Abstract
Myeloid sarcomas are tumour masses of myeloid leukaemic cells at extramedullary sites. These tumours can, on occasion, occur without concurrent or antecedent leukaemia. Myeloid sarcomas have been described at unusual locations including the female genital tract. An unusual case of therapy‐related acute myeloid leukaemia (t‐AML) presenting as isolated monoblastic myeloid sarcoma of the uterus in a patient who had received adjuvant chemotherapy for breast cancer is presented. Fluorescence in situ hybridisation analysis performed on paraffin‐wax‐embedded tumour tissue revealed a mixed‐lineage leukaemia (MLL) gene rearrangement, supporting the association of this malignancy with prior chemotherapy. This case illustrates that t‐AML can rarely present as isolated extramedullary tumours, and the detection of specific chromosomal abnormalities in these myeloid sarcomas can be useful for risk assessment and guiding definitive therapy.
Myeloid sarcomas are tumour masses of immature leukaemic myeloid cells occurring at extramedullary sites. Previously known by various terms such as chloroma, extramedullary myeloid tumour and extramedullary acute myeloid leukaemia, the term “myeloid sarcoma” was adopted by the 2001 World Health Organization Classification of Haematopoietic and Lymphoid Malignancies.1 Myeloid sarcomas may be the only manifestation of myeloid malignancy or may occur concurrently with leukaemia in the bone marrow. Myeloid sarcomas may also be the sole manifestation of relapse of previously treated myeloid leukaemia. Commonly involved sites of myeloid sarcoma include subperiosteal bone, lymph nodes and skin.1
Myeloid sarcomas comprise two major subtypes. Granulocytic sarcomas are the more common subtype and are composed of granulocytic precursors at various stages of differentiation. Monoblastic sarcomas are rare and consist of monoblasts and immature monocytes with an immunophenotype similar to the immature cells of acute monoblastic leukaemia.1
The mixed‐lineage leukaemia (MLL) gene, which maps to chromosome band 11q23 is a developmental regulator that is structurally altered in some leukaemias, including infantile acute leukaemia and therapy‐related leukaemia, following treatment with topoisomerase II inhibitors.2,3 Translocations of the MLL gene could result in its fusion with a variety of partner genes resulting in aberrant gene expression in haematopoietic stem cells and development of leukaemia. Other MLL abnormalities such as partial tandem duplication and amplification have also been described in acute myeloid leukaemia (AML) with normal cytogenetics.2 AML with MLL abnormalities usually have a myelomonocytic or monoblastic (French–America–British (FAB) M4 or M5) morphology.1 Leukaemias with MLL abnormalities have been reported after chemotherapy for breast cancer with regimens that include topoisomerase II inhibitors like doxorubicin and epirubicin.4
Myeloid sarcomas often cause diagnostic problems when they are the only manifestation of myeloid malignancy. We report an unusual case of therapy‐related AML (t‐AML) that presented as monoblastic sarcoma of the uterus without bone marrow disease. MLL gene rearrangement was shown in tumour tissue by fluorescence in situ hybridisation (FISH), thus confirming the association of this neoplasm with prior adjuvant chemotherapy for breast cancer.
Case report
A 49‐year‐old woman was diagnosed with adenocarcinoma of the breast (stage III, T3, N1, M0) in November 2001. She underwent a modified radical mastectomy followed by radiation therapy and adjuvant chemotherapy with cyclophosphamide, doxorubicin and 5‐fluorouracil (CAF), which was completed in August 2002. In January 2005, she presented with severe right flank pain. Imaging studies showed a right hydroureter and a bulky uterine mass measuring 12×11×16 cm. A positron emission tomography scan showed intense fluro‐deoxy‐gluccse uptake limited to the uterus. The patient underwent placement of a right ureteric stent and multiple core biopsies of the uterine mass.
