The treatment of localised prostate cancer remains controversial since there is no clear evidence of the superiority of any one treatment modality over any other. However, evidence is emerging that, in some patient groups, radical treatment may confer a survival advantage. The issue, therefore, appears to be one of treatment efficacy against complications and effects on quality of life.
Compared to watchful waiting, radical prostatectomy remains the only treatment modality where there is published evidence of a survival advantage. Goldstraw and Kirby, therefore, argue that surgery remains the gold standard treatment despite its slightly higher complication rates compared to external beam radiotherapy. Langley, however, demonstrates that brachytherapy now offers effective treatment of prostate cancer with oncological outcomes which are certainly comparable with those of surgery and he describes a lower risk of complications. Whether the complication rates are really more acceptable is perhaps less clear than he suggests since there is a danger in comparing figures from acknowledged centres of excellence with those from units performing much lesser numbers of procedures.
In considering new treatment modalities, healthcare systems have now become obliged to consider costs. Hitherto, new technologies including brachytherapy and laparoscopic or robotic techniques have proved expensive compared to traditional surgery. It is perhaps difficult, therefore, to see the NHS wishing to continue funding for procedures where an acceptable, cheaper alternative exists.
Krishna K Sethia
Consultant Urological Surgeon, Norfolk & Norwich University NHS Trust, Colney, Norwich NR4 7UY, UK
krishna.sethia@nnuh.nhs.uk
The Case for Radical Prostatectomy
Correspondence to: Miles A Goldstraw, Urology Surgical Assistant, The London Clinic, 149 Harley Street, London W1G 6BW, UK E: miles.goldstraw@gmail.com
The management of clinically localised prostate cancer is controversial. Walsh and Donker1 first described the anatomical basis for nerve-sparing radical prostatectomy in 1982 and this is considered by many to be the ‘gold standard’ treatment. However, other strategies exist including watchful waiting, external beam radiotherapy and brachytherapy. Which of these modalities is the optimal treatment is fiercely debated and there is only a scanty evidence base available with no large-scale, randomised trials of radical prostatectomy versus radiotherapy or brachytherapy.
Surgery versus watchful waiting
The most significant report comparing surgery with watchful waiting was produced by Bill-Axelson et al.2 in 2005. They reported the final results from the Scandinavian prostate cancer group study – a randomised, prospective study following nearly 700 men with early (moderately- and well-differentiated) prostate cancer over a 10-year period (1989–1999). Importantly, this is the only report to show a survival benefit with any singular modality treatment. During a median period of 8.2 years, death due to prostate cancer occurred in 14.4% of men assigned to watchful waiting versus 8.6% in the surgery group. Table 1 shows that the difference in the cumulative incidence of death due to prostate cancer increases from 2.0% after 5 years to 5.3% after 10 years, for a relative risk of 0.56. Admittedly these may be small benefits but, more significantly, results showed a considerable reduction in metastatic disease of 1.7% to 10.2% at 5 years and 10 years, respectively. Because clinical manifestations of disseminated disease virtually always precede death, this finding may herald a further lowering of the risk of death due to prostate cancer in the radical prostatectomy group after a longer period of follow-up.2
Table 1.
Cumulative incidence of the main end-points and corresponding relative risks
| End-point | Cumulative incidence | Absolute risk reduction (95% CI) | RR (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
| Radical-prostatectomy group | Watchful-waiting group | ||||||
| Total no. | %(95% CI) | Total no. | %(95% CI) | ||||
| Disease-specific mortality | |||||||
| At 5 years | 30 | 2.3 (1.2–4.6) | 50 | 4.3 (2.6–7.1) | 2.0 (−0.6 to 4.7) | ||
| At 10 years | 30 | 9.6 (6.5–14.2) | 50 | 14.9 (11.2–19.8) | 5.3 (−0.3 to 11.0) | 0.56 (0.36–0.88) | 0.01 |
| Distant metastases | |||||||
| At 5 years | 50 | 8.1 (5.7–11.6) | 79 | 9.8 (7.1–13.5) | 1.7 (−2.5 to 6.0) | ||
| At 10 years | 50 | 15.2 (11.4–20.3) | 79 | 25.4 (20.4–31.5) | 10.2 (3.1–17.2) | 0.60 (0.42–0.86) | 0.004 |
Taken from the data of Bill-Axelson et al.2 The absolute risk reduction and relative risks are for radical prostatectomy as compared with watchful waiting. RR denotes relative risk and CI confidence interval.
