Abstract
Background
Acid cysteine protease inhibitor (ACPI) is an intracellular protein, often linked to neoplastic changes in epithelium and thought to have an inhibitory role in malignant transformation.
Aim
To analyse the expression and prognostic role of ACPI in non‐small‐cell lung cancer (NSCLC).
Method
Histological samples from 199 patients with resected NSCLC were stained immunohistochemically for the expression of ACPI in normal and preneoplastic bronchial epithelium, and in various types of lung carcinomas.
Results
A normal bronchial epithelium showed positive staining for ACPI in the basal cells, whereas the upper two‐thirds of the dysplastic epithelium was ACPI positive. High staining for ACPI was found in 74% (91/123) of squamous‐cell carcinomas, whereas 16% (8/49) of adenocarcinomas and 30% of (8/27) large‐cell carcinomas showed the high expression of ACPI (p<0.001). Among squamous‐cell carcinomas, low expression of ACPI was correlated with poor tumour differentiation (p = 0.032). In the whole tissue, reduced expression of ACPI was associated with tumour recurrence (p = 0.024). In overall survival (OS) and disease‐free survival (DFS) analyses, the histological type of the tumour (both p<0.001) and stage of the tumour (p = 0.001, p = 0.013, respectively) were related to patient outcome. Low expression of ACPI in tumour cells was associated with poor OS and DFS (p<0.041, p = 0.004, respectively). In multivariate analysis, ACPI did not retain its prognostic value, whereas the traditional factors were the most important prognostic factors.
Conclusions
ACPI expression is linked with the malignant transformation of the bronchial epithelium and predicts a risk of tumour recurrence as well as poor rate of survival for the patients. However, ACPI does not have any independent prognostic value in NSCLC.
Cysteine proteases are proteolytic enzymes having cysteine in the structural centre of the molecule, and the protease activity is induced by the external reducing agent.1 All mammalian cysteine proteases belong to the cathepsin superfamily and are involved in various biological and pathological processes such as protein catabolism, inflammation and metastasis formation.2 Cystatins are members of a protein family with endogenous inhibitors of cysteine proteases such as catepsins B, H and L.3,4 Acid cysteine proteinase inhibitor (ACPI cystatin A) was the first identified mammalian cystatin, originally purified and biochemically characterised from rat skin.5 Furthermore, it has also been demonstrated in other benign squamous epithelia,4,6 and thought to be a major soluble protein in stratified squamous epithelium.7 In addition, it has been shown to be expressed in many other cells such as the dendritic cells of lymphoid tissue,8 and also in basal and myoepithelial cells of normal glandular epithelium of prostate and breast.9,10 In recent years, cystatins have been linked with many immunological reactions in various tissues by modulating cathepsin activation and antigen presentation.11
ACPI expression has previously been linked with neoplastic changes in squamous‐cell epithelium.12,13,14 However, the effect seems to be based on the inhibitory role of ACPI in malignant transformation.13 The reduced expression of ACPI parallels the change in the epithelium from normal to dysplastic, and finally to invasive carcinoma.14 This supports the notion that ACPI might act as a tumour supressor.15 Similar findings have also been demonstrated in adenocarcinoma.16 However, the expression of ACPI in the basal‐cell layer has been found to be retained in preneoplastic glandular epithelium, but disappears in invasive carcinomas.16
The role of ACPI in the progression of cancer has become evident in recent years.9,17,18,19,20 In squamous‐cell carcinomas, expression of ACPI is concentrated in better‐differentiated areas of the tumour.20 Reduced expression of ACPI has been a sign of more aggressive disease,17,18 but opposing results also exist.9,19 However, the expression of ACPI in different types of carcinomas seems to be very scanty9,16 and its clinical prognostic value is somewhat unclear. In lung tumours the prognostic role of ACPI has not been studied previously, but previous data suggest that lung tumour cells, in vitro, produce both cysteine proteases and cystatins, which are regulated differently in histologically different types of lung cancers.21
On the basis of previous reports from various other carcinomas,9,10,16 we hypothesised that the expression of ACPI might be significantly different in the various types of lung carcinomas. To clarify the biological and prognostic role of ACPI in resected non‐small‐cell lung cancer (NSCLC), we studied its expression immunohistochemically, both in preneoplastic lesions and in tumour cells of different histological types of carcinomas. The results were compared with the clinicopathological parameters and survival of the patients.