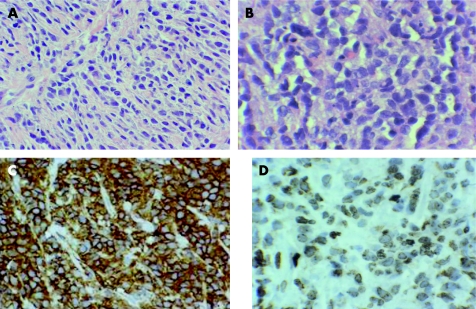
Biopsies showed a malignant haematopoietic neoplasm extensively infiltrating the uterine body and cervix. The infiltrate was diffuse and comprised of medium‐sized cells with hyperchromatic nuclei and fine chromatin. (fig 1A,B) An immunohistochemical study of paraffin‐wax‐embedded tissue showed that the neoplastic cells expressed CD15, CD43 (fig 1C), CD45, CD68 and lysozyme (fig 1D). The Ki‐67 proliferation rate was approximately 70%. The malignant cells did not stain for CD3, CD5, CD10, CD20, CD34, CD79a, CD117, pancytokeratin, terminal deoxynucleotidyl transferase and myeloperoxidase. A diagnosis of monoblastic myeloid sarcoma was made. Bone marrow examination showed normal haematopoiesis and no morphologic or flow cytometric evidence of malignancy. Cytogenetics of the bone marrow revealed a normal female karyotype.
Figure 1 (A) Medium‐sized cells infiltrate the uterine stroma in a diffuse and focally single‐cell pattern, (H & E low‐power view). (B) These cells have irregular nuclear outlines, delicate chromatin and inconspicuous nucleoli (H & E high‐power view). (C) The malignant cells show intense CD43 staining and (D) lysozyme expression in the cytoplasm by immunohistochemical examination.
The patient received induction chemotherapy with idarubicin (3 days) and then infusional cytarabine (7 days) with a subsequent ultrasound examination showing a normal‐sized uterus and decrease in right hydronephrosis. Currently, the patient is undergoing consolidation chemotherapy with high‐dose cytarabine while a matched unrelated donor is being identified for an allogeneic haematopoietic stem‐cell transplant.
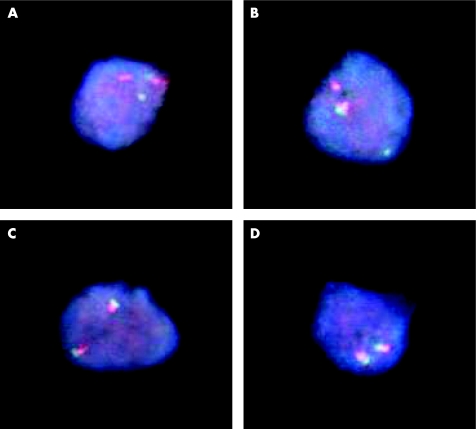
We further studied this patient's uterine biopsies with dual‐colour FISH studies for a MLL gene rearrangement. This analysis was performed on paraffin‐wax‐embedded uterine tissue. Of the 200 interphase nuclei screened, 171 (85.5%) contained aberrant FISH signals consistent with MLL gene rearrangement (fig 2).
Figure 2 (A,B) Representative nuclei extracted from paraffin‐wax‐embedded tissue showing abnormal and normal signal patterns for MLL. The LSI MLL break‐apart probe is labelled with spectrum green at the 5′ centromeric MLL gene break‐apart region and with spectrum orange at the 3′ telomeric region. (A,B) Positive 11q23 rearrangement pattern of one green/one orange (yellow) fusion signal, one orange signal and one green signal (1O1G1F). (C,D) Negative pattern of two orange/green (yellow) fusion signals.
