Abstract
Aim
To investigate whether nuclear and cytoplasmic Maspin expression is associated with distinct clinicopathological parameters and TP53 expression in a representative series of primary non‐small cell lung cancer (NSCLC).
Methods
Tissue microarrays (n = 487) were used to immunohistochemically analyse expression of Maspin and TP53. Cytoplasmic and nuclear expression of Maspin was scored on the basis of the percentage of positive tumour cells. Univariate analysis of clinicopathological variables potentially affecting tumour‐specific survival was performed.
Results
Immunohistochemical Maspin expression (nuclear and cytoplasmic) was informative in 72.3% (352/487) of cases. Cytoplasmic and nuclear Maspin immunoreactivity in ⩾10% of tumour cells was detected in 37.8% (133/352) and 65.3% (230/352) of informative cases, respectively. Nuclear and cytoplasmic Maspin staining was observed more frequently in primary squamous cell carcinomas than in other lung cancer types. Only nuclear Maspin immunoreactivity was significantly associated with positive TP53 staining. Cytoplasmic or nuclear Maspin expression was not associated with tumour‐specific survival.
Conclusion
Maspin expression was found both in the nucleus and the cytoplasm of NSCLC, more frequently in squamous cell carcinomas. However, no association with tumour‐specific survival could be demonstrated.
Maspin, a member of the Serpin family of protease inhibitors, is known to have tumour‐suppressor activity in several human tissues by inhibiting tumour cell motility and invasion in vitro, as well as tumour growth and metastasis in animal models.1 Zou et al2 have shown that, in primary breast cancer, Maspin is regulated by wild‐type TP53, defining a new category of molecular targets of TP53 that have the potential to negatively regulate tumour invasion and metastasis. Loss of Maspin expression correlated with increased tumour aggressiveness and poor prognosis in advanced breast and prostate cancer.1,3,4,5 In contrast, Maspin was overexpressed in pancreatic, ovarian, thyroid, gastric, lung, bladder, breast, skin and colon cancer.6,7,8,9,10,11,12,13,14,15,16 A possible role of Maspin in lung cancer biology was suggested by gene expression profiling.10 Several studies have investigated the prognostic significance of Maspin expression in lung cancer. In one study, cytoplasmic Maspin expression in primary non‐small cell lung cancer (NSCLC) was an independent negative prognostic factor for overall survival,17 whereas strong nuclear Maspin expression was associated with increased overall and disease‐free survival in patients with resectable NSCLC.11
Tissue microarrays (TMAs) are highly efficient tools for the investigation of large series of tumours with defined clinical characteristics of the patients.18,19 The aim of this study was to clarify whether nuclear and cytoplasmic Maspin expression is associated with a distinct clinicopathological profile, and to study the relationship of Maspin expression with TP53 alterations by using a large‐scale TMA of 487 primary NSCLCs.
Methods
Lung cancer TMA
A TMA was constructed as described previously20 and contained 487 formalin‐fixed, paraffin wax‐embedded specimens of patients with primary invasive NSCLC. All the 487 tissue cylinders (one cylinder per tumour; diameter 0.6 mm) were subsequently brought into two empty “recipient” paraffin wax blocks. Patients were treated at the University of Basel, Basel, Switzerland, and were studied retrospectively. One pathologist (PD) with expertise in lung pathology evaluated H&E‐stained slides of all tumours. Tumour stage and grade were assigned according to UICC (The International Union Against Cancer) and World Health Organization criteria. Clinical follow‐up data were available for 449 (92%) lung cancer patients with a median (range) follow‐up period of 27 (0–200) months. The Institutional Review Board of the University of Basel granted approval for the study.