Materials and methods
Clinicopathological data of the patients
Clinicopathological data were based on the previous studies of the same clinical material.22,23 Briefly, all patients with NSCLC were diagnosed and treated in eastern Finland between 1978 and 1995. The total number of patients who underwent resection of the tumour was 212. Both mean and median age of the patients was 63 years (range 42–78 years). The exact tumour–node–metastasis classification (UICC 2002) and stage of the tumour were recorded by reviewing the clinical, radiological and histopathological data in the patients' files. The follow‐up was carried out until death or until July 2002.
Histology
The material consisted of 123 squamous‐cell carcinomas (62%), 49 adenocarcinomas (25%) and 27 large‐cell carcinomas (13%). All tumours were re‐evaluated for the histological type and graded by two experienced histopathologists according to the World Health Organization classification.24
Immunohistochemistry for ACPI
Antibody
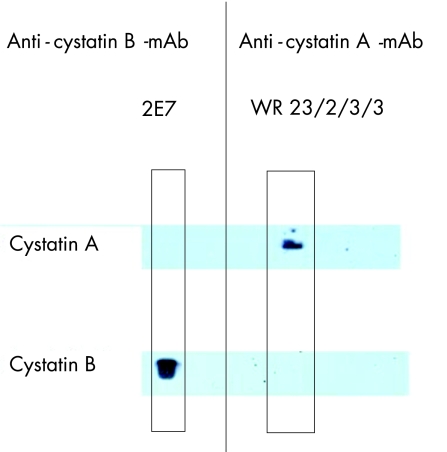
ACPI was originally purified from palatine tonsil and spleen tissues using papain affinity chromatography as described by Järvinen and Hopsu‐Havu.5 The anti‐ACPI (cystatin A) monoclonal antibody clone WR23/2/3/39 was raised in a Balb/c mouse following common protocols using cystatin A for immunisation. The antibody specifically reacts with human cystatin A in ELISA and western blotting (fig 1) and is now also commercially available (Sigma, Saint Louis, Missouri, USA and Alexis Biochemicals Corporation, San Diego, California, USA).
Figure 1 Demonstration of the specificity of the reaction of anti‐acid cysteine protease inhibitor (cystatin A) monoclonal antibody WR23/2/3/3 on western blot, using entry‐level enhanced chemiluminescent substrate. The antibody is detecting recombinant human cystatin A and shows no cross‐reactivity with homologous human cystatin B, which is recognised by a cystatin B‐specific monoclonal antibody RJWM 2E725 (Alexis Corporation, San Diego, California, USA).
Immunohistochemical staining
In brief, 5 μm thick sections were dewaxed in xylene, rehydrated with graded alcohols and washed with 0.1 M sodium phosphate buffer (pH 7.4). The antigen retriewal was processed in a microwave oven in citrate buffer (pH 6.0) for 3×5 min. Endogenous peroxidase activity was inactivated with 5% hydrogen peroxide for 5 min, and non‐specific binding was blocked with 1.5% normal horse serum in phosphate buffered saline (PBS). The slides were incubated overnight at 4°C, followed by washing with PBS for 2×5 min. The slides were then incubated with the biotinylated secondary antibody (anti‐mouse immunoglobulin G; ABC Vectastain Elite kit, Vector Laboratories, Burlingame, California, USA) for 35 min at room temperature, after which the slides were washed with PBS for 2×5 min, incubated for 45 min in preformed avidin‐biotinylated peroxidase complex (ABC Vectastain Elite kit, Vector Laboratories,) and washed 2×5 min with PBS. The colour was developed with 0.05% diaminobentzine tetrahydrocloride (Sigma) and 0.03% hydrogen peroxide in PBS. Finally the slides were counterstained with Mayer's haematoxylin, washed, dehydrated and mounted with Depex (BDH, Poole, UK).
