Abstract
Aim
To assess the biological significance of vascular endothelial growth factor (VEGF) A, VEGF receptor (Flk‐1) and cyclooxygenase 2 (COX2) expression with respect to microvessel density (MVD), proliferative activity (Ki‐67), expression of p53 and clinical presentation in a large cohort of nodal B cell lymphomas.
Methods
An immunohistochemical and morphometric study was performed on a validated tissue microarray containing 271 B cell lymphoma specimens, 197 of which included follow‐up data. Statistical assessment was done by Pearson's χ2 test, Spearman's rank correlation coefficient, analysis of variance and survival analysis.
Results
266 (98%) cases were evaluable. Strong VEGF expression was observed in only 20 diffuse large B cell lymphomas (DLBCLs). Flk‐1 and COX2 were expressed in 53 and 21 cases, respectively, mainly in DLBCLs, follicular lymphoma (FL) grade 3 and mantle cell lymphomas (MCLs), in a low proportion of cells. MVD decreased in the following order: DLBCLs, FLs, MCLs and small lymphocytic lymphomas/chronic lymphocytic leukaemia (SLL/CLLs). VEGF expression correlated with Ki‐67, p53 and COX2 expression in the whole cohort and in DLBCLs. Flk‐1 expression correlated with Ki‐67 in the cohort and in SLL/CLL and FL grade 1 and 2. COX2 expression correlated with Ki‐67 and p53. The analysed angiogenesis parameters did not correlate with clinical parameters or survival.
Conclusions
Angiogenesis plays a differential role in various B cell lymphomas. Aggressive lymphomas express the potential molecular therapeutic targets VEGF and COX2, and have higher MVD. In a few low proliferation‐fraction lymphomas, Flk‐1 might have a role in proliferative advantage. Therapeutic strategies aimed at angiogenesis should take into account lymphoma heterogeneity.
Neoplastic growth and progression in solid and haematological malignancies is associated with the formation of new blood vessels, known as tumour angiogenesis.1,2,3,4,5,6 Vascular endothelial growth factor (VEGF) A is one of the most important mediators of angiogenesis, and VEGF expression is stimulated by intratumoral hypoxia, which, in turn, depends on the proliferative activity of the tumour.1 VEGF binds to its receptors Flk‐1 and Flt‐1 with tyrosine kinase activity to induce endothelial cell proliferation (Flk‐1) and further capillary tube formation and monocyte migration (Flt‐1).1 The inducible enzyme cyclooxygenase 2 (COX2) is an additional important mediator of both angiogenesis and tumour growth,7 and one of the downstream actions of its prostaglandin substrates is VEGF production and release.8
Several studies have shown that serum angiogenic factor elevations (eg, VEGF, endostatin), VEGF expression and increased microvessel density (MVD) are predictive of poor prognosis and associated with higher tumour grade or high‐grade transformation in non‐Hodgkin's lymphomas.3,4,9,10,11,12,13,14,15 Other studies have failed to confirm these results.4,5,16,17,18 Decreased MVD may also predict resistance to chemotherapy in selected patients with non‐Hodgkin's lymphoma.19 Recently, COX2 was shown to be overexpressed in human B cell lymphomas and in lymphoma cell lines, and its inhibition induced apoptosis in vitro.20,21,22 Thus, the appraisal of angiogenesis in B cell lymphomas could be important for potential antiangiogenic treatment strategies such as application of bevacizumab, thalidomide, celecoxib or anti‐Flt‐1 antibodies.21,23,24,25,26,27 The interplay between lymphoma cells and tumour vessels is more complex, particularly in light of the maturity of vessels and the distinct angiogenic factor sources.15,28 Furthermore, lymphoma‐specific chromosomal aberrations such as t(8;14), t(11;14) and t(14;18) were discovered in tumour endothelial cells, delineating the histogenesis of B cell lymphoma vasculature.29
Several in situ studies have been performed on small paraffin‐embedded B cell lymphoma series to assess MVD and expression of VEGF, Flk‐1 and COX2, with somewhat controversial results.4,12,14,16,18,19,20,21,22,28,30,31,32,33,34 Therefore, we aimed to assess further the biological significance of VEGF, Flk‐1 and COX2 expression with respect to MVD, proliferative activity (Ki‐67), expression of p53 and clinical presentation in a large cohort of common nodal B cell lymphomas.35 We performed an immunohistochemical and morphometric study on a previously validated tissue microarray (TMA) containing 271 B cell lymphoma specimens.36
Materials and methods
Patients
A total of 271 formalin‐fixed, paraffin‐embedded nodal B cell lymphoma tissue samples from the archive of the Institute of Pathology at the Medical University of Innsbruck (Innsbruck, Austria), diagnosed between 1988 and 2000, were included in this study (table 1). Paraffin blocks were selected on the basis of preservation. All slides were reviewed and re‐classified according to the World Health Organization criteria.37 In 197 cases, follow‐up data and bone marrow involvement status at diagnosis were known: 80 patients died due to relapsed or primary resistant disease or histologically detectable progressive lymphoma (disease‐related deaths), 14 died due to cardiovascular events, and 5 due to trauma or infections (table 1); 98 patients were alive as of June 2005.
