the greater frequency of abdominoperineal resections performed in such patients or the inherent tumour biology of low rectal cancers has been debated. Investigators involved in the Dutch TME study, such as Quirke, have shown that abdominoperineal resection is more frequently associated with a poorer quality of mesorectum, an increased frequency of CRM involvement and a poorer prognosis compared to low anterior resection TME procedures.20 On the other hand, when Faerden et al prospectively studied 140 patients who underwent TME for rectal cancer, patients with tumours <6 cm from the anal verge had a higher rate of local recurrence compared to those with tumours >6 cm from the anal verge (18% vs 5%, p = 0.0014); however, there was no difference in the frequency of abdominoperineal resection between these groups.4 While multiple factors may work together to cause a propensity for aggressive behaviour in low rectal cancers, the inherent low volume of distal mesorectal soft tissue, with thin enveloping of the lowermost portion of the rectum, is a likely factor, regardless of the surgical technique performed.
While evaluation of CRM status in rectal cancer specimens is of the greatest importance for predicting prognosis, one should be aware that a considerable number of upper rectal cancers, especially those occurring anteriorly, may recur locally even while maintaining a fair distance from the CRM. Ludeman and Shepherd have suggested that tumour spread to the serosal surface is the likely pathogenetic explanation in such cases.12
Distal margin
The distal margin, although less important than the CRM in terms of frequency of involvement and impact on recurrence, is still important to assess. With conventional surgery, this margin is involved in <2% of cases compared to nearly 20% involvement of the CRM.8,21 Results from the Dutch TME trial showed that while in nearly 40% of cases the distance between the tumour and the distal margin was <2 cm, there was no statistical difference in recurrence between patients with a distal margin <2 cm compared to those with a distal margin >5 cm.8 Two issues to keep in mind when considering the distal margin are first, the extent of intramural and extramural continuous tumour growth, and second, discontinuous distal mesorectal spread through lymphatics.
The latter phenomenon is the greater issue and can be explained by the occurrence of lymph node metastases along the inferior mesenteric artery chain, causing retrograde lymph flow and secondary tumour spread in a downward, distal direction. Thus, in 20% of cases with positive nodes, there is lymphatic spread distal to the primary tumour; furthermore, in many such cases these positive distal nodes are located >2 cm away from the main tumour mass.22 By contrast, intramural distal spread >2 cm is seen in only 3.6% of cases.23 When Zhao et al recently looked at 45 cases, the rate of discontinuous tumour deposits within the distal mesorectum was 17.8% and they found that the extent of distal mesorectal spread was greater than the extent of intramural spread (3.6 cm vs 1.2 cm).24 From their data, they concluded that a 1.5 cm distal rectal wall margin and a 4 cm distal mesorectal margin are necessary to achieve adequate surgical clearance.
One final issue to keep in mind, when measuring the distal margin, is shrinkage artefact. Goldstein et al have shown that a 5 cm length of colorectum in vivo is equivalent to 3 cm after resection and 2.2 cm after fixation.25 Pinning of the specimen under tension on a corkboard helps to avoid shrinkage.14
Lymph nodes
Lymph node status probably constitutes the single most important determinant of overall survival in patients with rectal cancer, likely because of the associated risk of systemic spread more than local recurrence. The five‐year survival for rectal cancer patients with positive nodes is significantly lower than those with negative nodes (40% vs 68%).8 While some authors have stated that lymph node positivity has a similar impact to CRM positivity on the risk for local recurrence, many such studies have not employed the TME technique in a consistent manner. In order to combat such claims, the Colorectal Research Unit from Basingstoke, UK, led by Heald, recently published data collected prospectively from 170 patients who underwent TME for rectal cancer.3 While patients with positive lymph nodes did have a higher local recurrence rate compared to node negative patients, the recurrence rate among node positive patients was only 7.5%; this is a remarkably low recurrence rate for node positive rectal cancer, highlighting the fact that adequate excision of the mesorectum is of paramount prognostic importance.
