Abstract
Background
Spindle cell carcinoma (SpCC) is a rare microscopic type of cancer of the mouth and oropharynx. Although SpCC is thought to arise from squamous cell carcinoma (SCC), it carries a worse prognosis.
Aim
To find out the difference in immunohistochemical expression of cytokeratin, vimentin and smooth‐muscle actin, and mutational alterations in the K‐ras oncogene between the two tumours, in an attempt to characterise SpCC.
Methods
Immunohistochemical analysis was performed by standard avidin–biotin complex method in 35 cases each of SpCCs and SCCs. DNA extracted from paraffin wax‐embedded tumours was used for PCR followed by single‐strand conformation polymorphism for mutational analysis of K‐ras exon 1 and exon 2.
Results
In the SpCC group, cytokeratin positivity was significantly higher in epithelial areas (52.2%) than in spindle cell areas (16.1%), whereas vimentin was more positive in spindle cell areas (18.7%) than epithelial areas (2.7%). Cells intermediate between epithelial and spindle cell areas were consistently positive for both cytokeratin and vimentin. Cytokeratin was found to be significantly more positive in SCC (72.6%) than the squamous component and spindle cell component of SpCC. In this study, no mutation was detected in the K‐ras gene of either the SpCC or SCC group.
Conclusions
The spindle cell component of SpCC is intermixed with cells that are morphologically mesenchymal but express dual antigen‐positivity characteristic of epithelial (cytokeratin) and mesenchymal (vimentin) cells. These, possibly, are cells in transition suggesting that SpCC may be a sarcomatous metaplasia of SCC.
Spindle cell carcinoma (SpCC) of the head and neck is a biphasic neoplasm described by Virchow in 18651. It has both epithelial and sarcomatous components. Although the aetiology and clinical profile of this tumour are similar to squamous cell carcinoma (SCC), they differ in their biological behaviour and response to treatment. SpCC has been described as a more aggressive tumour with little response to radiotherapy2 in contrast with SCC. Thus, there is a need to know the origin of SpCC and to characterise it as an entity for better management and therapeutic intervention.
The immunohistochemical expression of this tumour has been studied recently.3,4 The epithelial component of SpCC expresses cytokeratin, whereas spindle cell areas express vimentin with variable positivity for cytokeratin. However, the origin has not been elucidated. A few molecular studies on p53, a tumour‐suppressor gene, have yielded conflicting results, thus compounding the controversy of origin of SpCC.5,6 On extensive Medline search, no study of mutations in K‐ras oncogene in SpCC of the head and neck were found. However, K‐ras has been studied in carcinosarcoma of the uterus to elicit the clonal origin of squamous and spindle cells.7,8 Also, K‐ras has been reported to harbour mutations in SCC of the head and neck in patients of Indian origin.9 Although SpCC is considered to be a variant of SCC, no study comparing the two tumours could be found. Thus, this study was undertaken to compare SpCC and SCC to elicit the differences and the origin of SpCC.
Materials and methods
This is a prospective study of 35 cases each of SpCC and SCC of the head and neck region (excluding salivary glands). Cases with spindle cell areas of >30% along with cytokeratin positivity were considered to be SpCC. However, tumours showing such change in <30% area were excluded from the analysis. The clinical profile of all the patients was noted. Immunohistochemical analysis and DNA extraction followed by amplification and mutational analysis were performed on duplicate sections in all these cases.