Table 1 Patient characteristics.
| Lymphoma subtype | Total number | Male | Female | Bone marrow involvement | Disease‐related deaths | Median survival (months) | Mean survival (months) | Mean follow‐up (range) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean age | n | Mean age | |||||||
| De novo DLBCL | 111 | 56 | 64 | 45 | 66 | 24/94 (25%) | 42/96 (44%) | 26 | 63 | 41 (1–177) |
| Secondary DLBCL | 18 | 11 | 59 | 7 | 69 | 9/17 (53%) | 11/16 (69%) | 19 | 51 | 44 (3–130) |
| MCL | 19 | 14 | 59 | 5 | 66 | 8/12 (67%) | 7/9 (78%) | 20 | 35 | 26 (4–107) |
| FL G3 | 15 | 5 | 52 | 10 | 57 | 2/8 (25%) | 1/9 (10%) | Not reached | 55 | 49 (2–105) |
| FL G1&2 | 70 | 32 | 57 | 38 | 57 | 26/51 (51%) | 11/47 (23%) | Not reached | 94 | 57 (8–129) |
| SLL/CLL | 38 | 28 | 62 | 10 | 71 | 21/22 (96%) | 9/20 (45%) | 82 | 76 | 68 (2–132) |
DLBCL, diffuse large B cell lymphoma; FL G, follicular lymphoma grade; MCL, mantle cell lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukaemia.
Construction of TMA
TMA was constructed as described previously.36 Each donor tissue block was punched twice.
Morphological analysis
Sections of TMA blocks of thickness 4 μm were transferred to an adhesive‐coated glass slide system (Instrumedics, Hackensack, New Jersey, USA) and stained with H&E, Giemsa stain and with the periodic acid Schiff reagent. Only cases containing unequivocal B cell lymphoma, as assessed by H&E morphology in combination with immunohistochemistry, were analysed. To avoid any bias due to morphological heterogeneity of follicular lymphomas (FLs), only cases with unequivocal CD10 or BCL6 and BCL2‐positive follicles were evaluated.36
Immunohistochemistry
Bound secondary antibodies were visualised by the standard avidin–biotin‐peroxidase technique using diaminobenzidine as chromophore. Table 2 shows the primary antibodies applied in this study, their dilutions and pretreatment conditions.
Table 2 Antibodies and antigen‐retrieval techniques applied.
| Antibody | Dilution | Retrieval | Source |
|---|---|---|---|
| CD3 | 1:50 | Pressure cooker, aqua destillata, 121°C, 5′ | Dako, Glostrup, Denmark |
| CD20 | 1:700 | Microwave oven, citrate buffer pH 6, 800 W, 10′ | Dako, Glostrup, Denmark |
| CD34 | 1:25 | Pressure cooker, aqua destillata, 121°C, 5′ | Dako, Glostrup, Denmark |
| CD79a | 1:1600 | Pressure cooker, aqua destillata, 121°C, 5′ | NeoMarkers, Fremont, California, USA |
| COX2 | 1:100 | Pressure cooker, target retrieval solution S 2368, 121°C, 5′ | Dako Corporation, Carpinteria, California, USA |
| Flk‐1 | 1:50 | Microwave oven, citrate buffer pH 6, 800 W, 15′ | NeoMarkers, Fremont, California, USA |
| Ki‐67 | 1:50 | Pressure cooker, aqua destillata, 121°C, 5′ | Dako, Carpinteria, California, USA |
| p53 (PAb240) | 1:50 | Pressure cooker, aqua destillata, 121°C, 5′ | Dako, Copenhagen, Denmark |
| VEGF A | 1:50 | Microwave oven, EDTA buffer pH 8, 800 W, 20′ | NeoMarkers, Fremont, California, USA |
COX2, cyclooxygenase 2; VEGF, vascular endothelial growth factor.