One point of note is that subgroup analysis revealed that the reduction in disease-specific mortality was greatest among, or even limited to, patients younger than 65 years. Although the study was not powered to analyse subgroups, some may argue about the benefit of older patients undergoing surgery. As a consequence, in most cases, radical prostatectomy should only be undertaken in those with an expected life-expectancy of ≥ 10-years.
Surgery versus radiotherapy
It is probably unrealistic now to expect a well-designed, prospective randomised study with long-term follow-up to be undertaken comparing surgery with radiotherapy. Furthermore, the studies that do exist are hampered by differences in case selection, short duration of follow-up and varying definitions of biochemical failure. One benefit of surgery is the ability to sample lymph nodes intra-operatively. Traditionally, if frozen section is performed and proves to be positive, the radical surgery is immediately abandoned. This automatically places the surgical arm of studies at an advantage compared to radiotherapy as this patient group has effectively been down-staged. Paulson et al.3 performed the only randomised study of surgery versus radiotherapy and this demonstrated improved surgical survival outcomes. This study was never widely accepted as it was set in 1982, in the pre-prostate specific antigen (PSA) era, and the relevance to contemporary practice is unclear. However, several large retrospective studies exist: D'Amico et al.4 performed analysis of over 2600 men with early prostate cancer and results supported a survival advantage in those treated with surgery. This study is also controversial and it has been pointed out that only 380 men were treated in the radiotherapy arm and conventional dose radiation was used (69–71 Gy). Higher dose conformal or intensity-modulated techniques are now being used and do improve results. Lu-Yao et al.5 performed the largest study to date using the surveillance, epidemiology, and end results (SEER) data of the National Cancer Institute. In an intention-to-treat analysis, the 10-year disease-specific survival was better for surgery compared with radiation in patients with well, moderately, and poorly differentiated disease. This is interesting as by using the intention-to-treat approach, the confounding factor of lymph node sampling and down-staging is taken into account. Admittedly, not all comparative studies find in favour of surgery and Martinez et al.6 reported no significant survival difference between radiotherapy and surgery groups but this involved a relatively small study group (382 patients) and a short median follow-up of 5.5 years.
Quality of life issues
Overall, the available evidence is in favour of a moderate survival advantage with surgery compared to radiotherapy. However, surgery may have significant morbidity, especially in terms of erectile dysfunction and incontinence. With the increasingly young age of patients undergoing radical prostate surgery, this potentially has a great impact on quality of life (QoL) issues. Comparative studies on QoL issues suggest that surgery has a higher rate of urinary incontinence compared to radiotherapy, 8% versus 1%, respectively. Both treatment modalities do result in impaired sexual function, but overall sexual function (interest, frequency, bothersome) is similar between the two groups.7 However, reported erectile dysfunction is more prevalent in the surgery group at 79.3% versus 63.5% at 5 years. Conversely, radiotherapy does result in more bowel-related problems mainly tenesmus, urgency and rectal bleeding.