Immunohistochemistry
Immunohistochemical studies utilised an avidin–biotin peroxidase method with a diaminobenzidine chromatogen. After antigen retrieval (in a microwave oven for 30 min at 250 W), IHC was carried out in a Ventana NEXES autostainer (Ventana, Tucson, Arizona, USA) following manufacturer's instructions. The following primary antibodies were used: anti‐TP53 (monoclonal, clone Bp53–12, Santa Cruz Biotechnology, Santa Cruz, California, USA; dilution 1:1000), and anti‐Maspin (monoclonal, clone G167–70, Becton Dickinson/Pharmingen, Heidelberg, Germany; dilution 1:1000). Negative controls without primary antibody were included in each experiment. One surgical pathologist (MW), who was blinded to the clinical data, evaluated all stainings. TP53 positivity was defined as strong nuclear staining in at least 10% of the tumour cells. Cytoplasmic and nuclear Maspin immunoreactivity was assessed semiquantitatively on the basis of the percentage of positive cells, as described previously.21 Maspin immunoreactivity was defined as positive if at least 10% of tumour cells showed at least moderate staining intensity. The cut‐off value of 10% was chosen for categorisation of cytoplasmic and nuclear Maspin expression, representing the best discrimination as derived from the receiver operator characteristic analysis. In detail, receiver operator characteristic curves obtained by plotting sensitivity versus (1 – specificity) were used to evaluate the prognostic performance of Maspin expression at various cut‐off points. The optimum cut‐off was calculated as the maximum value of sensitivity multiplied by specificity. Sensitivity and specificity were calculated according to standard formulae.
Statistical analysis
Statistical analyses were completed using SPSS V.10.0. p Values <0.05 were considered significant. Contingency table analysis and two‐sided Fisher's exact tests were used to study the statistical association between clinicopathological and immunohistochemical data. Survival curves comparing patients with or without any of the factors were calculated using the Kaplan–Meier method, with significance evaluated by two‐sided log rank statistics. For tumour‐specific survival, patients were censored at the time of their last tumour‐free clinical follow‐up appointment or at their date of death not related to the tumour. For multiple testing, the closed test principle was used.
Results
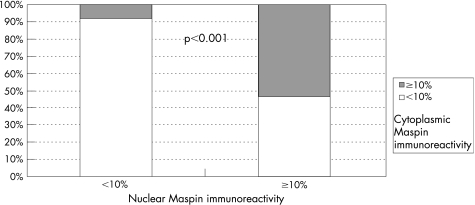
Investigation of immunohistochemical Maspin expression was informative in 72.3% (352/487) of cases. Cytoplasmic and nuclear Maspin immunoreactivity in ⩾10% of tumour cells was detected in 37.8% (133/352) and 65.3% (230/352) of informative cases, respectively. Figure 1A,B shows the representative Maspin immunostaining patterns. Negative nuclear and cytoplasmic Maspin immunoreactivity (<10%) is co‐associated significantly in 91.8% (112/122) of informative cases, whereas simultaneous cytoplasmic and nuclear Maspin expression in at least 10% of cells was found only in 53.5% (123/230) of informative cases (p<0.001; fig 2). For descriptive data analysis, all relevant variables were compared with the results of Maspin IHC (table 1).
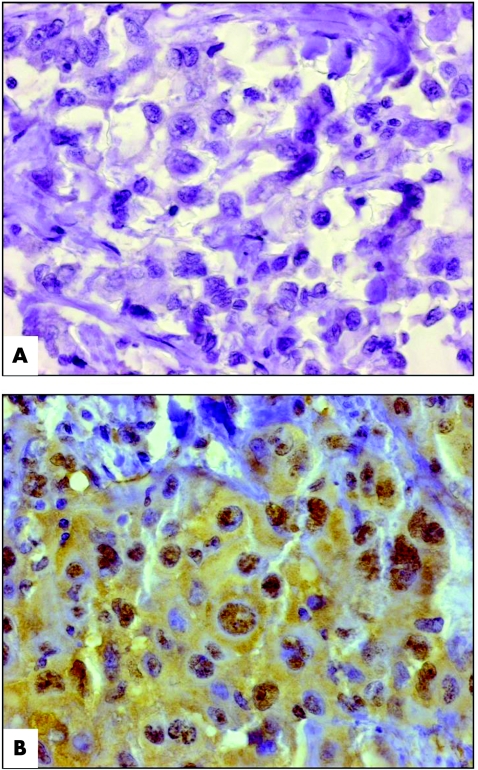
Figure 1 Immunohistochemical Maspin staining on the tissue microarray. (A) Representative example of primary non‐small cell lung cancer (NSCLC) with negative immunoreactivity for Maspin (original magnification ×400). (B) Representative section of primary NSCLC showing moderate nuclear and cytoplasmic immunoreactivity for Maspin (original magnification ×400).
Figure 2 Cumulative bar chart showing the association of negative nuclear and cytoplasmic Maspin immunoreactivity in non‐small cell lung cancer.
