Cutaneous adnexal tumours are largely benign lesions, and are typically classified according to their state of appendageal differentiation as eccrine, apocrine, follicular and sebaceous.1,2 However, rare tumours may display a mixture of eccrine, follicular, sebaceous and apocrine differentiation. Such tumours with varied differentiation are relatively rare.3,4,5,6,7,8,9 The term “combined adnexal tumours of the skin” was proposed by Apisarnthanarax et al.3 Other synonyms include “benign adnexal tumour with multi‐directional differentiation”8 and “benign adnexal tumour of mixed lineage”.1
Clinically, these tumours present as solitary, slowly enlarging dermal or subcutaneous nodules located in the head and neck and in the extremities.1,8 Histologically, they are characterised by well‐circumscribed, non‐encapsulated nodules composed of a lobular proliferation of epithelial cells displaying a spectrum of eccrine, apocrine, sebaceous and follicular differentiation.
We describe a composite adnexal tumour arising in the ventral aspect of the penis, differentiating into follicular and sweat ductal structures.
Case report
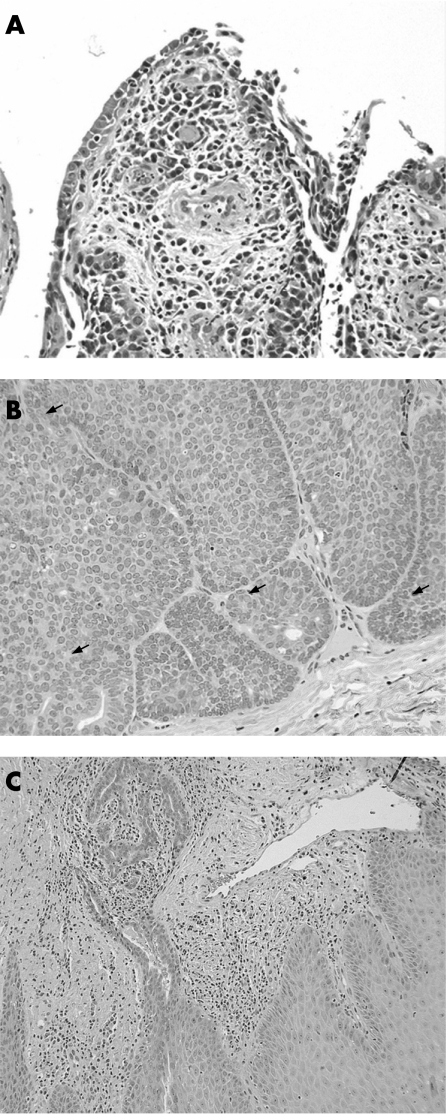
A 40‐year‐old man presented with an asymptomatic mass over the ventral aspect of the penis that had enlarged over a period of 5 years to a maximum size of 1.5 cm. The mass was excised and sent for histopathological examination. Low‐power examination revealed epithelial hyperplasia with hyperkeratosis of the surface epithelium. A relatively well‐circumscribed unencapsulated large nodular lesion was seen arising from the epidermis and extending downwards. The lesion was composed of partially solid and partially cystic areas (fig 1). One area was connected to the surface epithelium and showed cystic invaginations with numerous papillary projections, lined by two rows of cells. The luminal cells were mostly columnar, and showed decapitation secretions. The outer layer was composed of small cuboidal cells. Groups of plasma cells were seen within the papillary cores. The lesion in this area is compatible with syringocystadenoma papilliferum (SCAP) (fig 2A). The overlying epidermis in this area showed hyperplasia, parakeratosis and focal crust formation.
