Abstract
Background
The antiapoptotic and epithelial–mesenchymal transition activities of Twist have been implicated in the neoplastic transformation and the development of metastasis, respectively. Upregulation of Twist, described in several types of human cancer, also acts as a prognostic marker of poor outcome.
Aim
To investigate Twist expression in oesophageal squamous cell carcinoma (SCC) and its prognostic value in a Chinese cohort of patients with oesophageal SCC.
Methods
Twist expression in primary oesophageal SCC of 87 Chinese patients was investigated by immunohistochemical staining. Twist protein level in one immortalised normal oesophageal epithelial cell line and six oesophageal SCC cell lines was measured by western blot analysis. Twist mRNA level in 30 pairs of frozen specimens of primary oesophageal SCC and non‐neoplastic oesophageal epithelium from the upper resection margin of corresponding oesophagectomy specimen was also determined by semiquantitative reverse transcription‐PCR.
Results
It was found that Twist was upregulated in oesophageal SCC cell lines, and its mRNA and protein levels were both increased in oesophageal SCC and the non‐neoplastic oesophageal epithelium (p<0.001). In addition, a high level of Twist expression in oesophageal SCC was significantly associated with a greater risk for the patient of developing distant metastasis within 1 year of oesophagectomy (OR 3.462, 95% CI 1.201 to 9.978; p = 0.022).
Conclusions
Our results suggest that upregulation of Twist plays a role in the neoplastic transformation to oesophageal SCC and subsequent development of distant metastasis. Twist may serve as a useful prognostic marker for predicting the development of distant metastasis in oesophageal SCC.
Twist, a basic helix—loop–helix transcription factor, has been recently identified as a promoting factor for epithelial–mesenchymal transition (EMT),1 which enhances the metastatic potential of cancer cells.2 Epithelial cells lose their polarity and express genes specific for mesenchymal cells during EMT. Mobility of the cells is thus increased. Snail, which has also been described to promote EMT, works similar to Twist in gastrulation and neural crest development.3 It also acts as an inducer of cell movement and survival to promote cancer progression.4 Similar to Snail, Twist works as an EMT promoter, which downregulates E‐cadherin to increase cell mobility1,5 and also acts as an anti‐apoptotic factor to enhance the survival of a cancer cell.5
Snail has been shown to be overexpressed in 83% of cases with oesophageal squamous cell carcinoma (SCC).6 Upregulation of Slug, a member of the Snail repressor family and a suppressor of E‐cadherin expression,7 has been demonstrated to relate to poor clinical outcomes in oesophageal SCC.8
To our knowledge, there has not been any report on the prognostic significance of Twist in oesophageal SCC, the eighth most common cancers and the sixth most frequent cause of cancer‐related death worldwide.9 We set out to study Twist expression in archival clinical samples of oesophageal SCC by immunohistochemical methods and to correlate its expression level with the clinicopathological data of the patient cohort. Our study showed that Twist is generally upregulated in oesophageal SCC. In addition, a high‐level expression of Twist in the primary tumour correlated significantly with the risk of subsequent development of distant metastasis.
Materials and methods
Patients and specimens
The cohort comprised 87 Chinese patients with oesophageal SCC who had undergone total oesophagectomy in Queen Mary Hospital, Hong Kong, China, from 1998 to 2005. Excluded from the study were patients who had received treatment directed against oesophageal SCC before surgical resection of the primary tumour. All clinical data were prospectively collected. The archival formalin‐fixed and paraffin wax‐embedded tissue blocks of the oesophagectomy specimens were retrieved from the Department of Pathology, Queen Mary Hospital. The age of patients at the time of procurement of the tissue samples ranged from 22 to 87 (median 67) years. Median follow‐up was 13 months (range 1 to 65 months). Cancer stage at the time of oesophagectomy was classified according to the tumour–node–metastasis staging system. The primary tumours were divided into three grades: well, moderately and poorly differentiated according to the World Health Organization classification.10 Table 1 lists the clinicopathological data of the patients. In 30 of 87 oesophagectomy specimens, fresh frozen tissues of the primary tumour and the non‐neoplastic upper resection margin were available for molecular analysis.
