Abstract
Background
An increased level of serum C‐reactive protein (CRP) is a known prognostic factor for acute coronary events and sudden cardiac death, and it is associated with coronary calcification. CRP is expressed in coronary arteries, but its role in the development of coronary plaques is unclear.
Aim
To investigate CRP immunoreactivity in relation to the severity of coronary artery disease and plaque morphology in human left anterior descending coronary arteries (LAD).
Methods
A prospective, consecutive autopsy series of 66 patients (mean age 63.4 years) in Tampere University Hospital, Tampere, Finland.
Results
CRP immunoreactivity was seen in 59% of the cases. In logistic regression analysis with age, sex and body mass index as confounders, CRP immunoreactivity in LAD was associated with >50% stenosis and plaque calcification. All three cases with acute coronary thrombosis due to rupture or erosion of the plaque showed a clear immunopositive reaction. CRP‐positive cells were never detected in normal arteries, but were often found in early fibrous plaques (75%) and almost invariably present in the shoulder area of plaques with necrotic core (96%). CRP immunoreactivity adjacent to calcified areas in more stable plaques (71%) was less consistent with one‐third of these plaques showing no immunoreactivity.
Conclusions
CRP immunoreactivity is associated with the progression of atherosclerosis, and especially with unstable coronary plaques. The immunoreactivity could cease at the stable calcified stages of atherosclerosis.
Inflammation plays an important role in the pathogenesis of atherosclerosis1 and plaque instability.2 C‐reactive protein (CRP) is considered to be the most important acute‐phase reactant in people. Increased serum CRP level has been shown to be an independent risk factor for myocardial infarction, and it predicts cardiovascular events better than low density lipoprotein cholesterol.3,4 Although CRP is mainly produced in the liver as a response to trauma or infection, it has also been located in the coronary wall5,6,7,8 where it can be synthesised by macrophages and smooth muscle‐like cells.9 In human coronary arteries, CRP has been shown in early atherosclerotic lesions5,6,7,8 and in atherectomy samples.8,10 Coronary CRP expression in early stages of atherosclerosis is associated with increased serum CRP levels,5 and the expression is associated with early unstable disease.10 CRP has been associated with inflammation11; however, its role may be protective, as indicated by its actions on fibroblasts and platelets.12
Coronary calcification and plaque erosion and rupture have been shown to be risk factors for sudden cardiac death, but are not necessarily associated with each other.13 Recently, it has been suggested that calcifying of atherosclerotic plaques may stabilise and thus protect the vessel wall from a rupture.14,15 Inflammation in end‐stage atherosclerosis with calcified plaques and CRP immunoreactivity has not been studied.
To investigate whether CRP is present during all stages of coronary atherosclerosis, we studied CRP immunoreactivity in postmortem samples of coronary artery wall at different stages of atherosclerotis in relation to coronary stenosis, plaque morphology and cardiovascular state.
Methods
The study series comprises 66 consecutive autopsies at the Department of Forensic Medicine, Tampere University Hospital, Tampere, Finland, during 3 months in 2003. This is a subsample of the Tampere Coronary Study of 746 cases collected during 2002–4. There were no selection criteria based on age, sex or cause of death, but the interval for postmortem examination was set to 1–5 days. Indications for autopsy were sudden out‐of‐hospital death or suggested non‐natural death. This study was approved by the ethics committee of Tampere University Hospital (DNO 1239/32/200/01) and the National Authority for Medicolegal Affairs.
Of the original 66 cases, five were disqualified because of technical problems in cryosectioning. All five disqualified cases were aged ⩾80 years and had a rigid calcified left anterior descending coronary artery (LAD) that turned into powder when cryosectioned. The mean (SD) age of the study cases was 63.4 (19.3) years range 2–94 years (42 men and 19 women). Sixteen patients (26%) died from cardiac disease, mainly coronary heart disease with or without infarction, and 19 (31%) from non‐cardiac diseases. The remaining 26 (43%) were non‐natural deaths, mainly suicides and accidents. In addition to patients that died from cardiac deaths, coronary atherosclerosis was detected in nine cases with non‐cardiac diseases (natural deaths) and in 13 with non‐natural death. Therefore, 38 of the 61 cases (62%) had histologically proven coronary atherosclerosis.