Table 1 Clinicopathological and immunohistochemical parameters relative to Maspin immunohistochemistry.
| Variable | Categorisation | Analysable cases (n) | Nuclear Maspin IHC | Cytoplasmic Maspin IHC | ||||
|---|---|---|---|---|---|---|---|---|
| <10% | ⩾10% | p Value* | <10% | ⩾10% | p Value* | |||
| Clinicopathological data: | ||||||||
| Tumour stage | ||||||||
| pT1 | 67 | 27 | 40 | 0.208 | 41 | 26 | 0.403 | |
| pT2 | 217 | 77 | 140 | 139 | 78 | |||
| pT3 | 45 | 10 | 35 | 24 | 21 | |||
| pT4 | 5 | 1 | 4 | 2 | 3 | |||
| Lymph node status | ||||||||
| pN0 | 182 | 63 | 119 | 0.842 | 112 | 70 | 0.923 | |
| pN1 | 114 | 38 | 76 | 69 | 45 | |||
| pN2 | 32 | 13 | 19 | 21 | 11 | |||
| pN3 | 1 | 0 | 1 | 1 | 0 | |||
| Histological type of NSCLC | ||||||||
| Squamous | 209 | 62 | 147 | 0.043 | 105 | 104 | <0.001 | |
| Adeno | 74 | 35 | 39 | 65 | 9 | |||
| Bronchoalveolar | 16 | 7 | 9 | 14 | 2 | |||
| Undifferentiated, large cell | 53 | 18 | 35 | 35 | 18 | |||
| TP53 IHC | ||||||||
| <10% | 179 | 60 | 119 | 0.006 | 105 | 74 | 1.000 | |
| ⩾10% | 121 | 23 | 98 | 68 | 53 | |||
IHC, immunohistochemistry; NSCLC, non‐small cell lung cancer.
*Fisher's exact test (two‐sided), bold type representing significant data.
Remarkably, nuclear (p = 0.04) and cytoplasmic (p<0.001) Maspin staining in at least 10% of tumour cells was observed more frequently in primary squamous cell carcinomas of the lung than in other tumour types. However, Maspin expression was unrelated to tumour stage and nodal status. Only nuclear Maspin immunoreactivity in at least 10% of tumour cells was significantly associated with positive TP53 staining (p = 0.006; table 1).
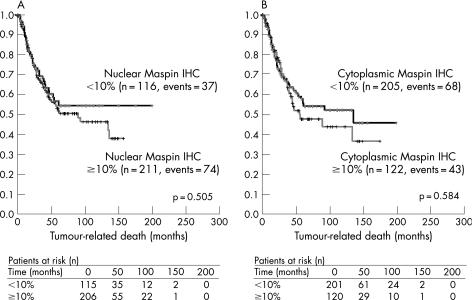
Tumour‐specific survival was compared between Maspin‐positive and Maspin‐negative cases by univariate log rank statistics. Higher tumour stage (p<0.001) and positive lymph node status (p<0.001) were significantly associated with shorter survival times. However, cytoplasmic or nuclear Maspin expression was not associated with tumour‐related survival when all tumours were included into the analysis (fig 3A,B). No association between Maspin IHC and tumour‐related survival was found in TP53‐positive and TP53‐negative cases, respectively. Finally, within the groups of patients with different histological subtypes of NSCLC, no such association could be shown.
Figure 3 Distribution of time (months) to tumour‐related death among patients with lung cancer with negative (<10%) and positive (⩾10%) (A) nuclear and (B) cytoplasmic Maspin immunoreactivity as estimated by the method of Kaplan and Meier. Below, numbers of patients with lung cancer at risk over time are provided. IHC, immunohistochemistry.
Discussion
We investigated nuclear and cytoplasmic Maspin expression in a large series of NSCLCs of all relevant histological subtypes. Using TMA technology, the results of TP53 and Maspin IHC were compared with the clinicopathological data. Despite the small size of the individual tumour specimens in TMAs, both histological features and molecular characteristics are highly representative of their tissues of origin (reviewed by Simon et al18).