Sections from the palatine tonsil were used as positive controls, which showed positive staining of ACPI in the dendritic reticulum cells and within the upper layers of the squamous‐cell epithelium of the tonsil.26 In negative controls, the slides were stained without the primary antibody. The negative controls showed no staining.
Evaluation of staining
Staining was analysed by two observers (TL, RP) who were unaware of the clinicopathological data. A tumour was graded positive if any tumour‐cell‐associated staining was noted. The intensity of ACPI staining in the tumour cells was scored as follows: 0 = negative, 1 = weak, 2 = moderate or 3 = strong. Strong staining intensity corresponded to that in control samples used as standards, and a weak intensity was similar to that seen in benign bronchial epithelium. Moderate intensity was considered as a staining intensity between weak and strong. The percentage of positively stained cancer cells of all cancer cells in the section tissue was evaluated using a continuous scale (0–100%). For statistical purposes, the stainings were further divided into two groups (high and low) according to the median (20%) value of positive tumour cells with moderate‐to‐strong staining intensity. This cut‐off level allowed the most clear‐cut separation between the high and low expressors of ACPI.
Statistical analyses
SPSS for Windows V.13.0 computer program package was used in statistical calculations. The frequency distributions of studied variables were compared using the χ2 test. In univariate survival analyses, the Kaplan–Meier method was used; the log‐rank test was used to examine the significance of the difference between drawn survival curves. Multivariate survival analysis was performed by using Cox's multivariate hazards model. Recurrence‐free survival time was defined as a time interval between the surgical operation and signs of recurrence.
Ethics
This study was a cohort from the study protocol previously approved by the ethics committee of Kuopio University Hospital, Kuopio, Finland and the Finnish Ministry of Social affairs and Health.23 Permission to use organic human tissue during the purification of ACPI (AR) was received from the University of Tromsö, Norway.
Results
There were 199 cases with satisfactory staining results; 13 cases were abandoned due to small amounts of cancer tissue in the tissue slide to be analysed. These 199 cases were comparable with the original cohort (n = 212) for distribution of clinicopathological characteristics (histological type of tumour, histological grade and stage).
ACPI in non‐neoplastic tissue and dysplastic epithelium
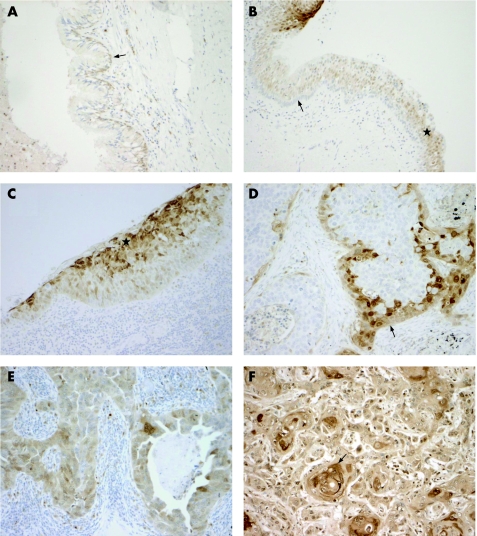
The normal bronchial epithelium was either ACPI negative or displayed low staining in the basal cells (fig 2A). The rest of the pseudostratified epithelium, including superficial ciliated cells and goblet cells, were usually ACPI negative. However, in some slides, the normal epithelium adjacent to dysplastic epithelium also showed ACPI positivity in the superficial layers (fig 2B).