The future
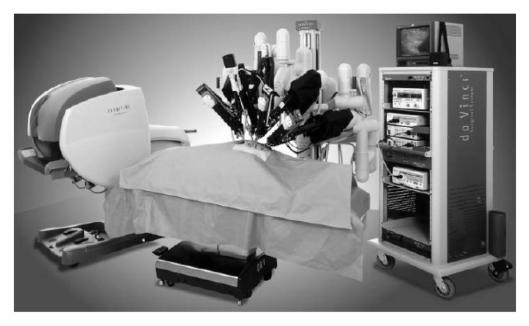
Continual refinements to the nerve-sparing prostatectomy are being made and potency rates continue to improve. Catalona et al.8 reported potency rates approaching 70% with bilateral nerve sparing surgery. Recently, the Vattikuti Institute (Detroit, MI, USA) has pioneered the development of the Robotic Radical Prostatectomy (RRP) using the Da Vinci robot (Intuitive surgical™).9 This technology allows three-dimensional stereoscopic vision with x10 magnification, increased dexterity and reduction of surgical tremor (Fig. 1). While this system shows similar oncological efficacy, reported benefits include reduced blood loss, decreased pain and reduced hospital stay. Theoretically, the enhanced visualisation of the neurovascular bundles at the time of RRP might be expected to result in improved erectile function. In December 2005, Menon et al.10 published 1-year follow-up of patients undergoing robotic radical prostatectomy (prostate fascia-sparing). These results revealed that an impressive 86% of men were potent with the use of phosphodiesterase 5 inhibitors.10
Figure 1.
The Da Vinci robot (Intuitive Surgical™). The device consists of a master console (left) and a slave system (right) controlling 3 or 4 telerobotic arms. Photo courtesy of Intuitive Surgical (Sunnyvale, CA, USA).
Conclusions
Radical prostatectomy remains the gold-standard treatment for clinically localised prostate cancer. Cumulative data suggest that it has a survival advantage over radiotherapy, but it is troubled by surgical morbidity especially erectile dysfunction and incontinence. Recent surgical advances, particularly with robotic radical prostatectomy, have shown encouraging results with minimised associated morbidity and maximised quality of life while still achieving surgical cure.
References
- 1.Walsh P, Donker P. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg A, Ruutu M, Haggman M, Andersson S, Bratell S, et al. Scandinavian prostate cancer group study. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–84. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 3.Paulson D, Lin G, Hinshaw W, Stephani S. Radical surgery versus radiotherapy for adenocarcinoma of the prostate. J Urol. 1982;128:502–4. doi: 10.1016/s0022-5347(17)53016-5. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico A, Whittington R, Malkowicz SB, Cote K, Loffredo M, Schultz D, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localised prostate carcinoma in the prostate specific antigen era. Cancer. 2002;95:281–6. doi: 10.1002/cncr.10657. [DOI] [PubMed] [Google Scholar]
- 5.Lu-Yao G, Yao S. Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet. 1997;349:906–10. doi: 10.1016/S0140-6736(96)09380-4. [DOI] [PubMed] [Google Scholar]
- 6.Martinez A, Gonzalez J, Chung A, Kestin L, Balasubramaniam M, Diokno A, et al. A comparison of external beam radiation therapy versus radical prostatectomy for patients with low risk prostate carcinoma diagnosed, staged, and treated at a single institution. Cancer. 2000;88:425–32. doi: 10.1002/(sici)1097-0142(20000115)88:2<425::aid-cncr25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Potosky A, Davis W, Hoffman R, Stanford J, Stephenson R, Penson D, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–67. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 8.Catalona W, Carvalhal G, Mager D, Smith D. Potency, incontinence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–8. [PubMed] [Google Scholar]
- 9.Menon M, Hemal A, VIP team Vattikuti Institute prostatectomy: a technique of robotic radical prostatectomy: experience in more than 1000 cases. J Endourol. 2004;18:611–9. doi: 10.1089/end.2004.18.611. [DOI] [PubMed] [Google Scholar]
- 10.Menon M, Kaul S, Bhandari A, Shrivastava A, Tewari A, Hemal A. Potency following robotic prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174:2291–6. doi: 10.1097/01.ju.0000181825.54480.eb. [DOI] [PubMed] [Google Scholar]
Permanent Low-Dose Rate Prostate Brachytherapy
Correspondence to Stephen EM Langley, Professor of Urology, St Luke's Cancer Centre, Royal Surrey County Hospital, Guildford GU2 5XX, UK E: slangley@prostatecancercentre.com. W: www.prostatecancercentre.com
Given the significant side-effects of incontinence and impotence with radical prostatectomy, an oncological operation with a reported positive margin rate of up to 30–40%,1 for a disease that for many will not be fatal if untreated,2 it is unfortunate that not all patients are suitable for prostate brachytherapy. Prostate brachytherapy has been shown to be a highly effective curative treatment for early prostate cancer with outcome results stretching to 13 years and meta-analysis studies revealing that it is just as effective in curing prostate cancer as surgery yet with a fraction of the side effects. This article will substantiate these claims.