Methods
Paraffin‐wax embedded FISH
Nuclei were isolated from paraffin‐wax‐embedded tissue for FISH analysis as described previously.5 Briefly, tissue cores were collected from the involved areas designated by a pathologist (KLC). Three cores were collected and placed into a 0.5 ml microcentrifuge tube using a 1.5 inch blunt‐tip needle. Xylene was added for 10 min at room temperature (RT) and removed carefully, and the procedure was repeated twice. The cores were passed through an alcohol rehydration series (95%, 75%, 50% ethanol in water, 2 min each, at RT) and digested with 100 μl of fresh 0.01% proteinase K solution, followed by incubation of 30 min in a 37°C bath and vortexed every 5 min for 3 s and centrifuged. The proteinase K solution was removed; the cells were washed with 100 μl of 1× PBS and 100 μl of cold Carnoy's fixative (75% methanol and 25% acetic acid). The fixed nuclei suspensions were dropped onto a 13 mm circle of silanised slide, dried for 15 min on a 65°C hotplate. The slides were pretreated with 10 mm citric acid at 90–95°C for 10 min, transferred to the 2×SSC solution for 15 min, followed by a pepsin/NaCl solution at 37°C for 15 min. Control and test slides were dehydrated in an alcohol series (70%, 80%, 95% ethanol in water, 2 min each at, −20°C), and dried in 37°C incubator. The slides were codenatured. Five μl of the MLL probe, Vysis (Vysis , Downer's Grove, Illinois, USA) mixture was applied to the slides, then covered with an 18 mm circular coverslip and sealed with rubber cement. The slides were placed on an 80°C hotplate for 8 min and then placed overnight in a humidified incubator at 37°C. Slides were washed in a 2×SSC/0.1% Igepal solution at 73°C for 2 min, rinsed in the same solution at RT for 2 min, air‐dried in the dark, counterstained with BioView 4',6‐diamidino‐2‐phenylindole (DAPI, BioView, Rehovot, Israel) and analysed manually using a Zeiss photomicroscope (Carl Zelss Inc, Axioplcn 2, Oberkoche, Germany). A total of 200 nuclei were scored from each test and control slide.
Discussion
The most unusual features of this case are the uterine location of the patient's tumour and the presentation of t‐AML as an isolated myeloid sarcoma. Myeloid sarcomas of the female genital tract, though uncommon, have been described previously.6,7,8 In a series of 11 well‐studied cases, the site of involvement was the ovary in seven cases, the vagina in three and the uterine cervix in one. In nine of these 11 cases, the female genital tract was the only site of disease at presentation.6 When the uterus is involved, the disease is usually limited to the cervix. Involvement of uterine body, as in our patient, is distinctly uncommon. As was the case in our patient, these tumours can be locally invasive and can cause ureteric obstruction.9 Myeloid sarcomas must thus be included in the differential diagnosis of unusual neoplasms that may involve the female genital tract and could be the presenting manifestation of AML.
The pathologic diagnosis of myeloid sarcomas in patients who do not have concurrent or antecedent leukaemia is often problematic. Immunophenotyping is crucial for making the correct diagnosis. The possibility of a haematopoietic neoplasm should be entertained in tumours of unclear origin and an appropriate set of immunohistochemical studies should be performed. CD43 is expressed in most myeloid sarcomas and is a useful marker although it can sometimes be expressed in lymphoid neoplasms. The immunophenotype of myeloid sarcomas generally reflects that of leukaemias of the corresponding lineage. Thus, granulocytic sarcomas express markers like CD13, CD33, CD117 and myeloperoxidase characteristic of AML with or without maturation. Monoblastic sarcomas, on the other hand, are myeloperoxidase negative but express CD68, CD163 and lysozyme. The latter three immunostains, when used together, appear to be most useful in confirming the diagnosis of monoblastic sarcoma.10,11,12
An association of granulocytic sarcomas with t(8;21)(q22;q22) and monoblastic sarcomas with 11q23/MLL rearrangements has been observed.1 Detecting an 11q23/MLL translocation in the tumour tissue was important in supporting the therapy‐related origin of the myeloid sarcoma in our patient. This case demonstrates the utility of using FISH on paraffin‐wax‐embedded tissue to detect clinically and pathologically suspected cytogenetic abnormalities, which is important for risk stratification and guiding definitive therapy.
In general, extramedullary disease is an adverse prognostic factor in AML. The major determinant of prognosis is the cytogenetic and other molecular abnormalities present in the leukaemic cells. Cases presenting as isolated myeloid sarcoma appear to invariably progress to AML.13 Thus it seems reasonable to recommend similar therapy as for AML with the same cytogenetic abnormality, which in the case of our patient would be an allogeneic haematopoietic stem‐cell transplant. The role of local therapy such as radiation in the definitive treatment of myeloid sarcoma is unknown.