For positive controls, normal tonsils, breast carcinoma and angiosarcoma samples were used. Except for VEGF, all markers were quantified as the mean proportion of positively stained tumour cells from the total tumour cells of both TMA cores. Three antiVEGF antibodies, two monoclonal and one polyclonal, from different manufacturers (clone VG1, Dako, Milan, Italy; clone VG1, NeoMarkers, Fremont, California, USA; and sc‐152, Santa Cruz Biotechnology, Santa Cruz, California, USA) were independently tested and compared. Staining specificity of the antiVEGF antibody sc‐152 was examined in an additional preabsorption experiment by applying the blocking peptide sc‐152P, as suggested by the manufacturer. Considering the expression of VEGF in positive control tissue and the B cell lymphoma TMA, only the monoclonal VEGF antibodies (clone VG1)38 worked reliably in our hands. VEGF staining was semiquantified, taking into consideration the intensity and proportion of positive cells. In weakly staining cases, the cases expressing VEGF in 1–10% of the tumour cells were considered score 1 positive, cases expressing VEGF in 11–49% of the tumour cells were considered score 2 positive, and those expressing VEGF in ⩾50% of the tumour cells were considered score 3 positive; analogously, strongly staining cases were considered score 4, 5 and 6 positive—that is, cases expressing VEGF strongly in 1–10% of the tumour cells were considered score 4 positive, cases expressing VEGF in 11–49% of the tumour cells were considered score 5 positive and those expressing VEGF in ⩾50% of the tumour cell were considered score 6 positive. VEGF in reactive background macrophages was also considered. For detection of p53, we used the monoclonal antibody PAb240, recognising an epitope mapped to amino acids 213–217 of the protein, which is inaccessible to binding for the antibody in the wild‐type or native configuration of p53, but becomes exposed on mutant forms of p53.39
Microvessel density
MVD was assessed, analogous to our previous report,40 by counting the number of CD34‐positive vascular lumina in the two TMA cores of each case, resulting in an assessed area of 0.57 mm2. In cases where only one core could be evaluated, results were extrapolated. In five selected cases of FL grade 1 and 2 (FL G1&2) and G3, and in five cases of reactive follicular hyperplasia, conventional tissue sections were stained for CD34 to count microvessels in 0.5 mm2 of unequivocal follicular and perifollicular tissue, using ×200 magnification.
Statistical analysis
Statistical analysis, including descriptive methods, was performed using the SPSS software V.10.0 for Windows. The Pearson χ2 test and the Spearman rank correlation coefficient were applied where appropriate to demonstrate correlations. Analysis of variance was used to compare means. Survival analysis (overall and disease‐specific survival, as well as freedom from treatment failures) was performed using the Kaplan–Meier method and compared by the log rank test. For continuous variables such as age, the Cox regression model was applied for survival analysis. p Values <0.05 were considered significant.
Results
Histopathology, B and T cell markers
Of the 271 arrayed cases, 266 (98%) were evaluable. Except for three diffuse large B cell lymphomas (DLBCLs) and one small lymphocytic lymphoma/chronic lymphocytic leukaemia (SLL/CLL) that were negative for CD20 but positive for CD79, all cases expressed CD20 (data not shown). B cell lymphoma populations were variably infiltrated by CD3‐positive reactive T cells (mean (standard deviation) fraction 9 (9.5)%). Although FLs had higher T cell fractions, differences in T cell amounts between the diagnostic groups were insignificant.