A complete medicolegal autopsy was performed on all cases, including dissecting all major branches of the coronary arteries, taking cardiac samples and recording any pathological changes in the heart. The calcification, thrombosis and stenosis percentages in the left main, left anterior, left circumflex and right arteries were registered. For analysis, a 1 cm specimen was taken from the proximal segment of the LAD, which is the predilection site of the plaque or narrowing.16
Tissue samples were formaldehyde‐fixed and cryosectioned at 10 μm thickness. Immunohistochemical staining was carried out using standard avidin–biotin techniques17 and a commercially available monoclonal antiserum for human CRP diluted at 1:200 (Sigma‐Aldrich, St Louis, Missouri, USA). To confirm the results, 6–9 sections per sample were studied at a time and each sample was stained twice. The second staining repeated the first result in all cases but two, which first showed weak staining with some background but when repeated twice there was no staining.
CRP immunohistochemical analysis was classified as negative when there was only faint background stain also visible in negative controls. For positive controls we used liver samples, and negative controls were obtained by omitting the antibody. The criteria for a positive result were: (1) strong colour equal to the positive control; (2) colour in particular anatomic structures or areas other than edges of the section or floating fragments; (3) no stain in the negative control sections of the same sample in the same analytical process clearly negative . The intensity of the staining was not further analysed. The positive class was divided into mildly positive with up to 10% plaque area stained, and strongly positive with >10% stained. An ocular grid was used to measure the approximate area of the plaque and staining. All cases with intracellular stain also had extracellular stain, and the classification was made based on the extracellular stain area. The plaques were classified microscopically into four categories: no plaque, increased fibrous plaque, atheroma with a necrotic core and calcified plaque with a necrotic core. For recognition of cell types in the coronary plaque, we counterstained some sections with H&E.
Statistical analyses between CRP immunoreactivity and coronary heart disease risk factors, and variables associated with the severity of coronary artery disease and plaque morphology were performed using χ2 test (categorical variables) or analysis of variance (continuous variables). Logistic regression analysis was used as a confirmatory test. Computation was carried out with SPSS v.10.0 for Windows.
Results
CRP immunoreactivity was detected in 36 of the 61 (59.0%) cases (Table 1). All positive cases showed atherosclerotic changes at microscopic examination, and of the 42 cases with atherosclerotic left coronary artery verified microscopically, only 6 (14%) were negative for CRP staining. In univariate analyses, CRP immunoreactivity was associated significantly with plaque morphology (p<0.001), plaque calcification (p<0.001), LAD stenosis percentage (p = 0.017) and cardiac volume (p = 0.014). All cases with acute left coronary thrombosis (n = 3) showed a clear immunopositive reaction (p = 0.070). CRP immunoreactivity also tended to be associated with age (p = 0.17) and cardiac death (p = 0.13). In the logistic regression model with age, sex and body mass index as confounders, specific CRP immunoreactivity in LAD was associated with >50% stenosis (odds ratio (OR) 7.1, p = 0.021) and presence of calcification (OR 4.7, p = 0.041). However, CRP immunoreactivity was not strongly associated with sudden cardiac death in this sample.
Table 1 Predictors of coronary artery C‐reactive protein immunoreactivity in the Tampere Coronary Study series.
| Negative | Positive | p Value | ||
|---|---|---|---|---|
| n = 25 | n = 36 | |||
| Gender (M/F) | 18/7 | 24/12 | 0.439* | |
| Mean (SD) age (years) | 59.4 (22.0) | 66.3 (16.9) | 0.172† | |
| Mean (SD) BMI (kg/m2) | 26.7 (5.8) | 26.5 (6.0) | 0.895† | |
| Cardiac death (+/−) | 4/21 | 12/24 | 0.111* | |
| LAD% | ||||
| None | 14 (60.9%) | 9 (39.1%) | ||
| <50% | 8 (40.0%) | 12 (60.0%) | ||
| ⩾50% | 3 (16.7%) | 15 (83.3%) | 0.017* | |
| LAD calcification (+/−) | 4/21 | 16/20 | 0.018* | |
| Heart weight (g) | ||||
| <550 | 9 (60.0%) | 6 (40.0%) | ||
| 551–800 | 9 (50.0%) | 9 (50.0%) | ||
| >801 | 3 (21.4%) | 11 (78.6%) | 0.096* |
BMI, body mass index; LAD, left anterior descending coronary artery.