Immunohistochemistry
For immunohistochemical study, 3 μm thick sections were cut on poly‐L‐lysine‐coated slides from paraffin wax blocks. Slides were dewaxed with xylene and rehydrated through graded alcohol. This was followed by blockage of endogeneous peroxidase activity using 1% hydrogen peroxide solution in methanol for 30 min, and the slides were washed in 10 mM phosphate‐buffered saline (pH 7.4). For antigen retrieval, they were immersed in 1 mM citrate/phosphate buffer and treated in a microwave oven at 100°C for 20 min. The slides were reacted with various prediluted primary antibodies overnight at 4°C in a humid chamber. The primary antibodies used in this study were: monoclonal antibody pancytokeratin (AE1/AE3, Dako, Glostrup, Denmark), vimentin (Dako) and smooth‐muscle actin (Dako). The reaction was amplified using biotinylated secondary antibody followed by avidin–biotin–peroxidase complex, both for 20 min each. The reaction was visualised using diaminobenzidine as the chromogen.10 The slides were then rinsed in tap water, counterstained in Mayer's haematoxylin and mounted in synthetic mountant. A positive reaction was seen as brown granular cytoplasmic staining. Percentage positivity was calculated in each case as the number of positive cells for every 500 cells counted.
DNA extraction and PCR
DNA was extracted from paraffin wax‐embedded tissue sections of all the cases. Sections were deparaffinised, rehydrated and scraped into digestion buffer, pH 7.0 (containing 1.5 mM MgCl2, 50 mM KCl, 100 mM Tris HCl and 2.5 μl Tween 20) with 10 μl Proteinase K (20 mg/ml; Roche, Indianapolis, Indiana, USA) and incubated for 3 h at 55°C with continuous mixing. DNA was isolated using the standard phenol/chloroform extraction method.11
PCR
PCR was performed on all specimens using K‐ras exon 1 primers 5′‐ ATG ACT GAA TAT AAA CTT GT ‐ 3′ and 5′ ‐ CTC TAT TGT TGG ATC ATA TT ‐ 3′ to amplify a 115bp product and K‐ras exon 2 primers 5′ ‐ GCA AGT AGT AAT TGA TGG AG ‐ 3′ and 5′ ‐ AGA AAG CCC TCC CCA GTC CT ‐ 3′ (synthesised by Genei, Bangalore, India) to amplify a 118 bp product of the gene. Amplification of the gene was performed in 25 μl reaction mixture containing 50–100 ng of genomic DNA, 10 mM Tris‐HCl (pH 8.0), 1.5 mM MgCl2, 50 mM KCl, 200 μM each deoxyribonucleoside triphosphate (dATP, deoxyguanosine triphosphate, deoxycytidine triphosphate (dCTP), deoxythymidine triphosphate), 10 pmol of each primer and 0.5 U Taq DNA polymerase. DNA was initially denatured at 94°C for 5 min, followed by 34 cycles of denaturation at 94°C for 30 s, primer annealing at 46°C for exon 1 (55°C for exon 2) for 30 s and extension at 72°C for 30 s. In the last cycle, extension at 72°C was allowed for 4 min. Amplified DNA was electrophoresed on 3% ethidium bromide‐stained agarose gel along with HaeIII‐digested φ X 174 DNA molecular mass marker and photographed under a UV transilluminator.
Single‐strand conformation polymorphism
Single‐strand conformation polymorphism was performed by radiolabelling the PCR products after regular amplification of 30 cycles for an additional 10 cycles where dCTP was replaced with [α‐32P]dCTP (specific activity, 4000 μCi/mmol; BARC, Mumbai, India) in the PCR mix. Thus for a typical PCR mix of 100 μl meant for 10 samples (10 μl each); 1 μl (10 μCi) of [α ‐P32]dCTP was added, with other reaction components being the same. For loading each sample, 1 μl radiolabelled PCR products was diluted with 9 volumes (10 times) of denaturing solution (95% formamide, 20 mM EDTA pH 8.0, 0.05% xylene cyanol and 0.05% bromophenol blue) and heat denatured for 5 min at 95°C, and chilled on ice for 5 min; 3 μl of this diluted product was then subjected to electrophoresis in a 6% polyacrylamide gel with 10% glycerol. The gel was run in 0.5×TBE (Tris/borate/EDTA buffer) for 12 h at 200 V in Base Ace sequencing gel apparatus (Stratagene GmbH, Heidelberg, Germany) at 17±1°C, dried and exposed to x ray film with intensifying screen at −70°C. Alterations in electrophoretic mobility shifts in single‐strand DNA bands were analysed by comparison with that of normal controls.12
Statistical methods
Quantitative data were analysed using Student's t test, whereas categorical data were analysed by χ2 test (if the data were not normally distributed, square root transformation was performed before the χ2 test). A p value of <0.05 was considered significant. The exact p value has been mentioned as far as possible.