Immunohistochemistry: VEGF, Flk‐1, COX2, MVD, Ki‐67 and p53
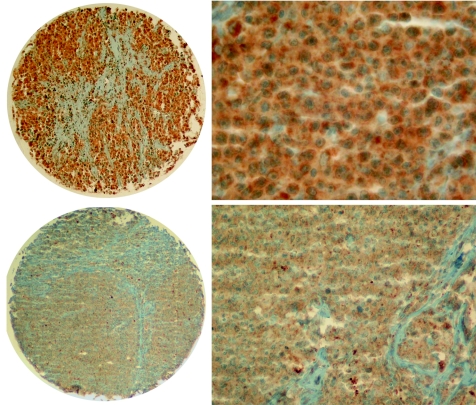
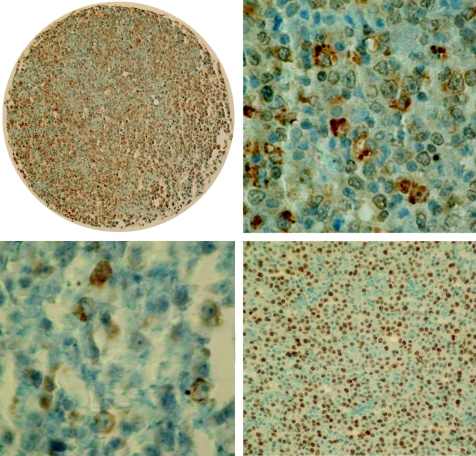
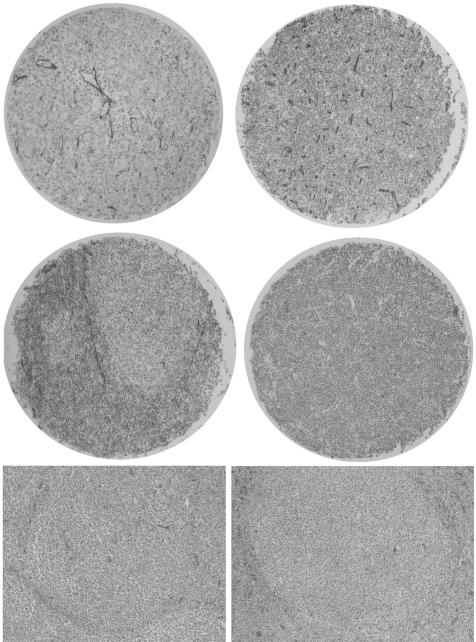
Quantitative staining results are shown in table 3. We found clear differences in VEGF, Flk‐1, COX2, Ki‐67 and p53 expression and MVD between the analysed B cell lymphomas, with various staining patterns and proportions of stained cells between the diagnostic groups. The staining for VEGF was very weak to weak, diffuse cytoplasmic in 164 (62%) cases, and strong only in 20 (8%, all DLBCLs) cases; in 82 (30%) cases we detected no stainable VEGF (fig 1). VEGF expression in intratumoral histiocytes did not significantly differ between the studied B cell lymphoma entities. All 53 (20%) cases, mainly DLBCLs, FL G3 and mantle cell lymphomas (MCLs), expressed Flk‐1 in a low proportion of cells (range 1–30%). The staining pattern for Flk‐1 was, as reported,41 moderate to strong granular cytoplasmic (fig 2, upper panel). COX2 expression was observed in 21 (8%) cases, mainly in DLBCLs, in a small proportion of cells (range 1–10%). COX2 staining pattern was moderate to strong granular cytoplasmic (fig 2, lower left). As expected, DLBCLs showed the highest proliferative activity (Ki‐67) and the highest expression of p53 (fig 2, lower right), the latter particularly pronounced in secondary DLBCLs (evolving from SLL/CLLs, FLs or marginal zone lymphomas). Both markers showed strong nuclear signals. All cases contained stainable microvessels. The staining for CD34 was strong, membranous and cytoplasmic, confined to tumour blood vessel endothelia (fig 3).
Table 3 Microvessel density, vascular endothelial growth factor, Flk‐1, cyclooxygenase 2, Ki‐67 and p53 expression in B cell lymphomas.
| Lymphoma subtype | n Evaluable | Mean (SD) microvessel count/0.57 mm2 | VEGF | Mean (SD) % expression in tumour cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean score | Number of cases with different scores | |||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | Flk‐1 | COX2 | Ki‐67 | p53 | ||||
| Primary DLBCL | 109 | 31 (17) | 1.8 | 22 | 36 | 29 | 4 | 12 | 5 | 1 | 4 (9) | 1.4 (4) | 45 (22) | 8 (19) |
| Secondary DLBCL | 18 | 29 (19) | 1.8 | 5* | 2 | 8 | 1 | 1 | 1 | 0 | 4 (10) | 0.6 (1.6) | 35 (24) | 12 (27) |
| MCL | 19 | 19 (7) | 1.2 | 5 | 8 | 4 | 2 | 0 | 0 | 0 | 1.5 (2) | 0.5 (2) | 17 (12) | 1.3 (5) |
| FL G3 | 15 | 22 (11) | 0.7 | 8 | 4 | 3 | 0 | 0 | 0 | 0 | 1.3 (2.3) | 0.0 (0.0) | 18 (10) | 1.2 (2) |
| FL G1&2 | 67 | 22 (8) | 0.9 | 31 | 20 | 16 | 0 | 0 | 0 | 0 | 0.6 (2) | 0.07 (0.6) | 8 (5) | 0.06 (0.3) |
| SLL/CLL | 38 | 19 (12) | 1.1 | 11 | 12 | 14 | 1 | 0 | 0 | 0 | 0.9 (3) | 0.0 (0.0) | 9 (13) | 0.5 (3) |
| Differences between diagnosis groups | p<0.001 | p<0.001 | p = 0.017 | p = 0.013 | p<0.001 | p = 0.01 | ||||||||
COX2, cyclooxygenase 2; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; FL G1&2, FL grade 1&2; MCL, mantle cell lymphoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukaemia; VEGF, vascular endothelial growth factor.
*To simplify statistical assessments, variables ⩾2 were rounded up.
Figure 1 Upper: strong vascular endothelial growth factor (VEGF) expression in diffuse large B cell lymphoma—overview (left, ×40) and detail (right, ×200). Lower: VEGF expression in mantle cell lymphoma—overview (left, ×40) and detail (right, ×100).