Brachytherapy procedure
Prostate brachytherapy involves the implantation of tiny radioactive seeds transperineally into the prostate gland (Fig. 1). The procedure takes 30–45 min to perform and may be delivered as a day-case. Initially, patients undergo a planning scan where detailed transrectal ultrasound images of the prostate are obtained. These images are used to produce a three dimensional model of the prostate from which the precise number and position of the seeds are determined. The seeds are then implanted into the prostate gland under a general anaesthetic transperineally using transrectal ultrasonography to guide the needles carrying the seeds to their pre-planned position.
Figure 1.
A patient being treated with brachytherapy.
Brachytherapy offers patients the ultimate in conformal radiotherapy with the radiation sources implanted in real-time into the cancerous gland; as the radiation is emitted from a point source, the surrounding tissues can be better spared. The minimum prescribed dose to the prostate gland is 145 Gy using Iodine-125 implants with about 50% of the gland receiving 150% of the dose (i.e. 217 Gy; see Fig. 2). Such doses of radiation have a biological equivalence much greater than that which can be delivered by any of the external beam techniques, including IMRT. In cases where there is an increased chance of local extension of the prostate cancer, a combined treatment using 45 Gy external beam radiation to the whole pelvis followed by a 110 Gy brachytherapy implant has been used with good effect.
Figure 2.
Post-implant CT scan taken with the patient catheterised at mid gland and apical levels of the prostate. The Iodine-125 sources are clearly seen within the prostate tissue. The dashed white line shows the periphery of the prostate. The dashed black isodose line is a simulation showing coverage of the prescribed radiation dose, 145 Gy. The grey continuous isodose line simulates that portion of the prostate receiving 150% of the prescription dose, 217 Gy. The dotted white isodose line simulates the 72 Gy isodose showing the rapid fall off in radiation to the surrounding structures.
Brachytherapy results
In 1988, the first 10-year results of transperineal ultrasound guided prostate brachytherapy were published from Seattle, USA. Examining their overall series of 634 patients, 402 treated with an implant alone (Iodine-125/Palladium-103) and 232 treated by an implant and external beam radiation reveals a progression-free survival rate of 77%.3 When stratified by risk factors (Table 1), low-, intermediate- and high-risk disease had progression-free survival rates of 87%, 74% and 45% respectively. Similar 10-year results have been recently reported by others with prostate specific antigen (PSA)-free survival rates of 79% from a cohort of 883 patients4 and results on 619 patients at 13 years continue to show a PSA-free survival of 77%.5
Table 1.