Figure 2 (A) Normal bronchial epithelium is mostly negative for acid cysteine protease inhibitor (ACPI), with only weak expression is seen in the basal cells (arrow). (B) Normal bronchial epithelium adjacent to the dysplastic epithelium. Negative ACPI staining in the basal‐cell layer (arrow) and positive staining in the upper parts of the epithelium (star). The strongly stained dysplastic epithelium is seen in upper left. (C) Dysplastic bronchial epithelium showing strong staining of ACPI in upper two‐thirds of the epithelium (star). (D) Squamous‐cell carcinoma showing a positive staining for ACPI in the peripheral area of the tumour epithelium (arrow). (E) Strong staining for ACPI in adenocarcinoma. (F) Well‐differentiated squamous‐cell carcinoma with strong ACPI staining. Note the concentration of staining signal around the keratin pearls (arrow). Original magnification×330.
Dysplastic bronchial epithelium or carcinoma in situ was observed in 10 cases. In weakly or moderately dysplastic epithelium in five cases, strong staining for ACPI was noted in the upper two‐thirds of the epithelium, whereas five cases with high‐grade dysplasia/carcinoma in situ lesions showed a strong staining signal only in the superficial layers of the epithelium (fig 2C).
Columnar cells of the normal peribronchial glands showed positive staining for ACPI in the basal‐cell layer. The peritumoural stroma was ACPI negative, whereas a positive staining signal was apparent in some inflammatory cells.
ACPI staining in cancer cells
Tumour cell‐associated ACPI staining was always located in the cell cytoplasm, and cell membrane‐associated positivity was noted in some cases (fig 2D,E). Positive ACPI staining in tumour cells was observed in 120 (60%) cases. In most cases, the positive signal was distributed heterogeneously in cell groups of different sizes inside the tissue sample. This was especially noted for squamous‐cell carcinomas. In 28 (23%) cases, positive ACPI staining was mainly focused towards the peripheral cell layers of the tumour epithelium (fig 2D). In large‐cell carcinomas and adenocarcinomas a similar peripheral staining signal was noted only in 1 (4%) and 2 (4%) cases, respectively. Occasional keratinisation was noted in 33 squamous‐cell carcinomas, which showed ACPI positivity concentrated in the keratin pearls or single keratinised cells (fig 2F).
ACPI as related to clinicopathological factors
High expression of ACPI was observed in 107 (54%) cases and low staining in 92 (46%) cases. High staining for ACPI was found in 91 (74%) squamous‐cell carcinomas, whereas 8 (16%) adenocarcinomas and 8 (30%) large‐cell carcinomas were highly positive for ACPI (p<0.001).
ACPI expression was not correlated with tumour differentiation in the whole tissue (n = 199), but for squamous‐cell carcinomas (n = 123), a significant correlation was found between low expression of ACPI and higher tumour grade (p = 0.032). Low expression of ACPI was associated with increased number of recurrences (p = 0.024). However, no correlation was found between ACPI expression and tumour size, lymph node status or stage of the disease (table 1).
Table 1 The relationship between the expression of acid cysteine protease inhibitor in tumour cells and clinicopathological factors.
| ACPI expression in tumour cells, n (%) | p Value | ||
|---|---|---|---|
| Low | High | ||
| Histology | |||
| Squamous‐cell carcinoma | 32 (35) | 91 (85) | |
| Adenocarcinoma | 41 (45) | 8 (7) | 0.001 |
| Large‐cell carcinoma | 19 (20) | 8 (8) | |
| Histological grade* | |||
| I–II | 35 (52) | 56 (57) | 0.635 |
| III | 32 (48) | 43 (43) | |
| Recurrence | |||
| Yes | 45 (56) | 37 (39) | 0.024 |
| No | 35 (44) | 58 (61) | |
| Nodal status | |||
| N0 | 62 (70) | 74 (69) | 0.999 |
| N1–3 | 27 (30) | 33 (31) | |
| Stage | |||
| I | 62 (68) | 70 (66) | 0.764 |
| II–IV | 29 (32) | 36 (34) | |
ACPI, acid cysteine protease inhibitor.
