Abstract
Aim
To validate tissue microarray (TMA) for endometrial cancer by comparing immunohistochemical staining results of triplicate core biopsies on TMA with the results of full‐section analysis.
Methods
The study material consisted of slides and selected tissue blocks of 41 patients with endometrioid cancer of the endometrium. A TMA was constructed. Both the TMA and the slides were stained with the same antibodies against progesterone receptor (PR), oestrogen receptor, p53 and epithelial membrane antigen (EMA). Concordance between results was expressed as the κ statistic.
Results
Concordance between the staining results of TMA and whole slides was good for PR (κ = 0.69), oestrogen receptor (κ = 0.78), p53 (κ = 0.81) and EMA (κ = 0.72). Concordance between the results on TMA and slides depends on the number of assessable cores per tumour. Three assessable cores per case result in outcomes that are at least 94% similar to those achieved using conventional tissue sections with a two‐class scoring system. This is independent of focal or diffuse staining patterns.
Conclusion
TMA is a useful tool for further analysis of the molecular pathways in endometrial cancer. The effect of selection has to be taken into account when the prognostic value of protein expression on TMA is determined.
The recent development of tissue microarray (TMA) technology has given rise to large‐scale retrospective studies using archival formalin‐fixed, paraffin‐wax‐embedded tissue.1 Currently, no data exist on validation of TMA in endometrial cancer. Because of the small size of tissue cores (0.6 mm) taken from paraffin wax‐embedded tumour specimens, focal expression patterns of investigated proteins could lead to significant differences in results between full‐section and TMA immunohistochemical analysis. It seems that this error could be reduced by using multiple tissue cores per specimen.2
In an effort to validate the TMA technique for endometrial cancer, we conducted a study to define the concordance between expression patterns in triplicate 0.6 mm core biopsies on TMAs in comparison to full‐section analysis. In all, 41 endometrial cancer specimens were arrayed. For this study, antigens with known focal expression (oestrogen receptor, progesterone receptor (PR) and p53), and also one with a known diffuse expression (epithelial membrane antigen (EMA)) were chosen. Readings of full sections have been compared with the results of three independent core biopsies from the same tissue block on TMA.
Materials and methods
The study material consisted of slides and selected tissue blocks from 41 patients with endometrioid cancer of the endometrium treated at the Academic Medical Centre, Amsterdam, The Netherlands. All histological specimens were reviewed by two of us (FWJ, GF) for histological type and grade.
Of each tumour, one representative H&E‐stained slide was selected. Ten slides were cut from the selected blocks. Three representative areas of interest with atypical infiltrative glands were encircled on each slide. In the corresponding paraffin block, 0.6 mm cores were punched out. These cores, each 3–4 mm high, were then embedded in the donor block using a manually operated TMA device (Beecher Instruments, Silver Springs, Maryland, USA). The spacing between the cores was 1 mm. The recipient block was cut into 4 μm thick sections and transferred to glass slides. The array consisted of 123 cores. Cores of liver, spleen and normal endometrium were used as controls. The avidin–biotin method was used for immunostaining. The unstained sections of the TMA and the slides were deparaffinised with xylol, and rehydrated through a series of graded alcohols. One section of the array and one slide were stained with H&E. Other sections of TMA and slides were stained with a panel of antibodies against PR (Dako, Pgr 636, California, USA), oestrogen receptor (Dako, 1D5), p53 (NeoMarkers, DO‐7+BP53‐12, Fremont, CA, USA) and EMA (Dako, E29).
The staining results were scored jointly by two observers (FWK, GF). Oestrogen receptor and PR staining was evaluated in two different ways. Firstly, the percentage of positive cells was determined on both the cores and the slides. The proportion of positive cells on slides and on TMA were then compared. Secondly, the mean percentage of positive cells of the cores and the percentage of positive cells of the slides were classified into two categories, positive (⩾10% of cells positive) and negative (<10% of cells positive). Comparison of slide results and TMA was then repeated.
The p53 and EMA staining of the tumour cells were marked as negative (<10% of cells showing staining), weakly positive (10–50% showing moderately intense staining) or strongly positive (>50% of cells showing moderately intense or 10–50% of cells showing very intense staining) on both slides and TMA.
