Abstract
Background
Red blood cell (RBC) rheology is altered in different diseases, including acute conditions such as patients in intensive care units (ICU) with sepsis or with an inflammatory reaction due to postoperative states or intracerebral haemorrhage, or chronic conditions such as diabetes mellitus or terminal renal failure. Several techniques are available to assess alterations in RBC rheology, especially deformability, but they are too cumbersome to be used on a large number of cells.
Objective
To develop a new, rapid flow cytometry technique for easy assessment of RBC shape in patients.
Methods
In flow cytometry, healthy human RBC shape shows a bimodal distribution related to the biconcave form. On this histogram, the second Pearson coefficient of dissymmetry (PCD) representing the asymmetry of this histogram and the spherical index (M2:M1) were calculated, both representing the spherical shape. This technique was used in healthy volunteers (n = 17) and in diseases characterised by abnormalities in RBC rheology, including terminal renal failure requiring haemodialysis (n = 28), diabetes mellitus (n = 18), sepsis (n = 19) and acute inflammatory states (postoperative, intracerebral haemorrhage, chronic obstructive pulmonary disease, epilepsy or severe drug intoxication; n = 21). Multivariate analysis was performed to determine the factors influencing RBC shape.
Results
Measurement of RBC shape was highly reproducible. A good correlation was observed between the PCD and the spherical index, except in the critically ill patients without sepsis. RBCs were more spherical in patients with terminal renal failure (PCD −0.56 (0.14), p<0.05), diabetes mellitus (PCD −0.59 (0.23), p<0.05), sepsis (PCD −0.58 (0.22), p<0.05) or an acute inflammatory state (PCD −0.65 (0.29), p<0.05) than in healthy volunteers (PCD −0.89 (0.12)). The spherical index was also increased in all populations compared with healthy volunteers (terminal renal failure 2.30 (0.20); diabetes mellitus 2.27 (0.38); sepsis 2.28 (0.37); acute inflammatory state 2.35 (0.42) vs healthy volunteers 2.72 (0.47); all p<0.05). Multivariate analysis demonstrated that the underlying pathology (sepsis, acute inflammatory state, diabetes mellitus, terminal renal failure) was the principal cause of these RBC shape abnormalities.
Conclusion
RBCs are characterised by an increased spherical shape in many disease states. The measure of the second PCD in flow cytometry is a new, easy method to investigate RBC shape in various diseases. This technique could facilitate the investigation of abnormalities of RBC rheology.
To effectively provide oxygen to the cells, the red blood cell (RBC) must be able to undergo considerable cellular deformation, since its diameter (8 μm in humans) far exceeds that of the capillaries (2–3 μm) through which it must pass.1 The RBC membrane is the most important determinant of RBC deformability and is composed of proteins (52% in weight), lipids (40%) and carbohydrates (8%). Membrane elasticity depends on complex structural interactions between the outer plasma membrane and the underlying protein cytoskeleton.
Reversible deformation of the RBC membrane involves a change in geometric shape, without change in surface area. With increased deformation, some of the spectrin molecules (an integral protein of the RBC membrane) can reach their maximal linear extension, and thereby the limit of reversible deformability.1 “Cellular deformability” is the term generally used to characterise the RBC's ability to undergo deformation during flow.2 The deformation response of an RBC to fluid forces is a complex phenomenon that is influenced by several different cell characteristics, including membrane material properties,3 cell geometry and cytoplasmic viscosity.4
Several methods have been reported to measure RBC deformability, but as measures depend on the technique used, quoted values are not comparable. Moreover, it is also possible to observe different RBC deformability distributions with the presence of hypodeformable and hyperdeformable cell fractions.5 The various techniques have been described in detail elsewhere.4 The most detailed characterisation of the membrane properties is obtained by aspiration of single RBCs into micropipettes with diameters in the range of 1–2 μm, so that the relationship between the applied negative pressure and the membrane tongue extension is quantitated. Using ektacytometry, RBCs are subjected to a laminar shear stress field in a couvette viscometer and the resultant change in cell shape is continuously monitored by laser diffractometry. Unstressed discoid cells generate a circular diffraction pattern. By measuring optical densities at two points along the major and minor axes of the elliptical diffraction pattern, a “deformability index” is generated, which is a direct measure of cell ellipticity. A numerical value of zero corresponds to non‐deformable cells, whereas move positive values correspond with increasing cellular deformability. The membrane fragmentation assay using the ektacytometer is particularly useful in documenting an altered mechanical integrity of the membrane owing to protein defects.6
Micropore filtration is limited by the possible occlusion of the filter pores by abnormally rigid RBCs or by white blood cells (WBCs),7 and has been replaced by the cell transit analyser, which is insensitive to the presence of WBCs whereas the passage of individual RBCs is monitored by a computerised system.