*χ2 test.
†t test.
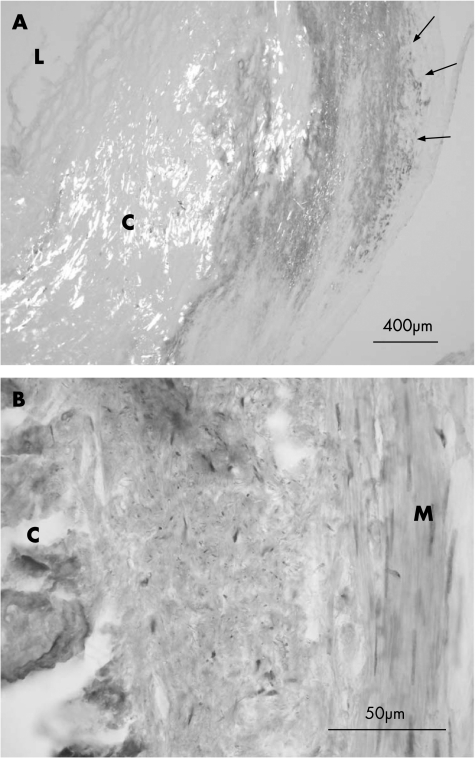
In all CRP‐positive cases, immunostaining was mostly extracellular and localised at the shoulder areas of the necrotic core of the atheroma plaques (fig 1). Intracellular CRP was detected in the outer perimeter of plaques and in the muscular layer. All cases with intracellular CRP also had strong extracellular staining (table 2). The CRP‐positive cells resembled smooth muscle‐like cells in shape and location. However, dual immunostaining to verify this was not possible, owing to the sensitive samples. In the deep cores of plaques, there were no immunopositive cells, but their was extracellular staining. In these regions in general, the cellular structure did not clearly indicate necrosis. The hard calcified core areas did not collect any stain, but their was intensive staining adjacent to most calcified cores.
Figure 1 Human coronary artery, immunohistochemical analysis of C reactive protein. (A) Immunopositive reaction is indicated with arrows. Calcified plaque (C) is visualised using polarised light. Immunoreaction is concentrated on the shoulder area of the plaque. (B) H&E counterstain. L, lumen; M, intima media.
Table 2 C reactive protein immunoreactivity at different stages of atherosclerosis in the left coronary artery.
| None | Cellular | Extracellular | Total | |
|---|---|---|---|---|
| No atherosclerosis | 19 | 0 | 0 | 19 |
| Early fibrotic plaque | 3 | 7 | 9 | 12 |
| Plaque with necrotic core | 1 | 18 | 22 | 23 |
| Calcified plaque | 2 | 2 | 5 | 7 |
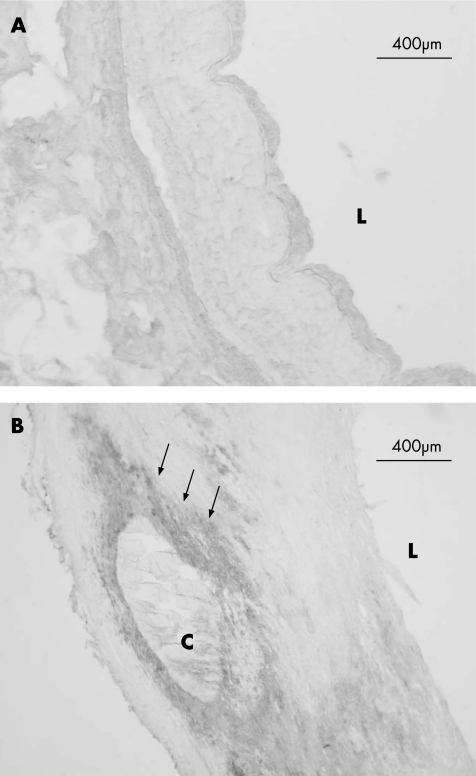
Neither extracellular nor intracellular CRP was detected in normal arteries without plaques (n = 19), but was often found in early fibrous plaques (75%, n = 12) and was almost invariably present in the shoulder area of plaques with a necrotic core (96%, n = 23). CRP immunoreactivity adjacent to calcified areas in more stable plaques (71%, n = 7) was less consistent, with one‐third of these plaques showing no immunoreactivity. In four cases with calcified plaque, specific staining was considerable, in one sample there was little specific staining and in two samples there was no specific staining, suggesting that the inflammatory process had possibly ceased in the in coronary arteries with the most severe of atherosclerosis. The samples with no CRP immunoreactivity did not otherwise differ in plaque morphology or in postmortem time. The results are shown in table 2. Figures 1 and 2 show typical staining results.