Figure 2 Upper: Flk‐1 expression in diffuse large B cell lymphoma—overview (left, ×40) and detail (right, ×400). Lower left: cyclooxygenase 2 expression in diffuse large B cell lymphoma (×400). Lower right: p53 expression in diffuse large B cell lymphoma (×100).
Figure 3 Upper left: microvasculature in diffuse large B cell lymphoma—overview (×40). Upper right: microvasculature in mantle cell lymphoma (×40). Middle left: microvasculature in follicular lymphoma (×40); note the higher perifollicular than follicular microvessel density (MVD). Middle right: microvasculature in small lymphocytic lymphoma (×40). Lower: MVD on conventional tissue slides from follicular lymphoma grade 2 (left) and grade 3 (right, ×40); note the higher perifollicular MVD in follicular lymphoma grade 3 (right).
MVD on conventional FL tissue slides
The neoplastic follicles of FLs G3 had similar microvascular network (MVD 19/0.5 mm2) as the FL G1&2 follicles (MVD 14/0.5 mm2) and reactive (MVD 15/0.5 mm2). An excessive perifollicular vascular network with a mean of 72 CD34‐positive lumina/0.5 mm2 was observed around neoplastic follicles of FL G3 compared with 31 around neoplastic follicles of FL G1&2 and reactive follicles (fig 3, lower panel, p = 0.003).
Correlations between factors
Strong expression of VEGF correlated with high proliferative activity (Ki‐67) and with p53 expression (table 4) in the whole cohort and in primary DLBCLs (data not shown). Expression of Flk‐1 correlated with proliferative activity in the whole cohort and in SLL/CLLs (p = 0.001) and FLs G1&2 (p = 0.041). All other correlations between factors studied are shown in table 4. Interestingly, VEGF expression in lymphoma cells correlated with the amount of CD3‐positive T cells in the whole cohort (p = 0.002, correlation coefficient 0.194) and in primary DLBCLs (p = 0.003), FL G1&2 (p = 0.014) and SLL/CLLs (p = 0.007).
Table 4 Correlations between vascular endothelial growth factor, Flk‐1, cyclooxygenase 2, Ki‐67 and p53 in B cell lymphomas.
| VEGF | Flk‐1 | Ki‐67 | p53 | |
|---|---|---|---|---|
| MVD | <0.001 | 0.027 | ||
| 0.223 | 0.140 | |||
| COX2 | 0.012 | <0.001 | <0.001 | |
| 0.154 | 0.236 | 0.247 | ||
| Ki‐67 | 0.001 | 0.021 | <0.001 | |
| 0.229 | 0.141 | 0.266 | ||
| p53 | 0.008 | |||
| 0.167 |
COX2, cyclooxygenase 2; MVD, microvessel density; VEGF, vascular endothelial growth factor.
Performed by the Pearson χ2 test, except for values in italics, which were performed by the Spearman rank correlation analysis; upper: p value, lower: correlation coefficient.
The analysed angiogenesis parameters did not correlate with age, sex or bone marrow involvement.
Survival analysis
Considering overall and disease‐specific survival, the diagnostic entities showed the anticipated profound differences.35 Advanced age at diagnosis was an important adverse factor for both overall (p<0.001) and disease‐specific survival (p<0.001) in all entities, as was bone marrow involvement in DLBCLs (p<0.001) and, in this cohort, male sex in MCLs (p = 0.029). p53 expression in >2% of lymphoma cells was an important adverse prognostic factor for overall (p<0.001) and disease‐specific survival (p = 0.007) and for success of treatment (p = 0.0379) in the whole cohort, particularly in DLBCL and MCL (data not shown).
Expression of the angiogenic factors studied and MVD were not of prognostic significance in the whole B cell lymphoma cohort or the different disease entities. Cardiovascular mortality did not correlate with VEGF expression in B cell lymphomas.
Discussion
Data on angiogenesis in B cell lymphomas are controversial,5 partially owing to the application of different lymphoma classification systems, the combined analysis of heterogeneous diseases, small study cohorts and the use of different detection models (serum levels, whole tumour mRNA extraction, RNA in situ hybridisation, immunohistochemistry with polyclonal and monoclonal antibodies). As B cell lymphomas encompass heterogeneous diseases, angiogenic events are likely to differ in the various entities. Our analyses improved on prior analyses by taking advantage of a validated B cell lymphoma TMA with clearly defined examination spot planimetry,36 reclassification in the current system of the B cell lymphoma cases studied,37 and application, except for Flk‐1, of monoclonal antibodies to characterise different aspects of angiogenesis aspects on a large B cell lymphoma cohort. As a result, we clearly demonstrated that MVD, and VEGF, Flk‐1 and COX2 expression vary significantly between different B cell lymphomas. We observed very weak to weak VEGF expression in 62% and strong VEGF expression in 8% of B cell lymphomas (all DLBCLs), confirming observations from smaller cohorts,11,12,16,32 but being at variance with others.4,5,18 This might be due to limitations of the TMA technology and to the application of other VEGF detection systems, particularly polyclonal antiVEGF antibodies that did not reliably work in our experience, or much higher primary antibody concentrations. Unfortunately, as scores applied in these other studies did not separately consider the amount and intensity of positively stained tumour cells and were not clearly defined, direct comparison with our results is not possible. Importantly, VEGF mRNA has been identified in DLBCL and MCL,12 whereas FLs almost completely lack VEGF mRNA expression.19 Together with the weak immunohistochemical expression of VEGF observed in about 50% of FL in a low proportion of tumour cells, this finding is relevant to the possibility of VEGF uptake by FL cells from other sources. Considering this alternative, the detected correlation between VEGF expression and the amount of CD3‐positive reactive T cells in B cell lymphomas, being the highest in FLs, could be of interest.