Risk group classification of prostate cancer patients
| Risk group | PSA | Stage | Gleason grade |
|---|---|---|---|
| Low | ≤ 10 | T1a–T2b | 2–6 |
| Intermediate (1 factor present) | > 10 | ≥ T2c | ≥ 7 |
| High (2 or more factors present) | > 10 | ≥ T2c | ≥ 7 |
The 10-year, disease-specific survival rates for 1561 men treated by brachytherapy, with or without EBRT and hormone therapy, have recently been published with a 96% survival, suggesting that the excellent results obtained using PSA as a surrogate marker appear to translate to actual clinical outcomes.6 The durability of brachytherapy has been demonstrated in 10- and 12-year results from Seattle. No further recurrences were identified in patients who were deemed biochemically free at 12 years compared to 10 years of follow-up.7
Reproducibility of brachytherapy results
The results achieved in the US appear exportable to the UK. In Guildford, UK, we have treated over 800 patients and prospectively assessed outcomes of both PSA-free survival using the ASTRO criteria as well as continence, potency and quality of life parameters using validated questionnaires.
The results of our first 300 patients treated with median follow-up of 45 months (range, 33–82 months) show an overall actuarial PSA-free survival of 93% at 5 years.8
Stratified by risk group, the actuarial survivals were 96%, 89% and 93% for low-, intermediate- and high-risk disease, respectively. There was no statistical difference in survival between hormone naive patients and those treated with 3 months neo-adjuvant therapy, (95% versus 93%, P = 0.3, respectively).
Comparison results
There are currently no randomised studies comparing brachytherapy to radical prostatectomy. In a study of over 6500 patients treated with brachytherapy, radiotherapy or radical prostatectomy, who were stratified according to a PSA and Gleason score, there was no significant difference between any of the primary treatment options in PSA-free survivals.9 Other, further, retrospective studies of 1305 and 2991 have also failed to demonstrate any clear superiority of one treatment over the other.10
Complications
Incontinence is rare, occurring in < 1% in our most contemporary series11 by comparison to radical prostatectomy series with rates as high as 27%.12
Erectile dysfunction seems significantly less common than with radical prostatectomy; impotence from UK and European studies is as high as 80% despite nerve-sparing techniques.13 Potters et al.14 reported on the potency of 482 patients pre-treatment and revealed a potency rate of 90% in men younger than 60 years treated with an implant alone. In our prospective study at Guildford, we have a 67% potency rate at 1 year in patients with an IIEF score ≥ 11/25, who were potent pre-treatment. A meta-analysis comparing erectile function outcomes reveals a 76% chance of remaining potent when treated by brachytherapy (reducing to 60% with combination therapy) compared to 34% after a nerve-sparing radical prostatectomy and 25% after a standard radical prostatectomy.15
Proctitis is relatively uncommon, occurring in about 5% of cases and, in our experience, has always resolved with conservative medication alone.8
The primary complication of prostate brachytherapy is a temporary deterioration of urinary function with increased irritative and obstructive symptoms. Patients are routinely treated with a-blockers. We have found that such urinary symptoms assessed by the IPSS are improved at 3 months and return to base line by 9 months.11 Temporary urinary retention can occur, usually for only 2–3 weeks; with careful case selection, the risk can be minimised to 5% or less.8,11
It is this temporary deterioration in urinary symptoms that limits the use of prostate brachytherapy. Patients with large prostate glands (+ 75 cc), poor urinary flow rates with moderate-to-severe urinary symptoms (IPSS < 15) and an inability to empty their bladder completely have exaggerated post-implant symptoms and, in the opinion of the author, are often best served by a laparoscopic radical prostatectomy so removing the obstructing gland as well as treating the cancer. However, with increasing awareness that early prostate cancer is normally a symptom-less disease and with the rise in PSA screening in the UK, the majority of patients with early prostate cancer are be suitable for prostate brachytherapy.
Summary
Brachytherapy is highly effective in the treatment of localised prostate cancer and results from the US have been reproduced in the UK. A recent review article in The Lancet discussing the clinical decision-making aspects of the treatment of early prostate cancer, highlighted the relatively low risk of urinary incontinence, rectal complications and sexual dysfunction following brachytherapy compared to radical prostatectomy.16 With many patients treated by brachytherapy in a day-case setting and being able to return to work within a few days, the advantages of brachytherapy are readily apparent. However, as with every treatment in medicine, case selection is critical to ensure satisfied patients with a low morbidity and high chance of cure.