Results of cores of each tumour were combined to give a tumour score. If the scores of the three cores from one tumour differed, the one that occurred most often determined the final score. If two of three cores were not assessable, the result was excluded.
Statistical analysis
For statistical analysis, scores were dichotomised. Weakly positive and negative became negative while strongly positive became positive.
The κ statistic was used to measure agreement between the scores on the TMA and scores on the slides. Agreement was considered poor if κ <0.2, slight if 0.21< κ <0.4, fair if 0.41< κ <0.6, good if 0.61< κ <0.8 and very good 0.81< κ <0.92.3 Calculations were performed with SPSS V.11. 5.
Results
Of the 123 tumour cores, 111 (90%) were assessable for oestrogen receptor and PR, 113 (92%) for p53 and 112 (91%) for EMA. Non‐assessable cores were either lost during processing or contained <10% tumour cells. For oestrogen receptor, four cases were lost. For PR, p53 and EMA, these numbers were four, five and three, respectively. On the oestrogen receptor TMA, eight cores contained <10% tumour. For PR, p53 and EMA these numbers were eight, five and eight, respectively. Cases were considered non‐assessable if two of three cores were lost. The rates of lost cases were 7% (3/41), 2% (1/41), 5% (2/41) and 2% (1/41) for the oestrogen receptor, PR, p53 and EMA arrays, respectively. Assessment was based on two cores in 15% (6/41), 24% (10/41), 15% (6/41) and 22% (9/41) of the cases on oestrogen receptor, PR, p53 and EMA TMA, respectively (table 1).
Table 1 Assessable cores.
| Marker | One core* | %† | Two cores‡ | %† | Three cores§ | %† | Total assessable | % |
|---|---|---|---|---|---|---|---|---|
| ER | 3 | 7 | 6 | 15 | 32 | 78 | 38 | 90 |
| PR | 1 | 2 | 10 | 24 | 30 | 73 | 40 | 98 |
| p53 | 2 | 5 | 6 | 15 | 33 | 81 | 39 | 95 |
| EMA | 1 | 2 | 9 | 22 | 31 | 76 | 40 | 98 |
EMA, epithelial membrane antigen; OR, oestrogen receptor; PR, progesterone receptor.
*Number of cases with one assessable core.
†n = 41 patients.
‡Number of cases with two assessable cores.
§Number of cases with three assessable cores.
In comparing the mean proportion of oestrogen receptor‐positive cells of the cores with the proportion of oestrogen receptor‐positive cells on slides, complete concordance was found in 47% of the cases. In 69%, the results differed by ⩽10%.
Of the 38 (68%) assessable cases, 26 (68%) were considered to show positive oestrogen receptor staining on slides and 22 (58%) cases showed positive staining on TMA (table 2, fig 1). In four cases, oestrogen receptor staining was positive on slides and negative on cores. None showed positive staining on the cores and negative staining on the slide. Considering the categories of positive or negative staining, the overall non‐concordance was 10% (4/38). The two methods correspond well with a κ of 0.78 (table 3).
Table 2 Oestrogen receptor scores on slides and tissue microarray.
| Oestrogen receptor slides | Oestrogen receptor scores | Total | |
|---|---|---|---|
| 0 to <10% | 1 to ⩾10% | ||
| 0<10% | 12 | 0 | 12 |
| 1⩾10% | 4 | 22 | 26 |
| Total | 16 | 22 | 38 |
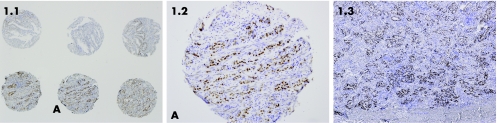
Figure 1 Oestrogen receptor expression on cores and slide. (A) Three cores at the bottom represent one patient. Oestrogen receptor staining is strong positive. (B) Core A enlarged. (C) Part of the representative slide of the same patient with strong positive oestrogen receptor staining.