In conclusion, t‐AML may rarely present with isolated extramedullary disease and it is important to define specific cytogenetic abnormalities in these cases using specialised techniques in order to guide optimal risk‐adapted therapy.
Take‐home messages
Therapy‐related acute myeloid leukaemia can present as isolated myeloid sarcoma
Paraffin‐wax‐embedded fluorescence in situ hybridisation is a valuable technique to risk‐stratify isolated myeloid sarcomas
Acknowledgements
This study was supported in part by Cancer Center Support Grant No 5P 30 CA33572 and Program Project Grant No 2 P01 CA030206‐24A1 from the National Institutes of Health, Bethesda, Maryland, USA.
Abbreviations
AML - acute myeloid leukaemia
FISH - fluorescence in situ hybridisation
MLL - mixed lineage leukaemia
RT - room temperature
t‐AML - therapy‐related acute myeloid leukaemia
Footnotes
Competing interests: None.
References
- 1.Myeloid sarcoma In: Jaffe ES, Harris NL, Stein H, Vardiman JW, ed. Tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2001104–105.
- 2.Li Z‐Y, Liu D‐P, Liang C‐C. New insight into the molecular mechanism of MLL‐associated leukemia. Leukemia 200519183–190. [DOI] [PubMed] [Google Scholar]
- 3.Super H J, McCabe N R, Thirman M J.et al Rearrangements of the MLL gene in therapy‐related acute myeloid leukemia in patients previously treated with agents targeting DNA‐topoisomerase II. Blood 1993823705–3711. [PubMed] [Google Scholar]
- 4.Leone G, Voso M T, Sica S.et al Therapy related leukemias: susceptibility, prevention and treatment. Leuk Lymphoma 200141255–276. [DOI] [PubMed] [Google Scholar]
- 5.Paternoster S F, Brockman S R, McClure R F.et al A new method to extract nuclei from paraffin‐embedded tissue to study lymphomas using interphase fluorescence in situ hybridization. Am J Pathol 20021601967–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliva E, Ferry J A, Young R H.et al Granulocytic sarcoma of the female genital tract: a clinicopathologic study of 11 cases. Am J Surg Pathol 1997211156–1165. [DOI] [PubMed] [Google Scholar]
- 7.Friedman H D, Adelson M D, Elder R.et al Granulocytic sarcoma of the uterine cervix‐Literature review of granulocytic sarcoma of the female genital tract. Gynecol Oncol 199246128–137. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez J A, Navarro J T, Rozman M.et al Primary myeloid sarcoma of the gynecologic tract: a report of two cases progressing to acute myeloid leukemia. Leuk Lymphoma 2002432151–2153. [DOI] [PubMed] [Google Scholar]
- 9.Steinbock G S, Morriseau P M, Vinson R K. Acute obstructive renal failure secondary to granulocytic sarcoma. Urology 19863268–270. [DOI] [PubMed] [Google Scholar]
- 10.Quintanilla‐Martinez L, Zukerberg L R, Ferry J A.et al Extramedullary tumors of lymphoid or myeloid blasts. The role of immunohistochemistry in diagnosis and classification. Am J Clin Pathol 1995104431–443. [DOI] [PubMed] [Google Scholar]
- 11.Audouin J, Comperat E, Le Tourneau A.et al Myeloid sarcoma: clinical and morphologic criteria useful for diagnosis. In J Surg Pathol 200311271–282. [DOI] [PubMed] [Google Scholar]
- 12.Chang C ‐ C, Eshoa C, Kampalath B.et al Immunophenotypic profile of myeloid cells in granulocytic sarcoma by immunohistochemistry. Am J Clin Pathol 2000114807–811. [DOI] [PubMed] [Google Scholar]
- 13.Breccia M, Mandelli F, Petti M C.et al Clinico‐pathological characteristics of myeloid sarcoma at diagnosis and during follow‐up: report of 12 cases from a single institution. Leuk Res 2004281165–1169. [DOI] [PubMed] [Google Scholar]