Figure 2 Human coronary artery, immunohistochemical analysis of C‐reactive protein. (A) A healthy artery. (B) Coronary atherosclerosis. Areas with immunopositive reaction are indicated with arrows. Necrotic core is noticeably visible. C, calcified plaque; L, lumen.
Values are n
Discussion
Localisation of CRP was investigated immunohistochemically in 61 human coronary arteries in an autopsy series. All findings supported the association of CRP immunoreactivity in the left coronary artery with advanced coronary atherosclerosis. Our results confirm the earlier finding of localisation of CRP in early coronary plaques, but not in healthy arteries.5,6,7 CRP immunoreactivity was most consistent in the shoulder area of plaques with a necrotic core, which is the type of atheroma often prone to plaque rupture and thus unstable. Association with unstable disease was further supported with the presence of CRP in all cases with coronary thrombosis. Some early fibrous plaques did not repeatedly stain for CRP, suggesting a stable condition in the artery. This is in line with earlier findings that atherectomy samples from patients with stable coronary disease do not express CRP.10
CRP immunoreactivity in late‐stage calcified atherosclerotic plaques has not received wide attention. Jabs et al8 showed that CRP was located in atherosclerotic lesions of venous coronary grafts and coronary arteries, but desobliterated coronary arteries did not show CRP gene expression, suggesting that local CRP expression could be limited to active sites of disease. Our data show that CRP immunoreactivity is less consistent in calcified plaques than in early fibrotic plaques. If CRP is associated with an inflammatory process and unstable atherosclerosis as suggested,5,9,10,11 calcification alone may not be a sufficient indicator of a stable plaque. However, the repeatedly negative staining results in calcified plaques for CRP in one‐third of our cases suggest that in end‐stage plaque the inflammatory process could have ceased.
Calcification has been associated with the lipid necrosis process of the atheroma, and could stabilise the vessel wall protecting against plaque rupture.14,15 CRP could have an active role in the atheroma calcification process. In an experimental model, CRP increased aortic wall calcification in tissue culture.18 There is also evidence that calcification could be a result of smooth muscle cell apoptosis in the atheroma,19 and that CRP could directly modify the apoptotic process.20 This is further supported by the finding that clinically stable atheromas had fewer apoptotic cells than clinically unstable atheromas.21 All this may indicate that, although CRP is a marker of an active atherosclerotic process, the actual role could be protective.
High serum CRP is known to be associated with high‐risk atherosclerotic disease. It has been reported that patients who died suddenly from severe coronary disease had significantly increased serum CRP levels measured by high‐sensitivity assay. It has also been shown that increased serum CRP is associated with ischaemic stroke.22 CRP is mainly produced in the liver, but the kidneys in renal cell carcinomas, cerebral neurons, respiratory epithelium, thymic epithelium, macrophages, adipocytes and vascular smooth muscle cells have also been shown to synthesise CRP.23,24,25,26,27,28,29,30,31 CRP mRNA has been detected in cultured human coronary artery smooth muscle cells, supporting the concept of a local production of CRP.29,32,33,34 This is further supported by the findings of high CRP mRNA levels in atheroma plaques29 and stimulation of CRP production in human smooth muscle cells by inflammatory cytokines.32
In our study, we found CRP extracellularly in the shoulder areas of necrotic plaque cores and to a lesser extent in smooth muscle‐like cells. Postmortem degradation may change the tissue structure and thus add to the proportion of the extracellular protein. However, our finding is in line with earlier studies using fresh, surgically removed atheromas.9,34 The localisation of CRP does not confirm the origin of the protein, which could either be produced locally or be the result of uptake of circulating CRP.