These current techniques for measuring in vitro deformability of individual RBCs are too complex to be used on large numbers of cells and their use is limited to the research field. For these reasons, we developed a new, rapid flow cytometry technique for easy assessment of RBC shape in patients.
As deformability depends also on cell geometry4 and because of the lack of studies about RBC shape in different pathologies, we developed a technique that gives information about the RBC shape estimated by the spherical index or by the second Pearson coefficient of dissymmetry (PCD) in a large number of cells. Interestingly, this technique is not influenced by sample temperature,8 unlike other techniques.9 In a preliminary study, we examined RBC shape in healthy volunteers and in critically ill patients with and without sepsis, and demonstrated a good correlation between these two indices.10 To validate this technique, we extended the flow cytometry studies to other diseases known to induce RBC rheology abnormalities including diabetes mellitus11 and terminal renal failure requiring haemodialysis.12
Patients and methods
Patients
Following approval by the André Vésale Hospital Ethics Committee, this study included 17 healthy volunteers (hospital employees of both genders), 18 patients with insulin‐dependent diabetes, 28 patients with terminal renal failure requiring iterative haemodialysis (but no diabetes) and 40 critically ill adult patients with sepsis (n = 19) or with acute inflammatory states (n = 21; but not diabetes or terminal renal failure). Criteria for severe sepsis included clinical and bacteriological evidence of infection and ⩾3 signs of a systemic response.13 Exclusion criteria for all patients were RBC transfusion in the previous 72 h, acute bleeding, haematological disorders, recent chemotherapy, burns, cardiogenic shock, cirrhosis, pregnancy, any drugs known to influence RBC shape and rheological properties (such as pentoxyfylline, ciclosporin, aspirin, nitrovasodilators). Erythropoietin administration was continued in patients with terminal renal failure. The Simplified Acute Physiologic Score14 was determined in patients in the intensive care unit (ICU).
Methods
Study protocol
In all subjects, blood samples were taken once and collected in EDTA tubes (2.5 ml of blood in 0.06 ml EDTA, 0.235 mol/l, Venoject, Terumo, Leuven, Belgium). For patients with terminal renal failure, blood samples were taken before haemodialysis to exclude a rheological effect of this procedure.15 In patients in the ICU, blood samples were drawn during the first 24 h of sepsis (in septic patients) or during the 24 h after ICU admission.
Blood analysis
Blood analysis included RBC count, haemoglobin concentration, mean corpuscular volume, leucocyte count, blood glucose, C reactive protein, sodium and urea concentrations; plasma osmolality was calculated. Blood lactate was measured in patients in the ICU and haemoglobin subtype 1c (HbA1c) in patients with diabetes.
Analysis of RBC shape
Data were collected on a Becton‐Dickinson FAC SCalibur cytofluorimeter (Becton‐Dickinson, San Jose, California, USA). RBC samples were analysed within 90 min after blood sampling. The forward light scatter channels (FSCs) were set on linear gain. Cell size is the principal component of the FSC signal. Both the general principles of the FACScan flow cytometer and the details used herein have been described elsewhere.16,17 For estimation of RBC shape, we expanded the technique described by Rolfes‐Curl et al17 using the FSC signal in iso‐osmolality, applying low shear stresses (12 μl/min RBC flow rate) to allow the RBCs to rotate in the flow18 without deformation due to shear stresses, as described by Cokelet and Goldsmith.19 Moreover, we did not add fluorescently labelled agglutinins that may alter RBC shape.
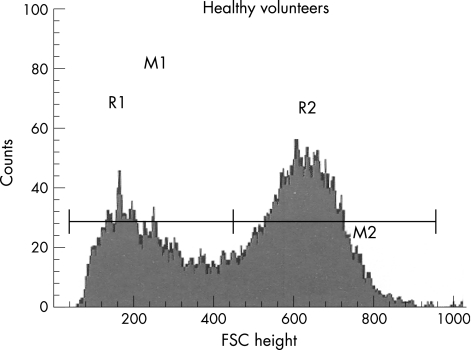
Whole blood (2 μl) was mixed with isotonic (286 mOsm) phosphate‐buffered saline at 25°C. The study was limited to 15 000 events and lasted for 15 s. In healthy volunteers, after the position of the obscuration bar was modified, the FACScan clearly viewed the flow of ellipsoid, biconcave RBCs as essentially two populations of cells and the FSC histograms showed a typically bimodal distribution of RBCs (fig 1).16,17 We fixed two gates of interest for these histograms: R1 and R2 (fig 1). The limit between R1 and R2 was the mean value in the valley between the two modes for the volunteer population. Two median values (M1 and M2) were calculated for each predetermined gate of interest (R1 and R2).
