Abstract
Follicular lymphoma (FL) is a neoplasm originating from germinal centre cells, corresponding to 25–40% of non‐Hodgkin's lymphomas. Transformation into diffuse large B cell lymphoma (DLBCL) occurs in about one‐third of cases. CD5 is expressed in B‐chronic lymphoid leukaemia/small lymphocytic lymphoma and mantle cell lymphoma, but can rarely be expressed in conjunction with CD10 in well‐documented cases of FL. In this report one case of grade 1 FL is described, which transformed into a DLBCL 6 months after initial diagnosis, with both tumours expressing CD5. In both specimens, neoplastic cells were strongly positive for CD20, CD79a, bcl‐2, bcl‐6 and CD5 in virtually all cells. CD10 was strongly positive in initial specimens and weakly positive in the DLBCL. Investigation using the PCR confirmed the derivation of the DLBCL from the FL as they presented the same immunoglobulin heavy chain gene rearrangement and the same BCL2‐JH break point.
Follicular lymphoma (FL) is a neoplasm originating from germinal centre cells, corresponding to 25–40% of non‐Hodgkin's lymphomas.1 At morphology it is mostly constituted by a majority of small cleaved lymphoid cells, with variable numbers of centroblasts with a nodular growth pattern.1 FL expresses B cell markers and CD10, whereas it is typically negative for CD5.1,2,3,4,5 Translocation t(14;18)(q32;q21) involving rearrangement of the BCL2 gene is present in 70–95% of the cases.1 Transformation into diffuse large B cell lymphoma (DLBCL) occurs in about one‐third of the cases, mainly in patients with adverse prognostic factors or in those who do not achieve complete remission after initial treatment.6
CD5 is a molecule present in T lymphocytes and in a subset of B lymphocytes and B lymphoproliferations.1,3,4,7 Rarely CD5 has been expressed in conjunction with CD10 in cases with well‐documented diagnoses of FL.8,9,10,11,12 It is our purpose to describe one case of FL which transformed into a DLBCL 6 months after initial diagnosis, both tumours strongly expressing CD5, with the same BCL2‐JH break point.
Case report
A patient in their 40s was referred to the hospital for an enlarged inguinal lymph node (2 cm), which was apparently isolated, without any other clinical and biological symptoms. The lymph node was removed and the diagnosis of grade 1 FL was made. She was considered as Ann Arbor stage I‐A and was not treated. After 6 months, the patient consulted for the presence of a spinal lymph node with rapid growth. This lymph node was removed and the diagnosis of DLBCL was retained. Both tumours were then compared from phenotypic and molecular points of view.
Materials and methods
Tissue specimens were fixed in 10% formalin and embedded paraffin wax. Histological sections stained with H&E from initial diagnosis and lymphoma transformation were reviewed. Immunohistochemical staining was performed using a Ventana immunostainer (Ventana‐Biotech, Tucso, Arizona, USA). The panel included antibodies to CD20, CD79a, CD3, CD5, CD10, CD21, CD23, bcl‐2, bcl‐6, CD138, MUM‐1, cyclin D1 and Ki‐67. Revelation was achieved using a streptavidin–biotin–peroxidase detection system and staining with diaminobenzidine.
For genetic analysis of immunoglobulin and BCL2‐JH rearrangements, 10 µm thick sections of both paraffin wax blocks were processed for PCR as described elsewhere.13,14 The sets of primers used were FR1c, FR2a and FR3a. PCR products were then sequenced to verify whether the amplified regions matched, using the procedure reported previously.15
Results
Histopathology of the first lymph node specimen was diagnostic of an FL, grade 1. In the specimen from tumour relapse a diffuse proliferation of large cells consistent with centroblasts was present, intermingled with frequent mitotic figures, diagnostic of a diffuse large cell lymphoma.