Take‐home messages
C‐reactive protein (CRP) immunoreactivity was detected in atherosclerotic coronary arteries, but never in normal arteries
CRP was most abundant extracellularly, located in the shoulder areas of atherosclerotic plaques. Intracellular stain was visible in smooth muscle cells
CRP immunoreactivity was almost always present in plaques with a necrotic core. Some arteries with calcified plaques did not stain for CRP
Inflammation plays an important role in the development of restenosis after atherectomy.35 A recent study on atherectomy materials suggests that it is the CRP of atheromas that influences the pathogenesis of unstable angina and restenosis after intervention.9 CRP could also function as a proinflammatory mediator during myocardial infarction by activating complement.36 Moreover, Calabró et al32 showed that human coronary artery smooth muscle cells can produce CRP in response to inflammatory cytokines, which all human cells cannot. It has also been suggested that CRP proteins could have a regulatory and even protective role in the atherosclerosis process.12
Our data suggest that CRP immunoreactivity in LAD is strongly associated with the progression of coronary atherosclerosis, and the development of vulnerable coronary plaques with necrotic cores. In calcified plaques, CRP immunoreactivity was not consistent. One possible explanation for this is that CRP immunoreactivity could cease at stable stages of coronary atherosclerosis. Coronary CRP may be a valid indicator of an acute coronary process in patients with sudden cardiac death.
Abbreviations
CRP - C reactive protein
LAD - left anterior descending coronary artery
Footnotes
Funding: This study was supported by grants from Elli and Elvi Oksanen Fund of the Pirkanmaa Fund under the auspices of the Finnish Cultural Foundation (Tampere), Medical Research Fund of Tampere University Hospital, Aarne Koskelo Foundation and Finnish Foundation for Cardiovascular Research (Helsinki).
Competing interests: None.
References
- 1.Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med 1999340115–126. [DOI] [PubMed] [Google Scholar]
- 2.van der Wal A C, Becker A E, van der Loos C M.et al Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 19948936–44. [DOI] [PubMed] [Google Scholar]
- 3.Ferreiros E R, Boissonnet C P, Pizarro R.et al Independent prognostic value of elevated C‐reactive protein in unstable angina. Circulation 19991001958–1963. [DOI] [PubMed] [Google Scholar]
- 4.Lagrand W K, Visser C A, Hermens W T.et al C‐reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation 199910096–102. [DOI] [PubMed] [Google Scholar]
- 5.Burke A P, Tracy R P, Kolodgie F.et al Elevated C‐reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation 20021052019–2023. [DOI] [PubMed] [Google Scholar]
- 6.Torzewski J, Torzewski M, Bowyer D E.et al C‐reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol 1998181386–1392. [DOI] [PubMed] [Google Scholar]
- 7.Rattazzi M, Puato M, Faggin E.et al C‐reactive protein and interleukin‐6 in vascular disease: culprits or passive bystanders? J Hypertens 2003211787–1803. [DOI] [PubMed] [Google Scholar]
- 8.Jabs W J, Theissing E, Nitschke M.et al Local generation of C‐reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation 20031081428–1431. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T, Hatakeyama K, Imamura T.et al Involvement of C‐reactive protein obtained by directional coronary atherectomy in plaque instability and developing restenosis in patients with stable or unstable angina pectoris. Am J Cardiol 200391287–292. [DOI] [PubMed] [Google Scholar]
- 10.Meuwissen M, van der Wal A C, Niessen H W.et al Colocalisation of intraplaque C reactive protein, complement, oxidised low density lipoprotein, and macrophages in stable and unstable angina and acute myocardial infarction. J Clin Pathol 200659196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson A M, Ryan M C, Boyle A J. The novel role of C‐reactive protein in cardiovascular disease: risk marker or pathogen. Int J Cardiol 2006106291–297. [DOI] [PubMed] [Google Scholar]
- 12.Schwedler S B, Filep J G, Galle J.et al C‐reactive protein: a family of proteins to regulate cardiovascular function. Am J Kidney Dis 200647212–222. [DOI] [PubMed] [Google Scholar]