Table 1 Clinical and pathological features of patients.
| Cases, n (%) | |
|---|---|
| Total | 87 |
| Sex | |
| Male | 67 (77) |
| Female | 20 (63) |
| T stage | |
| T1 | 2 (2) |
| T2 | 14 (16) |
| T3 | 53 (61) |
| T4 | 18 (21) |
| N stage | |
| N0 | 32 (37) |
| N1 | 55 (63) |
| M stage | |
| M0 | 72 (83) |
| M1 | 15 (17) |
| Histological grade | |
| Well differentiated | 13 (15) |
| Moderately differentiated | 50 (58) |
| Poorly differentiated | 23 (27) |
| Metastatic status, total | 64 |
| Non‐metastatic | 36 (56) |
| Metastatic (within 1 year) | 28 (44) |
Median (range) age of patients is 67 (22–87) years.
A group of 64 patients was selected for statistical analysis on Twist expression and development of metastasis within 1 year of oesophagectomy. In total, 23 patients were excluded from this analysis for two reasons. First, the patient was alive, with no known distant metastasis, but length of clinical follow‐up was <1 year. Second, the patient was dead within 1 year of oesophagectomy, and the metastatic potential of the primary oesophageal SCC could not be interpreted. The selected group of patients (n = 64) was further divided into two groups: the metastatic (metastatic lesion was detected at the time or within 1 year of oesophagectomy) and non‐metastatic groups (no metastatic lesion was detected until the end of this study with a minimum follow‐up of 1 year).
Cell lines
NE3 was maintained in keratinocyte serum‐free medium (Invitrogen). The cell lines EC1, EC18, and KYSE520 were maintained in RPMI 1640 (Invitrogen) with 10% fetal bovine serum (FBS). HKESC2 was maintained in 80% modified Eagle's medium with 20% FBS. KYSE150 and KYSE450 were maintained in 1:1 RPMI 1640/ (Invitrogen, Carlsbad, California, USA) Ham's F12 medium with 2% and 10% FBS, respectively.
Reverse transcription PCR
From the 87 patients included in this study, 30 paired tumour/non‐neoplastic frozen tissue samples were available for total RNA extraction. The presence or absence of tumour cells in these specimens was verified by histological assessment by a pathologist (K‐WC).
Total RNA was isolated from frozen sections using Trizol reagent according to the manufacturer's instructions (Invitrogen). First‐strand complementary DNA was synthesised using 2 μg of total RNA by the SuperScript First Strand Synthesis System (Invitrogen). Twist reverse transcription (RT)‐PCR was performed using TwRTF (5′‐CGG ACA AGC TGA GCA AGA TT‐3′) and TwRTR (5′‐CCT TCT CTG GAA ACA ATG AC‐3′) with cycling as follows: 10 min at 95°C followed by 36 cycles of 30 s at 95°C, 30 s at 55°C and 30 s at 72°C, with the final 7 min extension at 72°C. β‐Actin was used as an internal control. The PCR products were verified by 1% agarose gel electrophoresis. The intensity of the RT‐PCR product was analysed by Gelworks 1D Intermediate software V.3.01 (UVP). The intensity ratio of Twist RT‐PCR product to β‐actin RT‐PCR product was calculated. A ratio of 0–0.2 was regarded as negative (0), 0.2–0.5 as weakly positive (1) and >0.5 as positive (2), for statistical analysis.
Western blotting
Western blot was performed using primary antibodies against Twist (1:250, Santa Cruz Biotechnology, Santa Cruz, California, USA) as described previously.11 Actin (Santa Cruz Biotechnology) was used as a loading control.
Immunohistochemical staining
Immunohistochemical staining using Dako EnVision+ system‐horse radish peroxidase (Dako Corporation, Carpinteria, California, USA) was carried out according to the manufacturer's instructions. Polyclonal anti‐Twist (H‐81) antibody (Santa Cruz Biotechnology) was applied at a 1:500 dilution for overnight incubation at 4°C.