Results and observations
The age range of the patients with SpCC was 25–78 years mean (SD) (58.0 (12.1) years), whereas the age range of the patients with SCC was 28–80 years mean (SD) of (52.9 (12.9) years). There was no significant difference in the mean age of patients with SpCC and SCC (t = 1.71, p = 0.09). Male‐to‐female ratio was 8:1 in the SpCC group as compared with 5:1 in the SCC group. This difference was also not significant (χ2 = 0.47, p = 0.49).
The larynx (43%) was the most common site of involvement in the SpCC group followed by the oral cavity (37%). In 20% of cases, the oropharynx was involved. In the SCC group, the oral cavity was the most common site of involvement (57%) followed by the larynx (26%). The oropharynx was involved in 11% of cases. One patient each (3%) had SCC in the nasopharynx and scalp. The difference in the site of involvement in the SpCC and SCC group was not significant (χ2 = 3.78, p = 0.15). Cervical lymph node metastasis was found in 31% of cases of SpCC as compared with 25% cases of SCC. This difference was also statistically insignificant (χ2 = 0.28, p = 0.59)
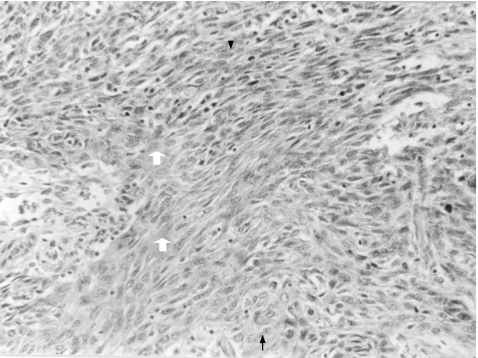
Morphologically, all our cases of SpCC showed a biphasic appearance, comprising epithelial and spindle cell areas. The epithelial component was identified as frankly invasive SCC merging with spindle cell areas. The spindle cell areas ranged from 30% to 85% in our cases. The spindle cell component was composed of short fascicles of spindle cells with moderate pleomorphism and frequent mitotic figures. The transition between spindle cell areas and epithelial areas was gradual and was characterised by a variable amount of intermediate or plump‐looking cells in all the cases of SpCC (fig 1).
Figure 1 Spindle cell carcinoma demonstrating spindle cells area (white arrow) intermixed with squamous cell component (black arrow). Few oval to plump spindle cells (arrow head) are also seen (H&E, ×250).
SCC was well differentiated in 23 (65.7%) cases, moderately differentiated in 10 (28.6%) cases and poorly differentiated in 2 (5.7%) cases.
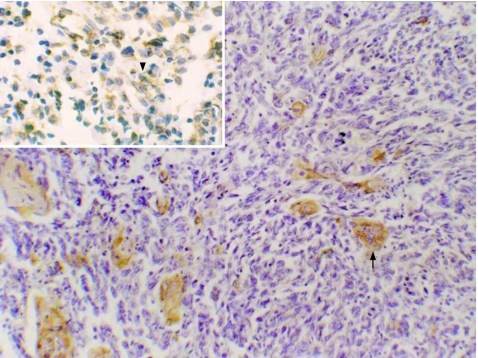
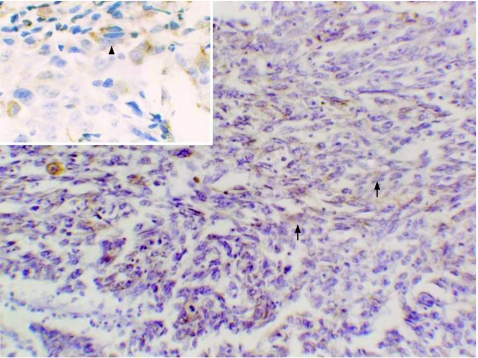
Immunohistochemical analysis showed that in the SpCC group, mean (SD) cytokeratin positivity in epithelial areas was 52.2% (23%) as compared with 16.1% (10.9%) in spindle cell areas. In spindle cell areas, the cytokeratin positivity was significantly higher in the plump‐looking cells than totally in spindled cells (fig 2). Mean (SD) vimentin positivity was 2.7% (4%) in epithelial areas as compared with 18.7% (11.5%) in spindle cell areas. The plump‐looking cells were consistently positive for vimentin in all cases of SpCC (fig 3). Mean (SD) smooth muscle actin positivity was 0.14% (0.9%) in epithelial areas, whereas it was 1.4% (2.5%) in spindle cell areas. All these differences in the immunohistochemical analysis of epithelial and spindle cell areas were significant (p<0.001 and p<0.05 respectively), as shown in table 1.