Gene expression profiling of primary NSCLC identified Maspin as a potential tumour‐suppressor gene in lung cancer.10 Our study found cytoplasmic Maspin expression predominantly in squamous cell carcinomas, consistent with the results of Yatabe et al.22 Pemberton et al23 found Maspin to be a predominantly soluble cytoplasmic protein associated with secretory vesicles at the cell surface of cultured human mammary myoepithelial cells, showing that Maspin is more ubiquitous than believed previously.23 According to Smith et al,11 the present study also found nuclear Maspin expression in NSCLC (table 1). The simultaneous expression of Maspin both in the nucleus and the cytoplasm of NSCLC cells in our study is in accordance with Lonardo et al,24 who have reported a combined nuclear–cytoplasmic staining, especially in squamous cell carcinomas of the lung. However, the association of nuclear Maspin expression with favourable morphological features in pulmonary adenocarcinoma, described by Lonardo et al,24 could not be validated. Furthermore, no association between Maspin IHC (nuclear or cytoplasmic) and tumour‐related survival was found within the subgroups of patients with different histological types of NSCLC.
Systematic quantification of Maspin expression in NSCLC was of particular interest as existing data on the prognostic significance of Maspin are conflicting. Nakashima et al25 suggested an association between cytoplasmic Maspin expression and a favourable prognosis in 78 patients with primary adenocarcinoma of the lung. Hirai et al17 showed cytoplasmic Maspin expression to be an independent negative prognostic factor for overall survival in 132 patients with NSCLC, with 3‐year post‐surgery survival of 30.8% for the Maspin‐positive group and 71.1% for the Maspin‐negative group (p = 0.007). Smith et al11 reported a significant association between nuclear Maspin expression and prolonged survival in 48 patients with NSCLC with resectable tumours. However, the results of Smith et al11 were of borderline significance (p = 0.05). In the present study, neither cytoplasmic nor nuclear Maspin expression correlated significantly with patient survival (fig 3A,B), using a large‐scale TMA with 487 cases of lung cancer of all histological types.
According to Zou et al,2 TP53 activates Maspin by binding directly to the TP53 consensus‐binding site of the Maspin promoter—that is, Maspin is one of the few TP53‐targeted genes involved in tumour invasion and metastasis. DNA‐damaging agents and cytotoxic drugs induced endogenous Maspin expression in cells containing wild‐type TP53. Maspin expression was refractory to DNA‐damaging agents in cells containing mutant TP53, thus defining a new category of molecular target for TP53 that has the potential to negatively regulate tumour invasion and/or metastasis.2 An association between nuclear Maspin expression and TP53 immunoreactivity in NSCLC could be shown. Cytoplasmic Maspin expression was not affected by the TP53 status. Similarly, Hirai et al17 did not find an association between cytoplasmic Maspin expression and TP53 IHC in NSCLC. On the basis of the hypothesis that biological effects of Maspin are linked to its subcellular localisation,24 association of nuclear, but not cytoplasmic, Maspin expression with nuclear TP53 staining seems inevitable.
In summary, Maspin expression was frequently found both in the nucleus and the cytoplasm of NSCLC in a large‐scale study using TMAs. However, no prognostic impact on tumour‐specific survival could be shown.
Acknowledgements
We thank Rudolf Jung for excellent technical assistance. This research was supported by a grant of the Dr Mildred Scheel Foundation for Cancer Research to Matthias Woenckhaus.
Abbreviations
IHC - immunohistochemistry
NSCLC - non‐small cell lung cancer
TMA - tissue microarray
Footnotes
Competing interests: None.