Table 2 Summary of TWIST expression results*.
| (A) | TWIST mRNA expression samples, n (%) | Total, n | |||
|---|---|---|---|---|---|
| Negative | Weakly positive | Positive | |||
| Non‐neoplastic | 24 (80) | 3 (10) | 3 (10) | 30 | |
| Tumour | 10 (33) | 11 (37) | 9 (30) | 30 | |
| (B) | TWIST protein expression samples, n (%) | Total, n | |||
|---|---|---|---|---|---|
| Negative | Weak | Moderate | Strong | ||
| Non‐neoplastic | 19 (58) | 13 (39) | 1 (3) | 0 (0) | 33 |
| Tumour | 17 (19) | 39 (45) | 25 (29) | 6 (7) | 87 |
| (C) | TWIST protein expression sample, n (%) | Total, n | |||
|---|---|---|---|---|---|
| Negative | Weak | Moderate | Strong | ||
| Non‐metastatic | 6 (17) | 21 (58) | 7 (19) | 2 (6) | 36 |
| Metastatic | 6 (21) | 7 (25) | 12 (43) | 3 (11) | 28 |
*(A) TWIST mRNA level, (B) TWIST protein level in non‐neoplastic squamous epithelium and tumour specimens, (C) TWIST protein level in oesophageal squamous cell carcinoma of non‐metastatic group and metastatic group.
Evaluation of immunohistochemical staining results
The stained sections were reviewed by two independent observers (K‐WC and Y‐PC) who had no knowledge of the patient data. Staining intensity was graded as follows: negative (0), weak (1), moderate (2) and strong (3). Staining extent was rated according to the percentage of positive cells. Samples with no stained tumour cells were rated as 0 and those with <25% of stained tumour cells were rated as 1, those with 25–50% of stained tumour cells were rated as 2 and those with >50% of stained tumour cells were rated as 3. The results of staining intensity and extent gave an overall staining score. An overall score of 0 was marked as negative, 1–2 as weak, 3–4 as moderate and 5–6 as strong. Negative or weak overall staining was regarded as low Twist expression, whereas moderate or strong overall staining was regarded as high Twist expression in the binary logistic regression analysis.
Statistical analysis
Statistical analysis was performed using SPSS V.11.5 software. Differences in Twist expression among different clinicopathological stages were analysed by χ2 test or Fisher's exact test where applicable. The association between Twist expression and the risk of developing metastatic disease was estimated by odds ratio (OR) and 95% CI using binary logistic regression analysis. Spearman's rank correlation test was applied to analyse the correlation between Twist mRNA level measured by RT‐PCR and Twist protein score as assessed by immunohistochemistry. A p value <0.05 was considered to be significant.
Results
Upregulation of Twist in oesophageal SCC cell lines
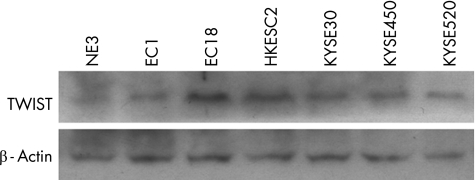
We examined Twist expression by RT‐PCR and western blot analysis in a normal immortalised cell line, NE3, and in six oesophageal SCC cell lines. As shown in fig 1, all six oesophageal SCC cell lines expressed higher levels of Twist than did NE3. This result suggests that Twist is upregulated in oesophageal SCC cells.
Figure 1 TWIST expression in immortalised normal oesophageal cell line and oesophageal squamous cell carcinoma (SCC) cell lines. Upper panel shows western blot analysis of TWIST expression in immortalised oesophageal cell line (NE3) and six oesophageal SCC cell lines. TWIST expression in NE3 is lower than in the six oesophageal SCC cell lines. Lower panel shows western blot analysis of β‐actin as control.
Upregulation of Twist in oesophageal SCC specimens
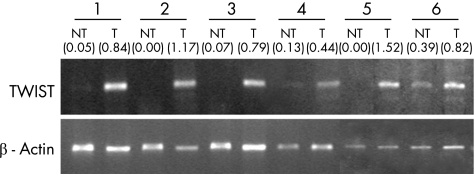
We compared Twist mRNA expression level in 30 frozen tumour specimens with their paired non‐neoplastic oesophageal epithelium at the upper resection margin by semiquantitative RT‐PCR. As shown in fig 2 and table 2A, Twist mRNA was detected in 67% (20 of 30) tumour specimens, whereas only 20% (6 of 25) of their non‐neoplastic oesophageal epithelium expressed Twist mRNA to the level that is detectable by RT‐PCR (p<0.001).