Figure 2 Spindle cell carcinoma showing cytokeratin‐positive squamous area (arrow) admixed with spindle area, which is cytokeratin negative. Inset shows cytokeratin positivity (arrow head) in plump spindle cells. (Immunostaining, ×400).
Figure 3 Spindle cell carcinoma demonstrating vimentin positivity in spindle cells (arrow). Inset shows vimentin positivity (arrow head) in plump spindle cells. (Immunostaining, ×400).
Table 1 Immunohistochemical profile (percentage positivity) of cases of spindle cell carcinoma.
| Marker | Epithelial areas (%) | Spindle areas (%) | p Value (Student's t test) |
|---|---|---|---|
| Cytokeratin | 52.2 (23) | 16.1 (10.9) | <0.001 |
| Vimentin | 2.7 (4) | 18.7 (11.5) | <0.001 |
| Smooth‐muscle actin | 0.14 (0.9) | 1.4 (2.5) | <0.001 |
Values are mean (SD)
In SCC cases, mean (SD) cytokeratin positivity was 72.6% (20%). None of the cases of SCC was positive for vimentin and smooth‐muscle actin.
Table 2 shows that the mean cytokeratin positivity was significantly higher in the SCC group as compared with epithelial areas of the SpCC group (p<0.001) and spindle cell areas of the SpCC group (p<0.001).
Table 2 Comparison of immunohistochemical profile of cases of spindle cell carcinoma and squamous cell carcinoma.
| Marker | SCC cases (%) | SpCC cases | Mean (SD) |
|---|---|---|---|
| Cytokeratin | 72.6 (20) | Epithelial areas | 52.2 (23)* |
| Spindle areas | 16.1 (10.9)* | ||
| Vimentin | 0 | Epithelial areas | 2.7 (4) |
| Spindle areas | 18.7 (11.5) | ||
| Smooth‐muscle actin | 0 | Epithelial areas | 0.14 (0.9) |
| Spindle areas | 1.4 (2.5) |
SCC, squamous cell carcinoma; SpCC, spindle cell carcinoma.
Values are mean (SD)
*p<0.001, Student's t test.
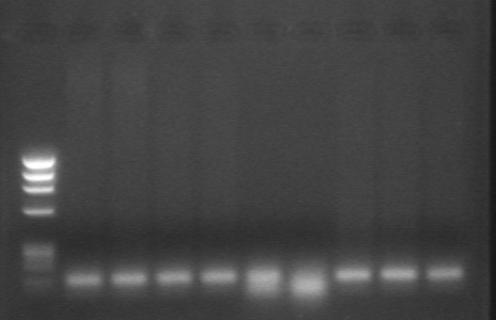
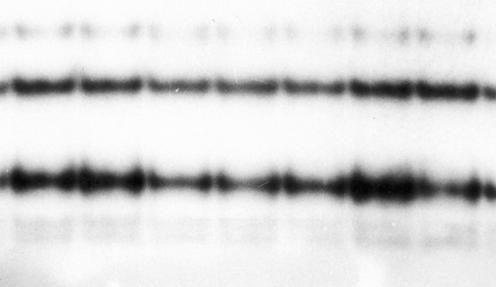
DNA was extracted successfully from formalin‐fixed paraffin wax‐embedded tissues of all cases of SpCC and SCC as visualised on ethidium bromide‐stained agarose gel electrophoresis. PCR was successful in only 26 (74%) cases of SpCC and 25 (71%) cases of SCC (fig 4). On single‐strand conformation polymorphism analysis, there was no band shift, suggesting the absence of mutations in exon 1 and exon 2 of the K‐ras oncogene in any of the cases (fig 5).