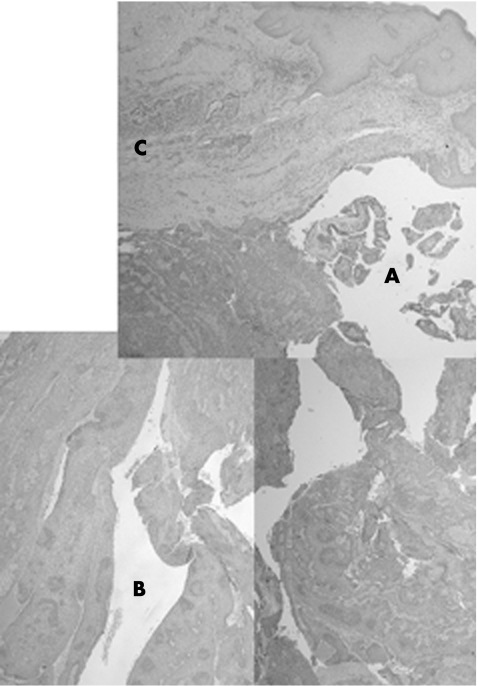
Figure 1 (A) Composite low‐power view showing partially solid and partially cystic areas (see fig 2 for corresponding areas), H&E ×2.5.
Figure 2 (A) Cystic area connected to the epidermis, with features of syringocystadenoma papilliferum, H&E ×20. (B) Solid area deep in the dermis, showing peripheral palisading, ductal structures and mitotic activity (arrows), H&E ×20. (A). Lateral area with eccrine ductal proliferation extending from an acrosyringeal structure, H&E ×10.
The solid area was located deeply in the dermis, and this formed the bulk of the lesion. It showed a lobular proliferation of epithelial cells, with peripheral palisading of basaloid columnar cells and a distinct eosinophilic rim/mantle. Normal mitotic figures were seen. Some cells within this solid area showed clearing of their cytoplasm, and other areas showed tiny tubular/ductal structures. A hyalinised/oedematous peripheral stroma was also noted. These features closely resembled those of a trichilemmoma (fig 2B).
A third area at both sides of the solid area showed proliferation of ductal structures arising from the eccrine ducts (fig 2C). These were lined by two rows of epithelial cells, and were encircled by an eosinophilic cuticle. Granular eosinophilic material was seen in some of these ductal structures.
No infiltrative borders were evident throughout the whole lesion.
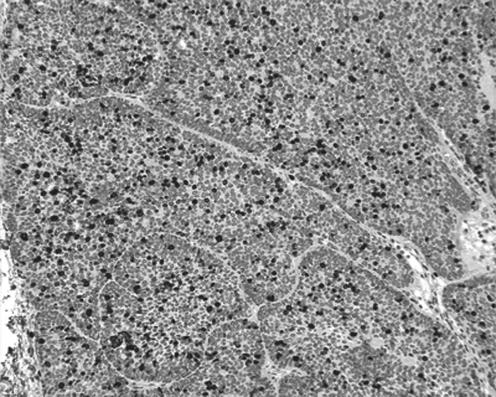
Immunohistochemical staining was performed (table 1); low‐molecular weight keratin, high‐molecular weight human kininogen, cytokeratin 7 and cytokeratin 14 had a mixed but mostly positive reaction in the three areas. P63 was positive in the solid and cystic areas. Epithelial membrane antigen and monoclonal CEA were positive in the area with eccrine ductal proliferation. Epithelial membrane antigen and carcinoembryonic antigen also showed focal positivity, especially in the tubular structures, in the cystic and solid areas. Periodic acid Schiff (PAS) showed a positive reaction with the peripheral hyalinised mantle surrounding the palisading cells of the solid area, and a positive cytoplasmic granular pattern in the clear areas. These granules were diastase sensitive, indicating glycogen deposition. Colloidal iron staining demonstrated mucin deposition in the peripheral stroma surrounding the solid area. MIB1 (a proliferation marker) showed an increased labelling index (∼30%), especially in the solid area (fig 3). CK20, Ber Ep4, oestrogen and progesterone immunostains were negative in the whole lesion.