Figure 2 Reverse transcription PCR (RT‐PCR) of TWIST in human oesophageal specimens. Upper panel shows representative TWIST RT‐PCR results in paired non‐neoplastic oesophageal epithelium (NT)/oesophageal squamous cell carcinoma (T) specimens. Non‐neoplastic specimens were the upper resection margin of oesophagectomy specimens. The absence of tumour cells was confirmed by a pathologist by examining H&E‐stained specimens. Lower panel shows β‐actin RT‐PCR results for normalisation. TWIST is upregulated in oesophageal tumour specimens compared with paired non‐neoplastic samples. The intensity ratio of the TWIST RT‐PCR product to β‐actin RT‐PCR product for each specimen is displayed in parenthesis. A ratio of <0.2 was regarded as negative, between 0.2 and 0.5 as weakly positive and >0.5 as positive.
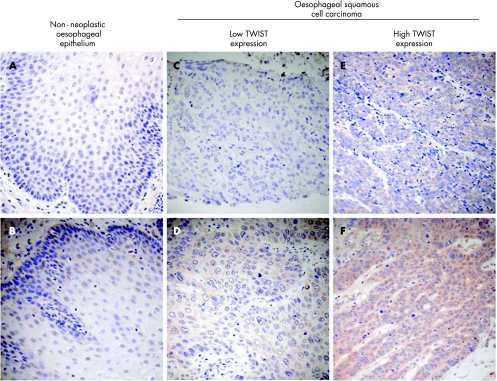
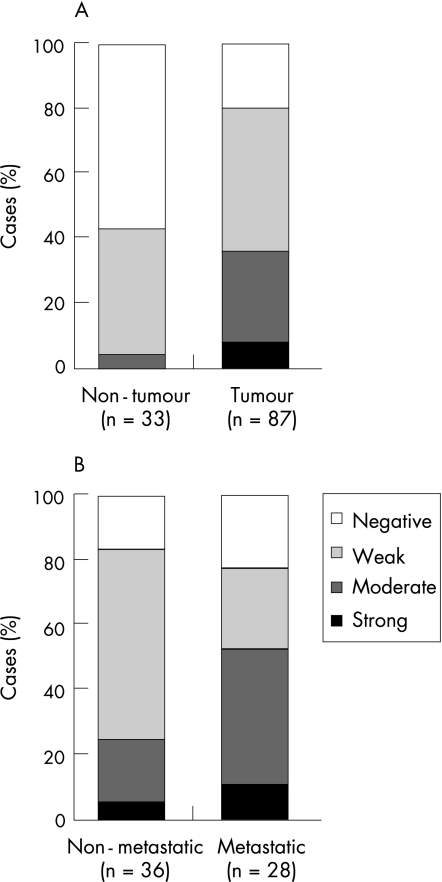
Twist protein level in 87 paraffin wax‐embedded tumours and 33 case‐matched non‐neoplastic oesophageal epithelium embedded in paraffin wax‐embedded (non‐neoplastic oesophageal epithelium at the upper resection margin) were compared by immunohistochemistry. As shown in figs 3 and 4A and in table 2, 36% (31 of 87) oesophageal SCC specimens showed moderate to strong Twist staining whereas only 3% (1 of 33) non‐neoplastic oesophageal epithelium showed similar staining extent. Twist protein level was significantly increased in oesophageal SCC specimens compared with non‐neoplastic oesophageal epithelium (p<0.001). The staining patterns observed were different between oesophageal SCC and non‐neoplastic oesophageal epithelium. In the Twist‐positive oesophageal SCC, Twist staining gave a diffuse cytoplasmic pattern whereas in Twist‐positive non‐neoplastic oesophageal epithelium, Twist staining was mainly localised to the perinuclear area.
Figure 3 Immunohistochemical staining of TWIST in human oesophageal specimens. (A,B) Representative TWIST staining in non‐neoplastic oesophageal epithelium. (A) Non‐neoplastic squamous cells show negative staining of TWIST and (B) TWIST‐positive non‐neoplastic cells show weak perinuclear staining. (C–F) Representative TWIST staining in oesophageal squamous cell carcinoma (SCC) tumour specimens. (C) Negative, (D) weak, (E) moderate and (F) strong TWIST staining were observed in our cohort of patients with oesophageal SCC. As many as 36% oesophageal SCC specimens express either moderate or strong level of TWIST, but only 3% of their non‐neoplastic counterparts express TWIST in the similar level.