Take‐home messages
Angiogenesis plays a differential role in various B cell lymphomas.
Aggressive lymphomas express the potential molecular therapeutic targets vascular endothelial growth factor and cyclooxygenase 2, and have higher microvessel density.
Therapeutic strategies aimed at angiogenesis should take into account lymphoma heterogeneity.
Both MVD and VEGF expression correlated with expression of Ki‐67 and p53 in the whole cohort and in primary DLBCLs, in accordance with previous reports pointing to the association of VEGF with morphological high‐grade lymphomas and transformation of low‐grade lymphomas.11,14 The correlation of VEGF expression with p53 (detected by PAb240, which preferentially identifies mutant forms of p53) suggests a possible association between VEGF expression and B cell lymphoma progression, as p53 mutations often accompany such progressions.42 VEGF expression in lymphoma cells did not correlate with MVD in our cohort, suggesting the importance of other VEGF sources (eg, platelets, serum) for angiogenesis in B cell lymphomas. Expression of VEGF and COX2 correlated in our study. As VEGF production and release is downstream controlled by prostaglandins,8 this correlation could indicate the functional importance of COX2 in single B cell lymphoma cases, particularly in DLBCLs, where COX2 is preferentially expressed. The correlation of COX2 expression induction with the loss of wild‐type p5343 was also supported by our observations.
Induction of angiogenesis is not the only known effect of VEGF, as it can stimulate Flk‐1‐expressing cells as a mitogen in an autocrine way.3 Importantly, only a few B cell lymphomas expressed Flk‐1, and there was no correlation between Flk‐1 expression and VEGF in lymphoma cells. This observation argues against functional autocrine loops through VEGF in most B cell lymphomas, except for single SLL/CLL and FL cases, in which Flk‐1 expression correlated with Ki‐67, indicating that Flk‐1‐mediated signalling could potentially have a role in proliferative advantage.
In FL cases, only cores from neoplastic follicles were a priori arrayed for TMA construction36; thus we could not address the perifollicular MVD in FLs.19,28,30,34 Therefore, we studied MVD in five selected FL G1&2, FL G3 and lymph nodes with follicular hyperplasia on conventional tissue sections. Our results for FL G1&2 support previous observations.19,28,30,34 In FL G3, although performed on a small series, our observations are in contrast with the observations of Koster et al,5 who detected no significant MVD differences between the different FL grades, perhaps owing to exclusive consideration of vessel hot‐spots.
We found no correlations between angiogenesis and clinical parameters in the whole cohort or in the diagnostic subgroups. Considering bone marrow involvement in DLBCL, MCL and FL, we therefore speculate that lymphoma dissemination is not related to angiogenesis. Although similar observations on the prognostic importance of angiogenesis have been reported previously,17,31 the predictive value of angiogenesis in B cell lymphomas still remains a matter of debate.4,5,12
Experimental and clinical observations report that inhibition of angiogenesis induces lymphoma regression.21,23,24,25,26,27 Our study points to the differential role of angiogenesis and the presence of potential molecular therapeutic targets such as VEGF and COX2 in single‐nodal B cell lymphomas. Particularly aggressive lymphomas express VEGF and COX2 and have higher MVD. In distinct low proliferation‐fraction lymphomas, Flk‐1 might have a role for proliferative advantage. Rational therapeutic strategies aimed at angiogenesis should take into account this B cell lymphoma heterogeneity.
Abbreviations
COX2 - cyclooxygenase 2
DLBCL - diffuse large B cell lymphoma
FL - follicular lymphoma
FL G1&2 - FL grade 1 and 2
MCL - mantle cell lymphoma
MVD - microvessel density
SLL/CLL - small lymphocytic lymphoma/chronic lymphocytic leukaemia
TMA - tissue microarray
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None declared.