*Among squamous‐cell carcinomas, a significant correlation was found between low expression of ACPI and higher tumour grade (p = 0.032).
Survival analyses
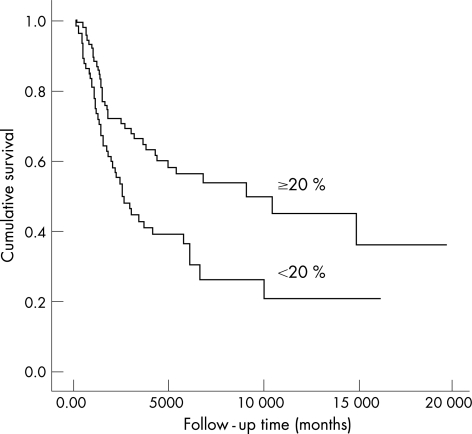
In overall survival (OS) and disease‐free survival (DFS) analyses, histological type of the tumour (p<0.001), and stage of the tumour (p = 0.001 and p = 0.013, respectively) were related to patients' outcome. The low expression of ACPI in tumour cells was also associated with poor OS and DFS (p<0.041 and p = 0.004, respectively, fig 3). In multivariate analysis, tumour type (p = 0.001 for OS and p = 0.009 for DFS) and stage of the tumour (p = 0.001 for OS and p = 0.20 for DFS) were most significant predictors of outcome. Expression of ACPI did not retain the prognostic value in either OS or DFS analyses.
Figure 3 A Kaplan–Meiyer curve demonstrating the difference in disease‐free survival between high (⩾20%, n = 107) and low (<20%, n = 92) expression of acid cysteine protease inhibitor in tumour cells (p = 0.004).
Discussion
The importance of ACPI in the development of malignant tumours has been demonstrated previously.2 However, studies emphasing its role in the large clinicopathological materials have been performed less widely.9,19 This study was undertaken to study the expression of ACPI in bronchial preinvasive lesions and various lung carcinomas, and, for the first time, to evaluate the prognostic value of ACPI in NSCLC. Normal bronchial epithelium was mainly devoid of ACPI, whereas dysplastic epithelium showed a more intense signal for ACPI. High ACPI expression was more often found with better‐differentiated squamous‐cell carcinomas compared with adenocarcinomas or large‐cell carcinomas. Low ACPI expression in tumour cells was associated with tumour recurrence and unfavourable outcome, whereas ACPI had no prognostic value in the multivariate analysis. The results are consistent with previous reports on other cancers, which have indicated the significance of ACPI in the tumour progression, probably acting as a tumour suppressor.15,17,27
It has been previously reported that ACPI is expressed in the basal‐cell layer of the epithelium in normal glandular structures.9,10,16 This is the first report demonstrating ACPI expression in bronchial epithelium. We observed that normal pseudostratified bronchial epithelium and peribronchial glands were either ACPI negative or showed a weak staining signal in the basal cells above the basement membrane, supporting previous findings.9,16 Interestingly, in some cases, the normal epithelium adjacent to dysplastic epithelium exhibited positive ACPI staining in the more superficial layers. This might suggest an early neoplastic transformation of the epithelium although it still seems to be histologically normal. This notion has not been demonstrated previously. ACPI expression has been noted only in the basal‐cell layers in premalignant prostate glandular epithelium.16 This may reflect the different nature of the pseudostratified bronchial epithelium compared with the simple columnar epithelium of other glands. This study supports previous results that expression of ACPI in bronchial low‐grade dysplasias is increased compared with normal bronchial epithelium,13,20 but is, however, decreased again in high grade dysplasias and in in situ carcinomas.14,20 The findings highlight the different role of ACPI in the malignant transformation of epithelium. The amount of ACPI increases as the cells show malignant features; however, the amount is somewhat suppressed as the malignant process advances further.