Table 3 Concordance between tissue microarray and slides.
| OR | PR | p53 | EMA | ||
|---|---|---|---|---|---|
| Three class scoring system | |||||
| Concordance | 92% | 95% | |||
| κ | 0.81 | 0.72 | |||
| Two class scoring system | |||||
| Concordance | 90% | 92% | 100% | 95% | |
| κ | 0.78 | 0.69 | 1 | 0.72 | |
EMA, epithelial membrane antigen; OR, oestrogen receptor; PR, progesterone receptor.
Disagreement between scoring results on cores and slides was more common for two‐core analysis than for three‐core analysis. A mismatch was noticed in 1 of 6 (17%) cases with two assessable cores compared with 3 of 32 (9%) cases with three assessable cores.
For the PR TMA, complete concordance was found in 40% of cases. In 65%, results differed by 10% at most. In total, 35 of 40 (87%) assessable cases showed positive staining (⩾10% cells positive) on slides. In all, 34 (85%) were positive on TMA (table 4).
Table 4 Progesterone receptor scores on slides and tissue microarray.
| PR slides | PR cores | Total | |
|---|---|---|---|
| 0 to <10% | 1 to ⩾10% | ||
| 0 <10% | 4 | 1 | 5 |
| 1 ⩾10% | 2 | 33 | 35 |
| Total | 6 | 34 | 40 |
PR, progesterone receptor.
In two cases, staining on slides was positive, but was negative on cores. In one case, it was the other way around, resulting in a non‐concordance of 8% (3/40).
The two methods corresponded well, with a κ of 0.69.
In the PR analysis, non‐concordance between cores and slides was also more common for two‐core analysis than for three‐core analysis. Mismatches were found in 2 of 10 (20%) cases with two assessable cores whereas only one was found in 1 of the 30 (3%) cases with three assessable cores (table 3).
In all, 38 cases on TMA were assessable for p53 staining. Two cases could not be analysed because of tissue loss. In one case the slide could not be evaluated. Both on slides and on TMA, five cases showed strong (defined as >50% of cells) staining (table 5).
Table 5 p53 scores on slides and tissue microarray.
| p53 slides | p53 cores | Total | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| <10% | 10–50% | >50% | ||
| 0 | 27 | 0 | 0 | 27 |
| <10% | ||||
| 1 | 3 | 3 | 0 | 6 |
| 10–50% | ||||
| 2 | 0 | 0 | 5 | 5 |
| >50% | ||||
| Total | 30 | 3 | 5 | 38 |
Of 38 assessable cases, 35 (92%) showed complete concordance (fig 2). In three non‐concordant cases, the difference was one class. Both methods showed a high level of correspondence, with a κ of 0.81. If only negative or positive expression is taken into account (defined as <50% or >50% of cells positive), concordance between slides and TMA is 100%, and κ is 1.0. Five cases (5/38 13%) showed overexpression both on slides and TMA.
Figure 2 p53 expression on cores and on slide. (A) Three cores at the bottom represent one patient. p53 staining is negative. (B) Core A enlarged. (C) Part of the representative slide of the same patient with negative p53 staining.
For p53 staining, in contrast to oestrogen receptor and PR, the three‐core analysis did not show a higher level of correspondence than the two‐core analysis. In seven cases with a two‐core analysis no mismatch occurred, compared with three mismatches in 32 cases with a three‐core analysis (table 3).
In all, 40 cases were assessable on the EMA TMA. One case could not be analysed because of tissue loss. In one case, the slide was non‐assessable. In total, 36 out of 39 (92%) assessable cases were strong positive on slides (defined as >50% of cells positive, table 6)
Table 6 Epithelial membrane antigen scores on slides and tissue microarray.
| EMA slides | EMA cores | Total | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| <10% | 10–50% | >50% | ||
| 0 | 0 | 0 | 0 | 0 |
| <10% | ||||
| 1 | 0 | 3 | 0 | 3 |
| 10–50% | ||||
| 2 | 0 | 2 | 34 | 36 |
| >50% | ||||
| Total | 0 | 5 | 34 | 39 |
EMA, epithelial membrane antigen.
Two of those were weakly positive on TMA. Three cases were weakly positive both on slides and on TMA. In all, 37 of 39 (95%) assessable cases showed complete concordance. In the two non‐concordant cases, the difference was one class. Concordance between scores on TMA and slides was good, with a κ of 0.72. Because none of the cases showed absent expression of EMA, κ was also 0.72 after dichotomisation of the results. Concordance varied with the number of analysed cores. In nine cases only two cores were available, showing a concordance of 79% (7/9). In the other 30 cases, three cores were available, giving 100% concordance (30/30, table 3).