While the current TNM guidelines state that at least 12 nodes should be examined before a patient can be classified as N0, these guidelines are based on studies that have not necessarily employed TME in a standardised manner.26 A study by Wang et al suggests a possible maximum or ideal number of nodes available for harvest within TME specimens, since they microscopically examined whole‐mount sections of serially sliced mesorectums (5 mm intervals) that were entirely embedded.17 In their study, 992 lymph nodes from 18 specimens were examined, averaging 32 per specimen, and 148 (15%) of these contained metastases. Interestingly, 922 (93%) of the total number and 104 (70%) of the positive lymph nodes were <5 mm in diameter. In practice, retrieval of even 12 nodes can be difficult to achieve in many cases, as shown by recent results of the Dutch TME trial, where 82% of node negative patients had <12 nodes examined, regardless of neoadjuvant radiotherapy.27 However, a high motivation to find as many nodes as possible must be maintained, since several studies support the concept that the more nodes that are examined, the more accurate the staging. Caplin et al showed that node negative patients with <7 nodes examined had a similar prognosis to node positive patients, and Tepper et al showed that patients with >14 nodes examined have a better recurrence‐free survival compared to those with <8 nodes examined.28,29 In addition, it is conceivable that setting a lower limit of 12 for the number of nodes that must be found, may lead to under‐staging as specimen prosectors look for the minimum number of nodes and potentially exclude harder‐to‐find nodes that are closer to the rectal wall.
Routine visual inspection, palpation and dissection is still the standard of practice for lymph node retrieval and the extent of examination, as well as the enthusiasm of the examiner, is one of the most important factors in determining the number of nodes retrieved. In order to address the challenge of lymph node yield, a number of adjunctive methods have been developed, including fat stretching, alcohol treatment, xylene clearance, wintergreen oil/cedar oil clearance and ether‐based methods.30,31,32,33,34 In fact, the most recent protocol for the examination of colorectal cancer specimens from the Cancer Committee of the College of American Pathologists recommends that if fewer than 12 nodes are found with traditional methods, then the use of “visual enhancement techniques” should be considered.10 While most of the above‐mentioned methods require special equipment, the use of noxious volatile compounds or prolonged treatment of pericolic fat (up to 3 weeks), Newell et al successfully used GEWF solution, which is an easily prepared, non‐noxious solution with a quick turn around performance (24 hours), to obtain a significant increase in lymph node yield.35
Take‐home messages
Careful macrosopic evaluation of total mesorectal excision (TME) specimens by pathologists plays a key role in the assessment of prognosis in patients with rectal cancer.
Assessment of the circumferential resection margin in the TME specimen is the most significant predictor of local recurrence.
The quality of the excised mesorectum is a key factor affecting the risk of local recurrence in patients with a negative circumferential resection margin.
Evaluation of the TME specimen provides important feedback on surgical technique to the surgeon.
Effects of neoadjuvant radiotherapy on the TME specimen
In several European countries, a short course of preoperative radiotherapy has become the standard of practice, based on results of the Dutch TME trial, which showed a reduction in the local recurrence rate among patients in the radiotherapy arm compared to those treated with surgery alone (2.4% vs 8.2%).36 While studies using long course neoadjuvant radiotherapy have shown a downstaging effect within rectal cancer specimens, even to the point of complete tumour loss in 23%, there does not appear to be a significant impact on staging for patients treated with short course radiotherapy, as long as they undergo surgery within 7–10 days of radiation.8,37,38 The Dutch TME trial has shown that preoperative short course radiotherapy has no significant impact on the rate of margin positivity.8 Patients who received radiotherapy had a similar rate of CRM positivity compared to those treated by surgery alone (16% vs 19%). With respect to the distal margin, patients had positive involvement of this margin in <2%, regardless of neoadjuvant radiation. Even short course preoperative radiotherapy appears to decrease the number of lymph nodes that can be harvested for examination in TME specimens.8 However, while the mean number of nodes per specimen examined in the Dutch TME trial decreased from 10 to 8 with neoadjuvant radiation, the number of positive nodes was similar, regardless of radiotherapy (mean of 2 nodes).