The expression of ACPI was more often higher in squamous‐cell carcinomas than in adenocarcinomas or large‐cell carcinomas. The results are in line with earlier findings shown in squamous‐cell and adenocarcinomas from other sites.10,13,16 The results also corroborate previous findings of lung carcinomas,4 (however not confirming the total positivity of squamous‐cell carcinomas) found in the study.4 Similar findings have also been published later,28,29 although in these studies only the total concentration of ACPI was measured in the tissue by an ELISA immunoassay, thus not giving the specific data on cellular expression in malignant epithelial cells. In squamous‐cell carcinomas low expression of ACPI was correlated with poorer tumour differentiation, as demonstrated previously.13,20 This could also reflect the unbalanced expression of proteinase and proteinase‐inhibitor complex,21,29 although the precise mechanisms remain unexplained.
The staining of ACPI in tumour cells was found to be located heterogeneously, forming clustering‐like stained areas, and intensity of the staining varied around the tumour epithelium, as also reported previously in breast cancer.9 Abundant ACPI expression was noted to be concentrated in areas showing prominent keratinisation.4 This reflects the notion that ACPI is mainly found among the most differentiated cells of the epithelium.3,14 Especially in squamous‐cell carcinomas, positivity was also found in the peripherial layers of the epithelium. Similar findings have been reported in cervical carcinomas.20 The peripheral positivity could be explained by maturation of tumour cells in the immediate infiltrating edge of the tumour.20
The prognostic role of ACPI has been studied in many tumours.9,18,19,30,31 However, despite the basic nature of the ACPI as a probable tumour suppressor,15 its prognostic significance in clinical materials has remained unclear.9,17,18,31 One explanation for this discrepancy might be the different laboratory methods used, which give different data concerning the amount of ACPI in the tumour tissue.9,19 Usually, decreased levels of ACPI have been linked with more advanced disease17,18 and also with poor outcome.17,19 However, increased ACPI expression was shown to be correlated with unfavourable prognosis in breast cancer.9 Our findings are consistent with others17,19 showing that low ACPI expression is associated with an increased tumour recurrence and poor disease‐free survival. In previous in vitro studies of lung carcinomas, the prognostic significance of ACPI has remained negative.28 To date, any comparable clinicopathological study of lung cancer has not been performed.
Take‐home messages
Cystatins are members of a protein family with endogenous inhibitors of cysteine proteases.
Acid cysteine protease inhibitor (ACPI, cystatin A) is the first identified mammalian cystatin.
ACPI expression has previously been linked with neoplastic changes in squamous-cell epithelium.
Low ACPI expression in tumour cells is associated with tumour recurrence and unfavourable outcome in non-small-cell lung cancer (NSCLC).
ACPI does not have any independent prognostic value in NSCLC.
To summarise, we showed that the expression of ACPI is linked with malignant transformation of bronchial epithelium. In this material, expression is higher in squamous‐cell carcinomas and correlates with tumour differentiation. The reduced ACPI expression in tumour cells is related to an increased risk of tumour recurrence predicting poor patient survival. However, ACPI seems not to be an independent prognostic marker in NSCLC.
Acknowledgements
The study was financially support by the grants from the Finnish Cultural Foundation, North‐Savo Cancer Foundation, the EVO funding from the Kuopio University Hospital and Suominen Foundation, Salo, Finland. The skilful technical assistance of Ms Aija Parkkinen and Ms Helena Kemiläinen in immunohistochemical studies is gratefully acknowledged.
Abbreviations
ACPI - acid cysteine protease inhibitor
OS - overall survival
DSF - disease‐free survival
PBS - phosphate buffer solution
NSCLC - non‐small‐cell lung cancer
Footnotes
Competing interests: None.