References
- 1.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]
- 2.Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280:975–80. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester JE, Blasko JC, Grimm PD, Meier R, Malmgren JA. Ten-year biochemical relapse-free survival after external beam radiation and brachytherapy for localized prostate cancer: the Seattle experience. Int J Radiat Biol. 2003;57:944–52. doi: 10.1016/s0360-3016(03)00739-9. [DOI] [PubMed] [Google Scholar]
- 4.Potters L, Huang D, Calugaru E, Fearn P, Lee L, Kattan MW. Importance of implant dosimetry for patients undergoing prostate brachytherapy. Urology. 2003;62:1073–7. doi: 10.1016/j.urology.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Ragde H, Gardo GL, Nadir B. Brachytherapy for clinically localized prostate cancer: thirteen-year disease-free survival of 769 consecutive prostate cancer patients with permanent implants alone. Arch Esp Urol. 2001;54:739–47. [PubMed] [Google Scholar]
- 6.Stock RG, Stone NN, Cesaretti JA, Rosenstein BS. Biologically effective dose values for prostate brachytherapy: Effects on PSA failure and posttreatment biopsy results. Int J Radiat Oncol Biol Phys. 2006;64:527–33. doi: 10.1016/j.ijrobp.2005.07.981. [DOI] [PubMed] [Google Scholar]
- 7.Ragde H, Korb LJ, Elgamal AA, et al. Modern prostate brachytherapy. Prostate specific antigen results in 219 patients with up to 12 years of observed follow-up. Cancer. 2000;89:135. [PubMed] [Google Scholar]
- 8.Kaksar SJ, Laing RW, Henderon A, Sooriakumaran P, Lovell D, Langley SEM. PSA relapse-free survival and toxicity following I-125 LDR prostate brachytherapy – The Guildford experience. Br J Urol. 2006 In press. [Google Scholar]
- 9.Vicini FA, Martinez A, Hanks G, Hanlon A, Miles B, Kernan K, et al. An interinstitutional and interspecialty comparison of treatment outcome data for patients with prostate carcinoma based on predefined prognostic categories and minimum follow-up. Cancer. 2002;95:2126–35. doi: 10.1002/cncr.10919. [DOI] [PubMed] [Google Scholar]
- 10.Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy AM, Carlson TP, et al. Radical prostatectomy, external beam radiotherapy < 72 Gy, external beam radiotherapy > 72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:25–33. doi: 10.1016/s0360-3016(03)00784-3. [DOI] [PubMed] [Google Scholar]
- 11.Henderson A, Cahill D, Laing RW, Langley SEM. 125Iodine prostate brachytherapy: outcome from the first 100 consecutive patients and selection strategies incorporating urodynamics. BJU Int. 2002;90:567–72. doi: 10.1046/j.1464-410x.2002.02983.x. [DOI] [PubMed] [Google Scholar]
- 12.Augustin H, Pummer K, Daghofer F, Habermann H, Primus G, Hubmer G. Patient self-reporting questionnaire on urological morbidity and bother after radical retropubic prostatectomy. Eur Urol. 2002;42:112–7. doi: 10.1016/s0302-2838(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 13.Steineck G. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–6. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 14.Potters L, Torre T, Fearn PA, Leibel SA, Kattan MW. Potency after permanent prostate brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2001;50:1235–42. doi: 10.1016/s0360-3016(01)01578-4. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:1063–8. doi: 10.1016/s0360-3016(02)03030-4. [DOI] [PubMed] [Google Scholar]
- 16.Jani AB, Hellman S. Early prostate cancer: clinical decision-making. Lancet. 2003;361:1045–53. doi: 10.1016/S0140-6736(03)12833-4. [DOI] [PubMed] [Google Scholar]