Figure 4 Summary of immunohistochemical staining of TWIST in primary oesophageal squamous cell carcinoma (SCC) specimens. (A) TWIST expression level in non‐neoplastic oesophageal epithelium and oesophageal SCC tumour specimens. (B) TWIST expression level in primary oesophageal SCC of non‐metastatic and metastatic groups.
Spearman's rank test was applied to correlate between Twist mRNA level and Twist protein level. The result showed that Twist mRNA expression level is correlated with Twist protein expression level in our patient cohort (r 0.643, p<0.001).
In our patient cohort (n = 87), a high level of Twist expression did not correlate with tumour–node–metastasis staging, histological grading of tumour or patient survival.
High level of Twist expression in primary oesophageal SCC tumour is associated with greater risk of distant metastasis development
It has been suggested that upregulation of Twist in cancer cells promotes EMT and metastasis.2 To investigate whether a high level of Twist expression in primary oesophageal SCC could be a predictor of development of distant metastasis, we studied Twist protein levels in primary oesophageal SCC in our cohort and examined their relationship with the occurrence of distant metastasis within 1 year.
As shown in fig 4B and table 2C, 54% (15 of 28) patients with moderate or strong Twist staining scores developed distant metastasis within 1 year whereas only 25% (9 of 36) patients with negative or weak Twist staining scores developed distant metastasis within 1 year. Oesophageal SCC primary tumours with higher Twist expression showed a trend to develop distant metastasis more frequently, although the difference was not significant (p = 0.054) when tumours were stratified into four groups by their Twist expression level. However, when the oesophageal SCC primary tumours were divided into two groups with low (negative or weak) and high (moderate or strong) Twist expression for binary regression analysis, high level of Twist expression in the primary oesophageal SCC was significantly associated with a higher risk for the patient to develop distant metastasis (OR = 3.462, 95% CI 1.201 to 9.978; p = 0.022).
Discussion
By comparing TWIST expression in human oesophageal SCC specimens with non‐neoplastic oesophageal epithelium, we found that Twist is upregulated in oesophageal SCC (p<0.001). Upregulation of Twist and its prognostic value have been described in several human cancers. In human breast cancer, increased Twist expression has been shown to correlate with metastasis development and shorter survival.12 Increased Twist expression has also been suggested to associate with poor outcome and shorter patient survival in patients with melanoma.13 In addition, Twist expression has recently been found to correlate with lymph node metastasis, distant metastasis and survival in nasopharyngeal carcinoma.14 This background information prompted us to investigate whether Twist expression in oesophageal SCC could be of any prognostic significance. This study showed that a high level of Twist expression in the primary oesophageal SCC was associated with an increased risk for the patient to develop distant metastasis. These results suggest that Twist could be used as a prognostic marker in predicting development of distant metastasis in patients with oesophageal SCC.
We demonstrated that Twist expression in the six human oesophageal SCC cell lines were higher than in the normal immortalised oesophageal cell line (fig 1) and that both Twist mRNA and protein levels in oesophageal SCC specimens were increased compared with non‐neoplastic oesophageal epithelium (figs 2 and 3). Furthermore, upto 81% of oesophageal SCC specimens showed positive Twist staining. These results suggest that upregulation of Twist could be involved in the neoplastic transformation of oesophageal epithelium. Twist is known to be antiapoptotic in other cancers.15 It is likely that upregulation of Twist plays a role in the neoplastic transformation of oesophageal epithelium through its antiapoptotic activity.
Upregulation of Twist in breast cancer cells1 and prostate cancer cells11 increased their invasiveness in vitro. By means of binary logistic regression analysis, we found that a high level of Twist expression in our cohort conferred a significantly higher risk of developing distant metastasis. The results lend support to the hypothesis that upregulation of Twist in oesophageal SCC cancer cells might enhance metastasis through EMT. As other two EMT promoters, Snail6 and Slug,8 have been shown to be upregulated in oesophageal SCC, it is worth investigating how Twist, Snail and Slug interact to promote metastasis in oesophageal SCC.
Take‐home messages
TWIST is significantly upregulated in oesophageal squamous cell carcinoma (SCC)
A high level of TWIST in the primary oesophageal SCC tumours was significantly associated with a higher risk of distant metastasis development in the corresponding patients
These findings suggest that TWIST might be involved in neoplastic transformation of oesophageal SCC through its antiapoptotic activity, and it might participate in the development of distant metastasis through its ability to promote epithelium–mesenchymal transition.