Discussion
Tissue microarray is a useful tool for rapid and efficient analysis of large numbers of paraffin‐wax‐embedded tissue. Tissue cores of 0.6 mm are punched out from the original blocks and arrayed in a recipient paraffin‐block. A major concern of this rather new technique is whether the heterogeneity of tumours will affect the validity of the results. Studies on gastric, bladder and breast cancers show that findings from routine sections can be generally reproduced in TMA.2,4,5
As endorsed in this study, concordance between results on TMA and slides depends on the number of cores per tumour on the TMA and also on the scoring system, the composition of the tumour and the protein tested.
Concordance improves when more cores are used. Camp's study on breast cancer shows that for oestrogen receptor and PR staining on TMA, one or two cores per case result in outcomes on TMA that are 95% similar to those achieved using slides.2 This rises to 98% with three assessable cores per case. It is likely that the influence of the number of cores on concordance depends on the expression pattern of the antigen. If an antigen is focally expressed, as with oestrogen receptor, PR and p53, concordance between slides and TMA will enhance when more cores are taken into account. If an antigen is diffusely expressed, as with EMA, the number of cores will probably not affect the outcome to a great extent. Both for oestrogen receptor and PR, the number of cores had a marked effect on the outcome. Oestrogen receptor staining corresponded in 91% of cases in the three‐core analysis, dropping to 83% in the two‐core analysis. For the PR staining, these percentages were 97% and 80%. This difference did not exist in the p53 analysis. Here the two‐core analysis showed 100% concordance compared with 94% in the three‐core analysis, which cannot be explained. For EMA, concordance was affected by the number of assessed cores in spite of the diffuse expression pattern. EMA staining results corresponded in 100% (30/30) of the cases in the three‐core analysis, dropping to 78% (7/9) in the two‐core analysis. Concordance between scoring results on TMA and slides improved when more cores are used. This applied to both the diffuse and the focal expression pattern.
Concordance between TMA and slides improved with fewer scoring categories. The validation studies on fibrosarcoma and stomach cancer showed a very high correlation between p53 staining results on both TMA and slides.6 In the study on fibrosarcoma, κ was 0.88 and in stomach cancer it was 0.94. In both studies, a classification system based on two categories was used. In our study, κ was 0.81 in a three‐class system and 1.0 in a two‐class system.
In our series, seven cases showed a discordant oestrogen receptor or PR expression pattern on TMA and slide. In six cases, the slides were positive and the matching cores on the TMA were negative. This is partly due to tumour‐related factors. Endometrioid adenocarcinomas of the endometrium can display various types of cellular differentiation. In a study by Lax et al,7 77 uterine endometrioid carcinomas were analysed. They concluded that 43 were pure endometrioid and 34 displayed additional types of cellular differentiation in at least 10% of the tumour. PR and oestrogen receptor expressions were higher in the glandular component compared with the other components. In those cases positive for p53, expression was in general limited to the glandular component. Although all the different components of the endometrioid cancers were not classified in this study, these were noticed while reviewing the slides. Because representative glands are selected for the construction of TMA, the steroid receptor expression measured on TMA might be more reliable than the one on the slides. On the whole slide, the ratio between atypical glands and other tissues and the protein expression in the different components determines the final outcome. On TMA, the expression of the glandular cells is measured exclusively because the selection of glands is made before the cores are punched out.
Expression of steroid receptors within endometrioid cancer glands is also heterogeneous,8,9 contributing to differences in staining results between slides and TMA and between cores of the same tumour.
Three cases showed discordant p53 expression on TMA compared with slides. None of these were strongly positive either on slide or on TMA. As only strong p53 expression is relevant, this discordance is of no importance.