References
- 1.Folkman J, D'Amore P A. Blood vessel formation: what is its molecular basis? Cell 1996871153–1155. [DOI] [PubMed] [Google Scholar]
- 2.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 19951479–19. [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy W T, Richter L, Frutiger Y.et al Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res 199959728–733. [PubMed] [Google Scholar]
- 4.Hazar B, Paydas S, Zorludemir S.et al Prognostic significance of microvessel density and vascular endothelial growth factor (VEGF) expression in non‐Hodgkin's lymphoma. Leuk Lymphoma 2003442089–2093. [DOI] [PubMed] [Google Scholar]
- 5.Koster A, Raemaekers J M. Angiogenesis in malignant lymphoma. Curr Opin Oncol 200517611–616. [DOI] [PubMed] [Google Scholar]
- 6.Podar K, Anderson K C. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood 20051051383–1395. [DOI] [PubMed] [Google Scholar]
- 7.Gately S, Li W W. Multiple roles of COX‐2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol 200431(Suppl 7)2–11. [DOI] [PubMed] [Google Scholar]
- 8.Hoper M M, Voelkel N F, Bates T O.et al Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol 199717748–756. [DOI] [PubMed] [Google Scholar]
- 9.Bono P, Teerenhovi L, Joensuu H. Elevated serum endostatin is associated with poor outcome in patients with non‐Hodgkin lymphoma. Cancer 2003972767–2775. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen L M, Klausen T W, Davidsen U H.et al Early changes in serum IL‐6 and VEGF levels predict clinical outcome following first‐line therapy in aggressive non‐Hodgkin's lymphoma. Ann Hematol 200584510–516. [DOI] [PubMed] [Google Scholar]
- 11.Ho C L, Sheu L F, Li C Y. Immunohistochemical expression of basic fibroblast growth factor, vascular endothelial growth factor, and their receptors in stage IV non‐Hodgkin lymphoma. Appl Immunohistochem Mol Morphol 200210316–321. [DOI] [PubMed] [Google Scholar]
- 12.Potti A, Ganti A K, Kargas S.et al Immunohistochemical detection of C‐kit (CD117) and vascular endothelial growth factor (VEGF) overexpression in mantle cell lymphoma. Anticancer Res 2002222899–2901. [PubMed] [Google Scholar]
- 13.Salven P, Orpana A, Teerenhovi L.et al Simultaneous elevation in the serum concentrations of the angiogenic growth factors VEGF and bFGF is an independent predictor of poor prognosis in non‐Hodgkin lymphoma: a single‐institution study of 200 patients. Blood 2000963712–3718. [PubMed] [Google Scholar]
- 14.Vacca A, Ribatti D, Ruco L.et al Angiogenesis extent and macrophage density increase simultaneously with pathological progression in B‐cell non‐Hodgkin's lymphomas. Br J Cancer 199979965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W L, Mourah S, Mounier N.et al Vascular endothelial growth factor‐A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T‐cell lymphoma and related to lymphoma progression. Lab Invest 2004841512–1519. [DOI] [PubMed] [Google Scholar]
- 16.Foss H D, Araujo I, Demel G.et al Expression of vascular endothelial growth factor in lymphomas and Castleman's disease. J Pathol 199718344–50. [DOI] [PubMed] [Google Scholar]
- 17.Ribatti D, Vacca A, Bertossi M.et al Angiogenesis induced by B‐cell non‐Hodgkin's lymphomas. Lack of correlation with tumor malignancy and immunologic phenotype. Anticancer Res 199010401–406. [PubMed] [Google Scholar]
- 18.Stewart M, Talks K, Leek R.et al Expression of angiogenic factors and hypoxia inducible factors HIF 1, HIF 2 and CA IX in non‐Hodgkin's lymphoma. Histopathology 200240253–260. [DOI] [PubMed] [Google Scholar]
- 19.Koster A, van Krieken J H, Mackenzie M A.et al Increased vascularization predicts favorable outcome in follicular lymphoma. Clin Cancer Res 200511154–161. [PubMed] [Google Scholar]
- 20.Li H L, Sun B Z, Ma F C. Expression of COX‐2, iNOS, p53 and Ki‐67 in gastric mucosa‐associated lymphoid tissue lymphoma. World J Gastroenterol 2004101862–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wun T, McKnight H, Tuscano J M. Increased cyclooxygenase‐2 (COX‐2): a potential role in the pathogenesis of lymphoma. Leuk Res 200428179–190. [DOI] [PubMed] [Google Scholar]