We observed that the pattern of Twist staining in oesophageal SCC specimens was different from that of the non‐neoplastic oesophageal epithelium. Although the staining was cytoplasmic in both, it was diffusely cytoplasmic in the Twist‐positive oesophageal SCC cells, whereas it was typically perinuclear in the non‐neoplastic oesophageal epithelium (fig 3). Subcellular localisation of Twist, in addition to its expression level, has been implicated in cell fate decisions.16 Further studies are required to validate our observations and to understand the mechanisms involved and the significance of this differential subcellular localisation of Twist expression as cells go through the process of neoplastic transformation to oesophageal SCC.
In conclusion, our study demonstrated that upregulation of Twist may play a role in the neoplastic transformation of oesophageal epithelium and that a high level expression of Twist in the primary tumour may further promote the development of metastasis. Our results showed that Twist might be a useful prognostic marker for predicting the development of distant metastasis in oesophageal SCC.
Abbreviations
EMT - epithelial–mesenchymal transition
FBS - fetal bovine serum
RT‐PCR - reverse transcription‐PCR
SCC - squamous cell carcinoma
Footnotes
Competing interests: None declared.
References
- 1.Yang J, Mani S A, Donaher J L.et al Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004117927–939. [DOI] [PubMed] [Google Scholar]
- 2.Huber M A, Kraut N, Beug H. Molecular requirements for epithelial‐mesenchymal transition during tumor progression. Curr Opin Cell Biol 200517548–558. [DOI] [PubMed] [Google Scholar]
- 3.Kang Y, Massague J. Epithelial‐mesenchymal transitions: Twist in development and metastasis. Cell 2004118277–279. [DOI] [PubMed] [Google Scholar]
- 4.Barrallo‐Gimeno A, Nieto M A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 20051323151–3161. [DOI] [PubMed] [Google Scholar]
- 5.Maestro R, Dei Tos A P, Hamamori Y.et al Twist is a potential oncogene that inhibits apoptosis. Genes Dev 1999132207–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeno S, Noguchi T, Fumoto S.et al E‐cadherin expression in patients with esophageal squamous cell carcinoma: promoter hypermethylation, Snail overexpression, and clinicopathologic implications. Am J Clin Pathol 200412278–84. [DOI] [PubMed] [Google Scholar]
- 7.Hajra K M, Chen D Y, Fearon E R. The SLUG zinc‐finger protein represses E‐cadherin in breast cancer. Cancer Res 2002621613–1618. [PubMed] [Google Scholar]
- 8.Uchikado Y, Natsugoe S, Okumura H.et al Slug expression in the E‐cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res 2005111174–1180. [PubMed] [Google Scholar]
- 9.Parkin D M, Bray F, Ferlay J.et al Global cancer statistics, 2002. CA Cancer J Clin 20055574–108. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton S R, Aaltonen L A. eds. World Health Organisation classification of tumours. Pathology and genetics of tumours of the digestive system Lyon: IARC Press, 2000
- 11.Kwok W K, Ling M T, Lee T W.et al Up‐regulation of Twist in prostate cancer and its implication as a therapeutic target. Cancer Res 2005655153–5162. [DOI] [PubMed] [Google Scholar]
- 12.Martin T A, Goyal A, Watkins G.et al Expression of the transcription factors snail, slug, and Twist and their clinical significance in human breast cancer. Ann Surg Oncol 200512488–496. [DOI] [PubMed] [Google Scholar]
- 13.Hoek K, Rimm D L, Williams K R.et al Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res 2004645270–5282. [DOI] [PubMed] [Google Scholar]
- 14.Song L B, Liao W T, Mai H Q.et al The clinical significance of Twist expression in nasopharyngeal carcinoma. Cancer Lett 2006242258–265. [DOI] [PubMed] [Google Scholar]
- 15.Puisieux A, Valsesia‐Wittmann S, Ansieau S. A Twist for survival and cancer progression. Br J Cancer 20069413–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M S, Lowe G, Flanagan S.et al Human Dermo‐1 has attributes similar to Twist in early bone development. Bone 200027591–602. [DOI] [PubMed] [Google Scholar]