Figure 4 Agarose gel electrophoresis of PCR product of K‐ras exon 1 in cases of spindle cell carcinoma run along with a marker DNA.
Figure 5 Single‐strand conformation polymorphism analysis for K‐ras exon 1 in cases of spindle cell carcinoma along with a normal control. No aberrant band could be seen, indicating that no mutation is present in any sample.
Discussion
SpCC has been described as a biphasic tumour with a predilection for the upper aerodigestive tract.4 The origin of spindle cells in this tumour has been debated since the time this entity was recognised.1 The question of origin of this tumour is not just of academic interest; it carries significance for the management of the patient. Appreciable difference has been found in the prognosis and response to treatment of this tumour as compared with that of conventional SCC.2 Thus, the question arises whether SpCC is totally different from SCC and should it be categorised separately?
We have carried out immunohistochemical analysis of three common tumour‐cell markers: cytokeratin, vimentin and smooth‐muscle actin. Cytokeratin positivity was significantly higher in SCC than in spindle cell and squamous cell components of SpCC. Also, in comparison with the spindle cell component, the squamous cell areas of SpCC had a higher mean positivity for cytokeratin. The spindle cell areas showed intermixed plump to oval cells with elongated nuclei exhibiting both cytokeratin and vimentin positivity. These cells with dual expression, which are otherwise morphologically mesenchymal cells, may represent cells in transition from squamous cells to spindle cells. The coexpression of cytokeratin and vimentin has been postulated to denote reversion to embryonic status.13 However, whether these are the reserve cells (or stem cells) is difficult to confirm at this stage. However, these are probably precursor cells that differentiated along a new pathway brought about by changes in signals generated by a mixture of cytokines and growth factors in the cell's environment. Tissue‐specific and differentiation genes are thought to be involved in this process, which are increasingly being identified in numerous other tumours.14 These cells also counter the contention that biphasic tumours are “collision tumours”—that is, parallel carcinoma and sarcoma developing in the same tumour, but separately.
As the immunohistochemical analysis has not been able to resolve the controversy surrounding the origin of SpCC, studies involving mutations in tumour‐suppressor genes and oncogenes have been undertaken in this regard. A study of oesophageal carcinosarcoma showed different genetic alterations in the p53 gene in the two cell types of this neoplasm, thus suggesting biclonal origin.5 However, this is in contrast with another study of sarcomatoid carcinoma of the upper respiratory tract, which demonstrated identical mutation in the p53 gene in spindle cell and epithelial components.6 Most of these molecular studies are case reports; there are hardly any reports of a long series. There is no published literature on mutations in the K‐ras oncogene in SpCC of the head and neck, although such studies have been conducted in uterine carcinosarcomas.7,8 In the present study, no mutation was detected in exon 1 and exon 2 of the K‐ras oncogene in SpCC and SCC. Although there are reports of K‐ras mutations in oral SCC (classical type) in patients of Indian origin,9,15 a study by Munirajan et al16 reported no mutation. The above two studies used different exons.15,16 Owing to their conflicting results, we decided to study the hot spot in K‐ras, which has been reported in relation to various other tumours.7,8 In our study, we did not find any mutation in these hot spots. Thus, the K‐ras gene may not be involved in the pathogenesis of SpCC or SCC.