For EMA, two cases were discordant. They both showed a weaker expression on TMA than on slides. It is known that EMA immunoreactivity is stronger in neoplastic endometrium than in hyperplastic or normal endometrium.10 Apart from that, overexpression of EMA depends on the histological subtype of endometrial cancer.11 Other subtypes such as serous–papillary, clear cell and adenosquamous endometrial cancer show significantly less overexpression than the endometrioid type. Because nearly 50% of the tumours contains different subtypes, it is plausible that selection of atypical glands affects the outcome when EMA expression on TMA and slides is compared.
Summarising this study, it can be said that the comparison of analysis results on the expression of steroid receptors, p53 and EMA on TMA and slides is convincing. Three cores per case result in outcomes that are at least 94% similar to those achieved using conventional tissue sections in a two‐class scoring system. Selection of areas on H&E‐stained full sections based on tumour morphology is important for the final result. The expression pattern on TMA might be more representative for the glandular part of the endometrioid cancer than that on the whole slide. The effect of selection has to be taken into account when the prognostic value of protein expression on TMA is determined.
As concordance between TMA and slide scoring results depends on both tumour‐related and antigen‐related factors, validation of this technique for each separate antigen on each different tumour is essential for future reliable use.
Take‐home messages
The comparison of analysis results on the expression of steroid receptors, p53 and EMA on TMA and slides is convincing.
Selection of areas on H&E‐stained full sections based on tumour morphology is important for the final result.
As concordance between TMA and slide scoring results depends on both tumour-related and antigen-related factors, validation of this technique for each separate antigen on each different tumour type is essential for future reliable use.
Abbreviations
EMA - epithelial membrane antigen
PR - progesterone receptor
TMA - tissue microarray
Footnotes
Competing interests: None declared.
References
- 1.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch M J, Sauter G, Kallioniemi O P. Tissue microarrays for high‐throughput molecular profiling of tumor specimens. Nat Med 19984844–847. [DOI] [PubMed] [Google Scholar]
- 2.Camp R L, Charette L A, Rimm D L. Validation of tissue microarray technology in breast carcinoma. Lab Invest 2000801943–1949. [DOI] [PubMed] [Google Scholar]
- 3.Dawson B, Trapp R G.Basic and Clinical Biostatistics. New York: Lange Medical Books/McGraw‐Hill, 2004
- 4.Gulmann C, Butler D, Kay E, Grace A, Leader M. Biopsy of a biopsy: validation of immunoprofiling in gastric cancer biopsy tissue microarrays. Histopathology 20034270–76. [DOI] [PubMed] [Google Scholar]
- 5.Nocito A, Bubendorf L, Maria T E, Suess K, Wagner U, Forster T, Kononen J, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knonagel H, Rist M, Anabitarte M, Hering F, Hardmeier T, Schoenenberger A G, Flury R, Jager P, Luc F J, Schraml P, Moch H, Mihatsch M J, Gasser T, Sauter G. Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol 2001194349–357. [DOI] [PubMed] [Google Scholar]
- 6.Hoos A, Urist M J, Stojadinovic A, Mastorides S, Dudas M E, Leung D H, Kuo D, Brennan M F, Lewis J J, Cordon‐Cardo C. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 20011581245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lax S F, Pizer E S, Ronnett B M, Kurman R J. Comparison of estrogen and progesterone receptor, Ki‐67, and p53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum Pathol 199829924–931. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron C, Ferenczy A, Toft D O, Shyamala G. Immunocytochemical study of progesterone receptors in hyperplastic and neoplastic endometrial tissues. Cancer Res 1988486132–6136. [PubMed] [Google Scholar]
- 9.Zaino R J, Clarke C L, Mortel R, Satyaswaroop P G. Heterogeneity of progesterone receptor distribution in human endometrial adenocarcinoma. Cancer Res 1988481889–1895. [PubMed] [Google Scholar]
- 10.Morse A R, Curran G J. Distribution of epithelial membrane antigen in normal and abnormal endometrial tissue. Br J Obstet Gynaecol 1985921286–1290. [DOI] [PubMed] [Google Scholar]
- 11.Coronado P J, Fasero M, Vidart J A, Puerta J, Magrina J, Furio‐Bacete V, Escudero M. A comparison of epithelial membrane antigen overexpression in benign and malignant endometrium. Gynecol Oncol 200182483–488. [DOI] [PubMed] [Google Scholar]