Conclusions
Pathologists' macroscopic evaluation of TME specimens is important for three main reasons: first, it provides feedback on surgical technique to the surgeon; second, assessment of the CRM is the most significant predictor of local recurrence; and third, the quality of the excised mesorectum is a key factor affecting the risk of local recurrence in patients with a negative CRM. The literature supports a specialised approach for the macroscopic assessment of TME specimens, which includes its evaluation in both the fresh and fixed states, allowing at least 48 hours of fixation of an intact specimen, with observations made on both the external appearance and cross‐sectional slices. Standardised protocols for the grossing of TME specimens should be available, so that pathologists, pathology residents and pathologists' assistants handle these specimens in the most consistent and effective manner possible.
Acknowledgements
The authors are grateful to Dr Ross McLean, Royal Alexandra Hospital, Edmonton, Canada for permission to use his images for figs 1, 4 and 5.
Abbreviations
CRM - circumferential radial margin
PME - partial mesorectal excision
TME - total mesorectal excision
Footnotes
Competing interests: None declared.
References
- 1.Heald R J, Ryall R D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 198611479–1482. [DOI] [PubMed] [Google Scholar]
- 2.MacFarlane J K, Ryall R D, Heald R J. Mesorectal excision for rectal cancer. Lancet 1993341457–460. [DOI] [PubMed] [Google Scholar]
- 3.Cecil T D, Sexton R, Moran B J.et al Total mesorectal excision results in low local recurrence rates in lymph node‐positive rectal cancer. Dis Colon Rectum 2004471145–1150. [DOI] [PubMed] [Google Scholar]
- 4.Faerden A E, Naimy N, Wiik P.et al Total mesorectal excision for rectal cancer: difference in outcome for low and high rectal cancer. Dis Colon Rectum 2005482224–2231. [DOI] [PubMed] [Google Scholar]
- 5.Hermanek P, Hermanek P, Klimpfinger M.et al The pathological assessment of mesorectal excision: implications for further treatment and quality management. In J Colorectal Dis 200318335–341. [DOI] [PubMed] [Google Scholar]
- 6.Nagtegaal I D, Marijnen C A M, Klein Kranenbarg E.et al Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 200226350–357. [DOI] [PubMed] [Google Scholar]
- 7.Marr R, Birbeck K, Garvican J.et al The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg 200524274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagtegaal I D, van Krieken J H J M. The role of pathologists in the quality control of diagnosis and treatment of rectal cancer—an overview. Eur J Cancer 200238964–972. [DOI] [PubMed] [Google Scholar]
- 9.Sterk P, Opitz T, Kasperk R.et al Studies of the vascular anatomy of the mesorectum support the concept of the total mesorectal excision. Tech Coloproctol 20004151–156. [Google Scholar]
- 10.Compton C C. Updated protocol for the examination of specimens from patients with carcinomas of the colon and rectum, excluding carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix: a basis for checklists. Cancer Committee. Arch Pathol Lab Med 20001241016–1025. [DOI] [PubMed] [Google Scholar]
- 11.Quirke P. The pathologist, the surgeon and colorectal cancer: get it right because it matters. Prog Pathol 19984201–213. [Google Scholar]
- 12.Ludeman L, Shepherd N A. Serosal involvement in gastrointestinal cancer: its assessment and significance. Histopathology 200547123–131. [DOI] [PubMed] [Google Scholar]
- 13.Compton C, Fenoglio‐Preiser C, Pettigrew N.et al American Joint Committee on Cancer prognostic factors consensus conference; colorectal working group. Cancer 2000881739–1757. [DOI] [PubMed] [Google Scholar]
- 14.Williams N, Dixon M, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients' survival. Br J Surg 198370150–154. [DOI] [PubMed] [Google Scholar]
- 15.Halvorsen T. Tissue sampling and histological grading in colorectal cancer. Are routine sections representative? APMIS 198997261–266. [DOI] [PubMed] [Google Scholar]