Table 1 Positive immunohistochemical stains in different areas of the removed lesion.
| Immunostain | Cystic area | Solid area | Area with eccrine ductal proliferation |
|---|---|---|---|
| P63 | ++ (periphery) | ++ (mixed, more at periphery) | − |
| HMWK | + | + (periphery) | + |
| LMWK | Mostly positive | Mostly positive | + |
| CK7 | + (periphery) | + (less in clear areas and periphery) | + |
| CK14 | + (periphery) | + (periphery) | + |
| mCEA | + (focal) | + (focal) | ++ |
| EMA | + (focal) | + (focal) | ++ |
| PAS | − | + (hyaline rim and in clear areas) | − |
| PASD | Not applicable | Diastase sensitive in clear areas | Not applicable |
| MIB1 (%) | 30 | 30 | − |
| Colloidal iron | − | + (peripheral stroma) | − |
CEA, carcinoembryonic antigen; CK7, cytokeratin 7; CK14, cytokeratin 14; EMA, epithelial membrane antigen; HMWK, high‐molecular weight kininogen; LMWK, low‐molecular weight keratin; PAS, periodic acid Schiff; PASD, periodic acid‐silver diamine; +, mostly positive; ++, diffusely strong positive; −, negative.
Figure 3 Increased labelling index in †the solid area, as illustrated by MIB1, H&E ×10.
The histological and immunohistochemical features of this lesion demonstrate a proliferating adnexal tumour, which shows a mixture of hair follicular and eccrine gland differentiation, partly resembling syringocystadenoma papilliferum, trichilemmoma and a tumour of proliferating eccrine ductal elements. This type of lesion is best described as a proliferating composite adnexal tumour.
There was no evidence of disease recurrence or metastases 4 months after complete excision.
Discussion
The occurrence of SCAP and composite adnexal tumours of the skin in the external male genitalia is extremely rare, and has only been reported in the scrotum,10,11,12 with no cases reported in the penis. In our patient, there was a composite adnexal tumour removed from the ventral aspect of the penis, showing a combination of SCAP, trichilemmoma and eccrine ductal proliferation.
SCAP is a benign adnexal tumour commonly occurring on the scalp or face, usually in association with nevus sebaceous of Jadassohn. It is characterised by two‐cell‐layered lining papillary projections and stroma filled with plasma cells. SCAP is variably considered to be derived from eccrine, apocrine or apoeccrine origins.1,2 Trichilemmoma is a benign adnexal tumour derived from the external hair sheath.1 It consists of multiple lobules of epithelial cells appearing like an outer root sheath, generally showing pronounced clear cell change. The lesion is bounded by a thickened basement membrane, and the cells in the outer layer are palisaded. When lesions are multiple, a clinical association with various tumours occurs, as in Cowden's disease.
SCAP has been described in association with other adnexal tumours, such as tubular apocrine adenoma,13 papillary eccrine adenoma,11 apocrine cystadenoma14 and tubulopapillary hidradenoma.15 The association of SCAP and trichilemmoma has been reported only three times previously, always in association with nevus sebaceous of Jadassohn, and the reported lesions were not located in the genital area.16,17,18
To our knowledge, the association between SCAP, trichilemmoma and eccrine ductal proliferation has not been reported previously. The occurrence of these three elements together in one lesion can be explained by the common embryological origin of these structures in the primary epithelial germ of the superficial ectoderm.2
Owing to the rarity of these tumours, and the unusual location seen in our patient, the biological behaviour of this lesion is uncertain. However, the patient did not show evidence of disease recurrence or metastases 4 months after excision.
Although this is the first description of a composite adnexal tumour of the skin arising in the penis, the present case also highlights the difficulties encountered in the classification of some benign skin adnexal neoplasms, where more than one cell lineage is encountered. We also recommend the terminology of “composite adnexal tumours of the skin”, to reflect their common embryological origin, and the potential variable combination of eccrine, apocrine, sebaceous and hair follicular differentiation.
Footnotes
Competing interests: None.
References
- 1.Weedon D. Tumors of cutaneous appendages. In: Weedon D. Skin pathology. 2nd edn. Edinburgh: Churchill Livingstone, 2002859–916.