- 22.Hazar B, Ergin M, Seyrek E.et al Cyclooxygenase‐2 (Cox‐2) expression in lymphomas. Leuk Lymphoma 2004451395–1399. [DOI] [PubMed] [Google Scholar]
- 23.Bertolini F, Fusetti L, Mancuso P.et al Endostatin, an antiangiogenic drug, induces tumor stabilization after chemotherapy or anti‐CD20 therapy in a NOD/SCID mouse model of human high‐grade non‐Hodgkin lymphoma. Blood 200096282–287. [PubMed] [Google Scholar]
- 24.Heider U, Kaiser M, Sterz J.et al Histone deacetylase inhibitors reduce VEGF production and induce growth suppression and apoptosis in human mantle cell lymphoma. Eur J Haematol 20067642–50. [DOI] [PubMed] [Google Scholar]
- 25.Pro B, Younes A, Albitar M.et al Thalidomide for patients with recurrent lymphoma. Cancer 20041001186–1189. [DOI] [PubMed] [Google Scholar]
- 26.Stopeck A T, Bellamy W, Unger J.et al Phase II trial of single agent bevacizumab in patients with relapsed, aggressive non‐Hodgkin's lymphoma (NHL): Southwest Oncology Group Study S0108 [abstract]. J Clin Oncol 2005236592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang E S, Teruya‐Feldstein J, Wu Y.et al Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood 20041042893–2902. [DOI] [PubMed] [Google Scholar]
- 28.Passalidou E, Stewart M, Trivella M.et al Vascular patterns in reactive lymphoid tissue and in non‐Hodgkin's lymphoma. Br J Cancer 200388553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streubel B, Chott A, Huber D.et al Lymphoma‐specific genetic aberrations in microvascular endothelial cells in B‐cell lymphomas. N Engl J Med 2004351250–259. [DOI] [PubMed] [Google Scholar]
- 30.Arias V, Soares F A. Vascular density (tumor angiogenesis) in non‐Hodgkin's lymphomas and florid follicular hyperplasia: a morphometric study. Leuk Lymphoma 200040157–166. [DOI] [PubMed] [Google Scholar]
- 31.Bairey O, Zimra Y, Kaganovsky E.et al Microvessel density in chemosensitive and chemoresistant diffuse large B‐cell lymphomas. Med Oncol 200017314–318. [DOI] [PubMed] [Google Scholar]
- 32.Ho C L, Sheu L F, Li C Y. Immunohistochemical expression of angiogenic cytokines and their receptors in reactive benign lymph nodes and non‐Hodgkin lymphoma. Ann Diagn Pathol 200371–8. [DOI] [PubMed] [Google Scholar]
- 33.Mazur G, Wrobel T, Dziegiel P.et al Angiogenesis measured by expression of CD34 antigen in lymph nodes of patients with non‐Hodgkin's lymphoma. Folia Histochem Cytobiol 200442241–243. [PubMed] [Google Scholar]
- 34.Ridell B, Norrby K. Intratumoral microvascular density in malignant lymphomas of B‐cell origin. APMIS 200110966–72. [DOI] [PubMed] [Google Scholar]
- 35.Mitterlechner T, Fiegl M, Mühlböck H.et al Epidemiology of non‐Hodgkin‐lymphomas in Tyrol/Austria from 1991 through 2000. J Clin Pathol 20065948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzankov A, Went Ph, Zimpfer A.et al Tissue microarray technology: principles, pitfalls and perspectives—lessons learned from hematological malignancies. Exp Gerontol 200540737–744. [DOI] [PubMed] [Google Scholar]
- 37.Jaffe E S, Harris N L, Stein H, Vardiman J W. eds. Pathology and genetics of tumours of the haematopoietic and lymphoid system. Lyon: IARC Press, 2001
- 38.Turley H, Scott P A, Watts V M.et al Expression of VEGF in routinely fixed material using a new monoclonal antibody VG1. J Pathol 1998186313–318. [DOI] [PubMed] [Google Scholar]
- 39.Gannon J V, Greaves R, Iggo R.et al Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J 199091595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stifter G, Heiss S, Gastl G.et al Over‐expression of tumor necrosis factor‐α in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol 200575485–491. [DOI] [PubMed] [Google Scholar]
- 41.Vidal S, Lloyd R V, Moya L.et al Expression and distribution of vascular endothelial growth factor receptor Flk‐1 in the rat pituitary. J Histochem Cytochem 200250533–540. [DOI] [PubMed] [Google Scholar]
- 42.Moller M B, Nielsen O, Pedersen N T. Frequent alteration of MDM2 and p53 in the molecular progression of recurring non‐Hodgkin's lymphoma. Histopathology 200241322–330. [DOI] [PubMed] [Google Scholar]
- 43.Subbaramaiah K, Altorki N, Chung W J.et al Inhibition of cyclooxygenase‐2 gene expression by p53. J Biol Chem 199927410911–10915. [DOI] [PubMed] [Google Scholar]