- 16.Nagtegaal I D, van de Velde C J H, van der Worp E.et al Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 2002201729–1734. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Zhou Z G, Wang Z.et al Mesorectal spread and micrometastasis of rectal cancer studied with large slice technique and tissue microarray. J Surg Oncol 200591167–172. [DOI] [PubMed] [Google Scholar]
- 18.Wibe A, Rendedal P R, Svensson E.et al Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 200289327–334. [DOI] [PubMed] [Google Scholar]
- 19.Lee S H, Hernandez de Anda E, Finne C O.et al The effect of circumferential tumor location in clinical outcomes of rectal cancer patients treated with total mesorectal excision. Dis Colon Rectum 2005482249–2257. [DOI] [PubMed] [Google Scholar]
- 20.Nagtegaal I D, van de Velde C J H, Marijnen C A M.et al Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol 2005239257–9264. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Zhou Z G, Wang C.et al Microscopic spread of low rectal cancer in regions of the mesorectum: detailed pathological assessment with whole‐mount sections. Int J Colorectal Dis 200520231–237. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa E, Yasutomi M, Shindou K.et al Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis Colon Rectum 199437219–223. [DOI] [PubMed] [Google Scholar]
- 23.Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter‐preserving surgery. Cancer 199576388–392. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G P, Zhou Z G, Lei W Z.et al Pathological study of distal mesorectal cancer spread to determine a proper distal resection margin. World J Gastroenterol 200511319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein N, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. Anat Pathol 1999111349–351. [DOI] [PubMed] [Google Scholar]
- 26.Sobin L, Greene F. TNM classification. Cancer 200192452. [DOI] [PubMed] [Google Scholar]
- 27.Marijnen C A, Nagtegaal I D, Klein Kranenbarg E.et al No downstaging after short‐term preoperative radiotherapy in rectal cancer patients. J Clin Oncol 2001191976–1984. [DOI] [PubMed] [Google Scholar]
- 28.Caplin S, Cerottini J, Bosman F.et al For patients with Dukes' B (TNM stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer 199883666–672. [PubMed] [Google Scholar]
- 29.Tepper J, O'Connell M, Niedzwiecki D.et al Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 200119157–163. [DOI] [PubMed] [Google Scholar]
- 30.Crucitti F, Doglietto G B, Bellantone R.et al Accurate specimen preparation is mandatory to detect lymph nodes and avoid understaging in colorectal cancer. J Surg Oncol 199251153–158. [DOI] [PubMed] [Google Scholar]
- 31.Cawthorn S J, Gibbs N M, Marks C G. Clearance technique for the detection of lymph nodes in colorectal cancer. Br J Surg 19867358–60. [DOI] [PubMed] [Google Scholar]
- 32.Haboubi N Y, Clark P, Kaftan S M.et al The importance of xylene clearance and immunohistochemistry in the accurate staging of colorectal carcinoma. J R Soc Med 199285386–388. [PMC free article] [PubMed] [Google Scholar]
- 33.Hyder J W, Talbott T M, Maycroft T C. A critical review of chemical lymph node clearance and staging of colon and rectal cancer at Ferguson Hospital, 1977 to 1982. Dis Colon Rectum 199033923–925. [DOI] [PubMed] [Google Scholar]
- 34.Koren R, Siegal A, Klein B.et al Lymph node‐revealing solution: simple new method for detecting minute lymph nodes in colon carcinoma. Dis Colon Rectum 199740407–410. [DOI] [PubMed] [Google Scholar]
- 35.Newell K J, Sawka B W, Rudrick B F.et al GEWF solution. Arch Pathol Lab Med 2001125642–645. [DOI] [PubMed] [Google Scholar]
- 36.Wiggers T, van de Velde C J H. The circumferential margin in rectal cancer: recommendations based on the Dutch total mesorectal excision study. Eur J Cancer 200238973–976. [DOI] [PubMed] [Google Scholar]
- 37.Walker J, Quirke P. Prognosis and response to therapy in colorectal cancer. Eur J Cancer 200238880–886. [DOI] [PubMed] [Google Scholar]
- 38.Marijnen C, Glimelius B. The role of radiotherapy in rectal cancer. Eur J Cancer 200238943–952. [DOI] [PubMed] [Google Scholar]