References
- 1.Abrahamson M, Alvarez‐Fernandez M, Nathanson C M. Cystatins. Biochem Soc Symp 200370179–199. [DOI] [PubMed] [Google Scholar]
- 2.Kos J, Lah T T. Cysteine proteinases and their endogenous inhibitors: Target proteins for prognosis, diagnosis and therapy in cancer (review). Oncol Rep 199851349–1361. [DOI] [PubMed] [Google Scholar]
- 3.Järvinen M, Rinne A, Hopsu‐Havu V K. Human cystatins in normal and diseased tissues—a review. Acta Histochem 1987825–18. [DOI] [PubMed] [Google Scholar]
- 4.Rinne A. Epidermal SH‐protease inhibitor in human neoplasm and their metastases. Path Res Pract 1980170172–179. [DOI] [PubMed] [Google Scholar]
- 5.Järvinen M, Hopsu‐Havu V K. Alpha‐N‐benzoylarginine‐2‐naphthylamide hydrolase (cathepsin B1?) from rat skin. II. purification of the enzyme and demonstration of two inhibitors in the skin. Acta Chem Scand B 197529772–780. [DOI] [PubMed] [Google Scholar]
- 6.Rinne A, Järvinen M, Räsänen O. A protein reminiscent of the epidermal SH‐protease inhibitor occurs in squamous epithelia of man and rat. Acta Histochem 197863183–192. [DOI] [PubMed] [Google Scholar]
- 7.Kominami E, Bando Y, Wakamatsu N.et al Different tissue distributions of two types of thiol proteinase inhibitors from rat liver and epidermis. J Biochem (Tokyo) 1984961437–1442. [DOI] [PubMed] [Google Scholar]
- 8.Rinne A, Alavaikko M, Järvinen M.et al Demonstration of immunoreactive acid cysteine‐proteinase inhibitor in reticulum cells of lymph node germinal centres. Virchows Arch B Cell Pathol Incl Mol Pathol 198343121–126. [DOI] [PubMed] [Google Scholar]
- 9.Kuopio T, Kankaanranta A, Jalava P.et al Cysteine proteinase inhibitor cystatin A in breast cancer. Cancer Res 199858432–436. [PubMed] [Google Scholar]
- 10.Söderström K O, Laato M, Wu P.et al Expression of acid cysteine proteinase inhibitor (ACPI) in the normal human prostate, benign prostatic hyperplasia and adenocarcinoma. Int J Cancer 1995621–4. [DOI] [PubMed] [Google Scholar]
- 11.Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci 2002591503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinne A, Järvinen M, Räsänen O.et al Demonstration of an epidermal SH‐protease inhibitor in normal epithelium and in some human neoplasms—an immunological study (author's transl). Acta Histochem Suppl 198022325–329. [PubMed] [Google Scholar]
- 13.Rinne A, Järvinen M, Räsänen O.et al Acid and neutral cysteine proteinase inhibitor in normal uterine portio and in squamo‐epithelial metaplasia, dysplasias and infiltrative carcinoma of the uterine portio. Exp Pathol 19842667–70. [DOI] [PubMed] [Google Scholar]
- 14.Pöllänen R, Pyykkönen K, Järvinen M.et al Immunolocalization of cystatin A in condylomatous and dysplastic lesions of the human uterine cervix: Correlation with the presence and type of human papillomavirus infection. Int J Gynecol Pathol 199514217–222. [DOI] [PubMed] [Google Scholar]
- 15.Calkins C C, Sloane B F. Mammalian cysteine protease inhibitors: Biochemical properties and possible roles in tumor progression. Biol Chem Hoppe Seyler 199537671–80. [PubMed] [Google Scholar]
- 16.Mirtti T, Alanen K, Kallajoki M.et al Expression of cystatins, high molecular weight cytokeratin, and proliferation markers in prostatic adenocarcinoma and hyperplasia. Prostate 200354290–298. [DOI] [PubMed] [Google Scholar]