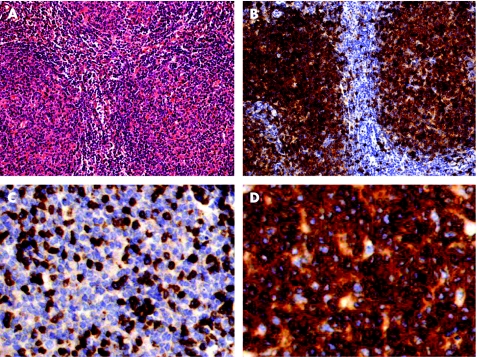
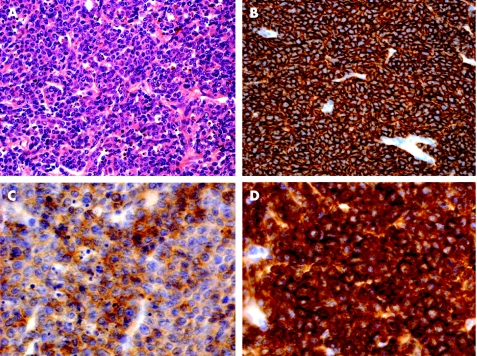
In both specimens, neoplastic cells were strongly positive for CD20, CD79a, bcl‐2, bcl‐6 and CD5 in virtually all cells. CD10 was strongly positive in initial specimens and weakly positive in the DLBCL (figs 1, 2). Neoplastic cells were negative for CD3, CD21, CD23, cyclin D1, MUM‐1 and CD138 in both specimens. Proliferation marker Ki‐67 was expressed in <10% of the cells in the initial biopsy and in >70% in the second biopsy.
Figure 1 (A) Follicular lymphoma, grade I in the first specimen (H&E). (B) Expression of CD10 by neoplastic cells. (C) Only reactive T lymphocytes express CD3. (D) Strong expression of CD5 in neoplastic cells (A and B: ×200; C and D: ×1000).
Figure 2 (A) Diffuse large B cell lymphoma in the second specimen (H&E). (B) Strong expression of CD20, (C) weak expression of CD10 and (D) strong expression of CD5 (A and B: ×400; C and D: ×1000).
PCR investigations confirmed a common clonal derivation for both tumours with the same pattern of rearrangement for immunoglobulin heavy genes (FR1c−, FR2a+ approximate length 250 kilobase pairs, FR3a−) and BCL2‐JH approximate length 190 kilobase pairs. Sequencing of PCR products showed the same pattern in both specimens, FL and DLBCL, with fusion of BCL2‐JH: BCL2‐MBR position 3063–JH segment J6 2951.
Discussion
The case reported here corresponds to a rare CD5+ FL, which progressed into a CD5+ DLBCL within 6 months. The initial diagnosis of FL, grade 1, is assured by morphological features, immunoexpression of CD10 and bcl‐6. DLBCL presented a germinal centre profile, CD10+/bcl‐6+/MUM‐1−/CD138−, which is in accordance with the progression from an FL.
Among reports on the expression of CD5 in FL, two reports present data from molecular analysis. In the first, expression of CD5 in 4 of 11 cases of “floral” FL is reported.8 In three cases, expression of CD5 was shown by immunohistochemistry, one with weak to moderate intensity on paraffin wax sections and two with weak intensity on frozen sections; no immunohistochemical expression of CD5 was shown in one case. In three cases, variable intensity of CD5 expression was detected by flow cytometry, including two also positive by immunohistochemistry. Molecular analysis demonstrated clonal heavy chain immunoglobulin gene rearrangement in three and BCL2‐JH products derived from the t (14;18) translocation in all four CD5‐positive cases.
Another report was based on three FLs, all with weak expression of CD5 both on paraffin wax sections and by flow cytometry.10 All three cases revealed clonal heavy chain immunoglobulin gene rearrangement, and two showed BCL2 gene rearrangement. These authors further supported the rarity of CD5 expression in large series of FL studied by flow cytometry. This technique was considered more suitable for this conclusion, as positivity for CD5 might be underestimated by immunohistochemistry, owing to the low intensity of staining of this marker in these FL. In contrast with these studies, our case presented strong expression of CD5 in both FL and DLBCL. At last, PCR investigations clearly showed the same clonal origin of both tumours, with shared patterns of immunoglobulin heavy gene and BCL2‐JH gene rearrangements, supporting the clonal malignant progression.
Take‐home messages
CD5, a pan‐lymphoid T marker, is typically expressed in two B‐lymphoid neoplasms: small lymphocytic lymphoma/chronic lymphoid leukaemia and mantle cell lymphoma.
CD5 may be rarely expressed in other B‐lymphoid neoplasms, as in the case reported herein of follicular lymphoma, which transformed into a diffuse large B‐cell lymphoma (DLBCL) 6 months after initial diagnosis.
Although the biological significance of CD5 expression in FL and DLBCL is still unclear, it might be associated with adverse clinical course.