- 13.Taylor A J, Burke A P, O'Malley P G.et al A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death. Circulation 20001011243–1248. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Virmani R, Younis H.et al The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 20011031051–1056. [DOI] [PubMed] [Google Scholar]
- 15.Nandalur K R, Baskurt E, Hagspiel K D.et al Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. Am J Roentgenol 2005184295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y X, Cliff W J, Schoefl G I.et al Coronary C‐reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis 1999145375–379. [DOI] [PubMed] [Google Scholar]
- 17.Coggi G, Dell'Orto P, Viale G. Avidin‐biotin methods. In: Polak JM, van Noorden S, eds. Immunocytochemistry, modern methods and applications 54th edn. Bristol: Wright, 1986
- 18.Warrier B, Mallipeddi R, Karla P K.et al The functional role of C‐reactive protein in aortic wall calcification. Cardiology 200510457–64. [DOI] [PubMed] [Google Scholar]
- 19.Proudfoot D, Skepper J N, Hegyi L.et al Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 2000871055–1062. [DOI] [PubMed] [Google Scholar]
- 20.Blaschke F B, Bruemmer D, Yin F.et al C‐reactive protein induces apoptosis in human coronary vascular smooth muscle cells. Circulation 2004110579–587. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Eriksson P, Kimura T.et al Apoptosis and angiogenesis are induced in the unstable coronary atherosclerotic plaque. Coron Artery Dis 200516191–197. [DOI] [PubMed] [Google Scholar]
- 22.Di Napoli M, Papa F, Bocola V. C‐reactive protein in ischemic stroke: an independent prognostic factor. Stroke 200132917–924. [DOI] [PubMed] [Google Scholar]
- 23.Jabs W J, Lögering B A, Gerke P.et al The kidney as a second site of human C‐reactive protein formation in vivo. Eur J Immunol 200333152–161. [DOI] [PubMed] [Google Scholar]
- 24.Diehl E E, Haines G K, 3rd, Radosevich J A.et al Immunohistochemical localization of modified C‐reactive protein antigen in normal vascular tissue. Am J Med Sci 200031979–83. [DOI] [PubMed] [Google Scholar]
- 25.Jabs W J, Busse M, Kruger S.et al Expression of C‐reactive protein by renal cell carcinomas unaffected surrounding renal tissue. Kidney Int 2005682103–2110. [DOI] [PubMed] [Google Scholar]
- 26.Yasojima K, Schwab C, McGeer E G.et al Human neurons generate C‐reactive protein and amyloid P: upregulation in Alzheimer's disease. Brain Res 200088780–89. [DOI] [PubMed] [Google Scholar]
- 27.Ramage L, Proudfoot L, Guy K. Expression of C‐reactive protein in human lung epithelial cells and upregulation by cytokines and carbon particles. Inhal Toxicol 200416607–613. [DOI] [PubMed] [Google Scholar]
- 28.Klein L, Klein T, Ruther U.et al CD4 T cell tolerance to human C‐reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium. J Exp Med 19981885–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasojima K, Schwab C, McGeer E G.et al Generation of C‐reactive protein and complement components in atherosclerotic plaques. Am J Pathol 20011581039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouchi N, Kihara S, Funahashi T.et al Reciprocal association of C‐reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003107671–674. [DOI] [PubMed] [Google Scholar]
- 31.Calabro P, Chang D W, Willerson J T.et al Release of C‐reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol 2005461112–1113. [DOI] [PubMed] [Google Scholar]
- 32.Calabró P, Willerson J T, Yeh E T. Inflammatory cytokines stimulated C‐reactive protein production by human coronary artery smooth muscle cells. Circulation 20031081930–1932. [DOI] [PubMed] [Google Scholar]
- 33.Maier W, Altwegg L A, Corti R.et al Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin‐6 and serum amyloid A but decreased C‐reactive protein. Circulation 20051111355–1361. [DOI] [PubMed] [Google Scholar]
- 34.Sun H, Koike T, Ichikawa T, Hatakeyama K.et al C‐reactive protein in atherosclerotic lesions: its origin and pathophysiological significance. Am J Pathol 20051671139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno P R, Bernardi V H, Lopez‐Cuellar J.et al Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina. Circulation 1996943098–3102. [DOI] [PubMed] [Google Scholar]
- 36.Nijmeijer R, Lagrand W K, Lubbers Y T.et al C‐reactive protein activates complement in infarcted human myocardium. Am J Pathol 2003163269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]