References
- 1.Zou Z, Anisowicz A, Hendrix M J.et al Maspin, a serpin with tumor‐suppressing activity in human mammary epithelial cells. Science 1994263526–529. [DOI] [PubMed] [Google Scholar]
- 2.Zou Z, Gao C, Nagaich A K.et al p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem 20002756051–6054. [DOI] [PubMed] [Google Scholar]
- 3.Hojo T, Akiyama Y, Nagasaki K.et al Association of maspin expression with the malignancy grade and tumor vascularization in breast cancer tissues. Cancer Lett 2001171103–110. [DOI] [PubMed] [Google Scholar]
- 4.Maass N, Nagasaki K, Ziebart M.et al Expression and regulation of tumor suppressor gene maspin in breast cancer. Clin Breast Cancer 20023281–287. [DOI] [PubMed] [Google Scholar]
- 5.Zou Z, Zhang W, Young D.et al Maspin expression profile in human prostate cancer (CaP) and in vitro induction of Maspin expression by androgen ablation. Clin Cancer Res 200281172–1177. [PubMed] [Google Scholar]
- 6.Sood A K, Fletcher M S, Gruman L M.et al The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res 200282924–2932. [PubMed] [Google Scholar]
- 7.Maass N, Hojo T, Ueding M.et al Expression of the tumor suppressor gene Maspin in human pancreatic cancers. Clin Cancer Res 20017812–817. [PubMed] [Google Scholar]
- 8.Akiyama Y, Maesawa C, Ogasawara S.et al Cell‐type‐specific repression of the maspin gene is disrupted frequently by demethylation at the promoter region in gastric intestinal metaplasia and cancer cells. Am J Pathol 20031631911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogasawara S, Maesawa C, Yamamoto M.et al Disruption of cell‐type‐specific methylation at the Maspin gene promoter is frequently involved in undifferentiated thyroid cancers. Oncogene 2004231117–1124. [DOI] [PubMed] [Google Scholar]
- 10.Heighway J, Knapp T, Boyce L.et al Expression profiling of primary non‐small cell lung cancer for target identification. Oncogene 2002217749–7763. [DOI] [PubMed] [Google Scholar]
- 11.Smith S L, Watson S G, Ratschiller D.et al Maspin—the most commonly‐expressed gene of the 18q21.3 serpin cluster in lung cancer—is strongly expressed in preneoplastic bronchial lesions. Oncogene 2003228677–8687. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto S, Maass N, Takimoto Y.et al Expression and regulation of tumor suppressor gene maspin in human bladder cancer. Cancer Lett 2004203209–215. [DOI] [PubMed] [Google Scholar]
- 13.Reis‐Filho J S, Torio B, Albergaria A.et al Maspin expression in normal skin and usual cutaneous carcinomas. Virchows Arch 2002441551–558. [DOI] [PubMed] [Google Scholar]
- 14.Umekita Y, Ohi Y, Sagara Y.et al Expression of maspin predicts poor prognosis in breast‐cancer patients. Int J Cancer 2002100452–455. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Yoshida H, Tomoda C.et al Maspin expression is directly associated with biological aggressiveness of thyroid carcinoma. Thyroid 20041413–18. [DOI] [PubMed] [Google Scholar]
- 16.Bettstetter M, Woenckhaus M, Wild P J.et al Elevated nuclear maspin expression is associated with microsatellite instability and high tumour grade in colorectal cancer. J Pathol 2005205606–614. [DOI] [PubMed] [Google Scholar]
- 17.Hirai K, Koizumi K, Haraguchi S.et al Prognostic significance of the tumor suppressor gene maspin in non‐small cell lung cancer. Ann Thorac Surg 200579248–253. [DOI] [PubMed] [Google Scholar]
- 18.Simon R, Mirlacher M, Sauter G. Tissue microarrays. Biotechniques 20043698–105. [DOI] [PubMed] [Google Scholar]
- 19.Bubendorf L, Nocito A, Moch H.et al Tissue microarray (TMA) technology: miniaturized pathology archives for high‐throughput in situ studies. J Pathol 200119572–79. [DOI] [PubMed] [Google Scholar]
- 20.Kononen J, Bubendorf L, Kallioniemi A.et al Tissue microarrays for high‐throughput molecular profiling of tumor specimens. Nat Med 19984844–847. [DOI] [PubMed] [Google Scholar]
- 21.Dietmaier W, Bettstetter M, Wild P J.et al Nuclear Maspin expression is associated with response to adjuvant 5‐fluorouracil based chemotherapy in patients with stage III colon cancer. Int J Cancer 20061182247–2254. [DOI] [PubMed] [Google Scholar]
- 22.Yatabe Y, Mitsudomi T, Takahashi T. Maspin expression in normal lung and non‐small‐cell lung cancers: cellular property‐associated expression under the control of promoter DNA methylation. Oncogene 2004234041–4049. [DOI] [PubMed] [Google Scholar]
- 23.Pemberton P A, Tipton A R, Pavloff N.et al Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem 1997451697–1706. [DOI] [PubMed] [Google Scholar]
- 24.Lonardo F, Li X, Siddiq F.et al Maspin nuclear localization is linked to favorable morphological features in pulmonary adenocarcinoma. Lung Cancer 2005 [DOI] [PubMed]
- 25.Nakashima M, Ohike N, Nagasaki K.et al Prognostic significance of the maspin tumor suppressor gene in pulmonary adenocarcinoma. J Cancer Res Clin Oncol 2004130475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]