The biological significance of the expression of CD5 in FL is unclear. It might be due to imbalance in the production or response to interleukins in the development of B cells modulating CD5 expression, it could be related to the activation state of B lymphocytes or it could just represent a fortuitous aberrant detection of a marker during neoplastic transformation.8,10,11 Notably, it was recently suggested that cases of CD5+ FL coexisting with CD5+ DLBCL were associated with an aggressive clinical course.12
In conclusion, CD5 may be rarely expressed in FL. This expression should be evaluated in conjunction with other morphological, immunophenotypic and genetic features, to differentiate FL from other small B cell neoplasms, which may present a nodular growth pattern. Furthermore, expression of CD5 might represent an adverse prognostic feature in these FL.
Acknowledgements
We acknowledgee the financial support from Fundação de Amparo à Pesquisa de São Paulo (FAPESP, Brazil), Fundo de Apoio ao Ensino e Pesquisa (FAEP‐UNICAMP, Brazil) and University of Toulouse (France). We also thank Mr Michel March and Daniel Roda for excellent technical assistance. JV is researcher of the Conselho Nacional de Pesquisa Cientifica (CNPq, Brazil).
Abbreviations
DLBCL - diffuse large B cell lymphoma
FL - follicular lymphoma
Footnotes
Competing interests: None declared.
References
- 1.Harris N L, Jaffe E S, Stein H.et al A revised European‐American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994841361–1392. [PubMed] [Google Scholar]
- 2.Treasure J, Lane A, Jones D B.et al CD43 expression in B cell lymphoma. J Clin Pathol 1992451018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh N, Wright D H. The value of immunohistochemistry on paraffin wax embedded tissue sections in the differentiation of small lymphocytic and mantle cell lymphomas. J Clin Pathol 19975016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C C, Raikow R B, Sonmez‐Alpan E.et al Classification of small B‐cell lymphoid neoplasms using a paraffin section immunohistochemical panel. Appl Immunohistochem Mol Morphol 200081–11. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, McKenna R W, Kroft S H. Assessment of CD10 in the diagnosis of small B‐cell lymphomas: a multiparameter flow cytometric study. Am J Clin Pathol 2002117291–300. [DOI] [PubMed] [Google Scholar]
- 6.Bastion Y, Sebban C, Berger F.et al Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol 1997151587–1594. [DOI] [PubMed] [Google Scholar]
- 7.Dono M, Cerruti G, Zupo S. The CD5+ B‐cell. Int J Biochem Cell Biol 2004362105–2111. [DOI] [PubMed] [Google Scholar]
- 8.Tiesinga J J, Wu D, Inghirami G. CD5+ follicular lymphoma. Immunophenotyping detects a unique subset of “floral” follicular lymphoma. Am J Clin Pathol 2000114912–921. [DOI] [PubMed] [Google Scholar]
- 9.Barekman C L, Aguilera N S I, Abbondanzo S L. Low‐grade B‐cell lymphoma with coexpression of both CD5 and CD10. A report of 3 cases. Arch Pathol Lab Med 2001125951–953. [DOI] [PubMed] [Google Scholar]
- 10.Barry T S, Jaffe E S, Kingma D W.et al CD5+ follicular lymphoma. A clinicopathologic study of three cases. Am J Clin Pathol 2002118589–598. [DOI] [PubMed] [Google Scholar]
- 11.Dong H Y, Gorczyca W, Liu Z.et al B‐cell lymphomas with coexpression of CD5 and CD10. Am J Clin Pathol 2003119218–230. [DOI] [PubMed] [Google Scholar]
- 12.Manazza A D, Bonello L, Pagano M.et al Follicular origin of a subset of CD5+ diffuse large B‐cell lymphomas. Am J Clin Pathol 2005124182–190. [DOI] [PubMed] [Google Scholar]
- 13.Rauzy O, Galoin S, Chale J J.et al Detection of t(14;18) carrying cells in bone marrow and peripheral blood from patients affected by non‐lymphoid diseases. J Clin Pathol Mol Pathol 199851333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thériault C, Galoin S, Valmary S.et al PCR analysis of immunoglobulin heavy chain (IgH) and TcR‐γ chain gene rearrangements in the diagnosis of lymphoproliferative disorders: results of a study of 525 cases. Mod Pathol 2000131269–1279. [DOI] [PubMed] [Google Scholar]
- 15.Galoin S, Al Saati T, Schlaifer D.et al Oligonucleotide clonospecific probes directed against the junctional sequence of t(14;18): a new tool for the assessment of minimal residual disease in follicular lymphoma. Br J Haematol 199694676–684. [DOI] [PubMed] [Google Scholar]