Take‐home messages
Spindle cell carcinoma shows plump spindle cells in transition from squamous cell carcinoma, and thus may be considered to arise as a sarcomatous transformation of squamous cell carcinoma. This transformation may explain the increased aggressiveness seen in spindle cell carcinoma.
To conclude, SpCC appears to be epithelial in origin and may arise from conventional SCC by sarcomatous transformation (metaplasia). The sarcomatous transformation is probably brought about by multiple growth factors in the cell's environment induced by tissue‐specific and differentiation genes, increasingly being identified in other tumours. This may also be the reason for enhanced aggressiveness of SpCC.
Abbreviations
dCTP - deoxycytidine triphosphate
SCC - squamous cell carcinoma
SpCC - spindle cell carcinoma
Footnotes
Competing interests: None declared.
References
- 1.Virchow R.Die krankhaften geschwulste. Vol 2. Berlin, Germany: Hirschwald, 1864–, 1865181–182.
- 2.Leonardi E, Dalri P, Pusiol T.et al Spindle cell squamous carcinoma of head and neck region: a clinicopathologic and immunohistochemical study of eight cases. ORL 198648275–281. [DOI] [PubMed] [Google Scholar]
- 3.Lewis J E, Olsen K D. Spindle cell carcinoma of the larynx: review of 26 cases. Hum Pathol 199728664–673. [DOI] [PubMed] [Google Scholar]
- 4.Takata T, Ito H, Ogawa I.et al Spindle cell squamous carcinoma of the oral region: an immunohistochemical and ultrastructural study of six cases. Virchows Arch A Pathol Anat Histopathol 1991419177–182. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa S, Nishimaki T, Suzuki T.et al Histogenetic heterogeneity in carcinosarcoma of the esophagus: report of a case with immunohistochemical and molecular analyses. Dig Dis Sci 199944905–909. [DOI] [PubMed] [Google Scholar]
- 6.Ansari‐Lari M A, Hoque M O, Califano J.et al Immunohistochemical p53 expression patterns in sarcomatoid carcinomas of the upper respiratory tract. Am J Surg Pathol 2002261024–1031. [DOI] [PubMed] [Google Scholar]
- 7.Wada H, Enomoto T, Fujita M.et al Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumours. Cancer Res 1997575379–5385. [PubMed] [Google Scholar]
- 8.Watanabe M, Shimizu K, Kato H.et al Carcinosarcoma of the uterus: immunohistochemical and genetic analysis of clonality of one case. Gynecol Oncol 200182563–567. [DOI] [PubMed] [Google Scholar]
- 9.Saranath D, Chang S E, Bhoite L T. High frequency mutation in codon 12 and codon 61 of ras oncogene in chewing tobacco‐related human oral carcinoma in India. Br J Cancer 199163573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu S M, Raine L, Fanger H. Use of avidin‐biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 198129577–580. [DOI] [PubMed] [Google Scholar]
- 11.Sepp R, Szabo I, Uda H.et al Rapid techniques for DNA extraction from routinely processed archival tissue for use in PCR. J Clin Pathol 199447318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maniaitis T, Fritsch E F, Sambrook J.Molecular cloning: a laboratory manual. New York, USA: Cold Spring Harbor Laboratory, 1982280
- 13.Lane E B, Hogan B L M, Kurkinen M.et al Co‐expression of vimentin and cytokeratin in parietal endoderm cells of early embryo. Nature 1983303701. [DOI] [PubMed] [Google Scholar]
- 14.Reddi H A. BMPs: actions in flesh and bone. Nat Med 19973837. [DOI] [PubMed] [Google Scholar]
- 15.Saranath D, Panchal R, Nair R.et al Oncogene amplification in squamous cell carcinoma of the oral cavity. Jpn J Cancer Res 198980430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munirajan A K, Mohanprasad B K, Shanmugam G.et al Detection of rare point mutation at codon 59 and relatively high incidence of H‐ras mutation in Indian oral cancer. Int J Oncol 199813971–974. [DOI] [PubMed] [Google Scholar]