- 17.Strojan P, Budihna M, Smid L.et al Prognostic significance of cysteine proteinases cathepsins B and L and their endogenous inhibitors stefins A and B in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 200061052–1062. [PubMed] [Google Scholar]
- 18.Sinha A A, Quast B J, Wilson M J.et al Prediction of pelvic lymph node metastasis by the ratio of cathepsin B to stefin A in patients with prostate carcinoma. Cancer 2002943141–3149. [DOI] [PubMed] [Google Scholar]
- 19.Smid L, Strojan P, Budihna M.et al Prognostic value of cathepsins B, D and steffins A and B in laryngeal carcinoma. Eur Arch Otorhinolaryngol 1997254150–153. [DOI] [PubMed] [Google Scholar]
- 20.Eide T J, Järvinen M, Hopsu‐Havu V K, Maltau J, Rinne A. Immunolocalization of cystatin A in neoplastic, virus and inflammatory lesions of the uterine cervix. Acta Histochem 199293241–248. [DOI] [PubMed] [Google Scholar]
- 21.Heidtmann H H, Salge U, Abrahamson M.et al Cathepsin B and cysteine proteinase inhibitors in human lung cancer cell lines. Clin Exp Metastasis 199715368–381. [DOI] [PubMed] [Google Scholar]
- 22.Pirinen R, Leinonen T, Böhm J.et al Versican in non‐small cell lung cancer: Relation to hyaluronan, clinicopathologic factors, and prognosis. Hum Pathol 20053644–50. [DOI] [PubMed] [Google Scholar]
- 23.Pirinen R T, Hirvikoski P, Johansson R T.et al Reduced expression of alpha‐catenin, beta‐catenin, and gamma‐catenin is associated with high cell proliferative activity and poor differentiation in non‐small cell lung cancer. J Clin Pathol 200154391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumours of the lung In: Travis W, Brambilla E, Muller‐Hermelink H, Harris C, eds. Tumours of the lung, pleura, thymus and heart. Lyon: IARCPress, 20049–122.
- 25.Alakurtti K, Weber E, Rinne R.et al Loss of lysosomal association of cystatin B proteins representing progressive myoclonus epilepsy, EPM1, mutations. Eur J Hum Genet 200513208–215. [DOI] [PubMed] [Google Scholar]
- 26.Rinne A, Järvinen M, Dorn A.et al Low‐molecular cysteine protease inhibitors in the human palatine tonsil. Anat Anz 1986161215–230. [PubMed] [Google Scholar]
- 27.Zajc I, Sever N, Bervar A.et al Expression of cysteine peptidase cathepsin L and its inhibitors stefins A and B in relation to tumorigenicity of breast cancer cell lines. Cancer Lett 2002187185–190. [DOI] [PubMed] [Google Scholar]
- 28.Ebert E, Werle B, Julke B.et al Expression of cysteine protease inhibitors stefin A, stefin B, and cystatin C in human lung tumor tissue. Adv Exp Med Biol 1997421259–265. [DOI] [PubMed] [Google Scholar]
- 29.Krepela E, Prochazka J, Karova B.et al Cysteine proteases and cysteine protease inhibitors in non‐small cell lung cancer. Neoplasma 199845318–331. [PubMed] [Google Scholar]
- 30.Sinha A A, Quast B J, Wilson M J.et al Ratio of cathepsin B to stefin A identifies heterogeneity within gleason histologic scores for human prostate cancer. Prostate 200148274–284. [DOI] [PubMed] [Google Scholar]
- 31.Lah T T, Kokalj‐Kunovar M, Drobnic‐Kosorok M.et al Cystatins and cathepsins in breast carcinoma. Biol Chem Hoppe Seyler 1992373595–604. [DOI] [PubMed] [Google Scholar]