- 2.Klein W, Chan E, Seykora J T. Tumors of the epidermal appendages. In: Elder DE, editor‐in‐chief. Lever's histopathology of the skin. 9th edn. Philadelphia: Lippincott Williams & Wlikins, 2005867–926.
- 3.Apisarnthanarax P, Bovenmyer D A, Mehregan A H. Combined adnexal tumor of the skin. Arch Dermatol 1984120231–233. [PubMed] [Google Scholar]
- 4.Zaim M T. Sebocrine adenoma. An adnexal adenoma with sebaceous and apocrine poroma‐like differentiation. Am J Dermatopathol 198810311–318. [DOI] [PubMed] [Google Scholar]
- 5.Poomeechaiwong S, Bonelli J E, Golitz L E. Mixed tumor of the pilosebaceous type: mixed tumor of the skin with apocrine, follicular and sebaceous differentiation. J Cutan Pathol 19881533–38. [Google Scholar]
- 6.Requena L, Sanchez Y E, Santa Cruz D J. Apocrine type of cutaneous mixed tumor with follicular and sebaceous differentiation. Am J Dermatopathol 199214186–194. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez Yus E, Requena L, Simon P.et al Complex adnexal tumor of the primary epithelial germ with distinct patterns of superficial epithelioma with sebaceous differentiation, immature trichoepithelioma, and apocrine adenocarcinoma. Am J Dermatopathol 199214245–252. [DOI] [PubMed] [Google Scholar]
- 8.Wong T Y, Suster S, Cheek R F.et al Benign cutaneous adnexal tumors with combined folliculosebaceous, apocrine, and eccrine differentiation: clinicopathologic and immunohistochemical study of eight cases. Am J Dermatopathol 199618124–136. [DOI] [PubMed] [Google Scholar]
- 9.Iwenofu O H, Crowson A N. Pathologic quiz case: cystic tumor of the left eyebrow in a 42‐year‐old woman. Arch Pathol Lab Med 20041281181–1182. [DOI] [PubMed] [Google Scholar]
- 10.Mammino J J, Vidmar D A. Syringocystadenoma papilliferum. Int J Dermatol 199130763–766. [DOI] [PubMed] [Google Scholar]
- 11.Coyne J D, Fitzgibbon J F. Mixed syringocystadenoma papilliferum and papillary eccrine adenoma occurring in a scrotal condyloma. J Cutan Pathol 200027199–201. [DOI] [PubMed] [Google Scholar]
- 12.Goshima J, Hara H, Okada T.et al Syringocystadenoma papilliferum arising on the scrotum. Eur J Dermatol 200313271. [PubMed] [Google Scholar]
- 13.Ansai S, Watanabe S, Aso K. A case of tubular apocrine adenoma with syringocystadenoma papilliferum. J Cutan Pathol 198916230–236. [DOI] [PubMed] [Google Scholar]
- 14.Schewach‐Millet M, Trau H. Congenital papillated apocrine cystadenoma: a mixed form of hidrocystoma, hidradenoma papilliferum, and syringocystadenoma papilliferum. J Am Acad Dermatol 198411374–376. [DOI] [PubMed] [Google Scholar]
- 15.Hsu P J, Liu C H, Huang C J. Mixed tubulopapillary hidradenoma and syringocystadenoma papilliferum occurring as a verrucous tumor. J Cutan Pathol 200330206–210. [DOI] [PubMed] [Google Scholar]
- 16.Coskey R J. The spectrum of organoid nevi. Cutis 198229290–294. [PubMed] [Google Scholar]
- 17.Yoon D H, Jang I G, Kim T Y.et al Syringocystadenoma papilliferum, basal cell carcinoma and trichilemmoma arising from nevus sebaceus of Jadassohn. Acta Dermatol Venereol 199777242–243. [DOI] [PubMed] [Google Scholar]
- 18.Castilla E A, Bergfeld W F, Ormsby A. Trichilemmoma and syringocystadenoma papilliferum arising in naevus sebaceous. Pathology 200234196–197. [DOI] [PubMed] [Google Scholar]