Figure 1 Flow cytometry study with FACScan. Red blood cell (RBC) distribution histograms relating number of events and forward light scatter channel (FSC) distribution (size estimation). In normal, biconcave, ellipsoid RBCs, the FSC distribution (size estimation) is bimodal (16). R1 and R2 (gate determination) are determined manually. M1 and M2 are medians of the gates R1 and R2.
The M2:M1 ratio, termed the spherical index, and the second PCD20 (asymmetry of the global histogram = 3×(mean−median/σ)) express the sphericity of the RBC. A negative PCD (which indicates a left asymmetry) and a decrease in M2:M1 correlate with the sphericity of the RBC. The cytometer settings were kept constant and the stability of the FSC channel was checked with calibrated size beads (CaliBrite Beads, Becton‐Dickinson) between each blood sample studied (interassay variability 3%).
Statistical analysis
SigmaStat v 3.5 software package (Systat Software Inc, San Jose, California, USA) was used. Non‐parametric statistical tests were used, including the Kruskal–Wallis test and the Bonferroni test for pairwise comparisons. The correlations were evaluated by the Spearman test. Data are presented as mean (SD) or median value (25th–75th) as appropriate. A p value <0.05 was considered significant.
The multilinear regression analysis used a stepwise backward selection of the variables. The standardised regression coefficients are given for each model. The model included individual characteristics (volunteers, terminal renal failure, diabetes mellitus, sepsis or acute inflammatory state, age, RBC count, leucocyte count, platelet count, blood sugar, C reactive protein, sodium and urea concentrations and calculated osmolality). PCD and the spherical index were the dependent variables.
Results
Patients
Table 1 presents the characteristics of the subjects. The healthy volunteers were significantly younger than the patients. The causes of sepsis were pneumonia (58%), peritonitis (21%) and other causes (21%, pyelonephritis, septicaemia and encephalitis). Major causes of ICU admission in patients without sepsis were postoperative inflammatory reactions (38%), intracerebral haemorrhage (14%), drug intoxication, chronic obstructive pulmonary disease and epilepsy. Of the 18 patients with diabetes, 16 had type 2 diabetes mellitus. In all, 80% of the terminal renal failure was due to nephroangiosclerosis.
Table 1 Characteristics of the subjects studied.
| Septic (n = 19) | Non‐septic (n = 21) | Diabetes (n = 18) | Renal failure (n = 28) | Healthy volunteers (n = 17) | |
|---|---|---|---|---|---|
| Age (years) | 69 (13)* | 66 (15)* | 63 (17)* | 72 (9)* | 47 (19) |
| SAPS II | 49 (18)† | 29 (18) | — | — | — |
| Hb (g/dl) | 10.7 (10.0–12.1)* | 12.4 (10.5–14.1)* | 14.0 (13.5–15.2) | 11.9 (11.0–12.7)* | 14.1 (13.7–14.8) |
| RBC (/mm3) | 3.5 (3.2–4.1)* | 3.8 (3.3–4.6)* | 4.6 (4.4–4.9) | 3.9 (3.6–4.0)* | 4.6 (4.4–4.8) |
| MCV (fl3) | 91.5 (2.7) | 91.8 (3.0) | 89.6 (3.6) | 93.9 (3.6)* | 89.0 (3.8) |
| WBC (/mm3) | 11.1 (6.6–14.9) | 11.1 (7.8–15.0)* | 7.5 (6.4–8.8) | 6.7 (5.7–8.2) | 7.1 (6.2–7.8) |
| CRP (mg/dl) | 18.1 (11.0–24.0)* | 5.5 (1.5–7.7)* | 0.8 (0.6–1.0) | 0.7 (0.4–1.1) | 0.1 (1.5–7.7) |
| Glucose (mg/dl) | 142 (124–179)* | 126 (119–150)* | 192 (128–288)* | 104 (90–115) | 100 (95–103) |
| Na+ (mEq/l) | 132.0 (131–137)* | 138.0 (133.8–140.0) | 136.0 (135.0–140.0) | 137.0 (135.5–138.5) | 139.0 (133.8–140.0) |
| Osm (mOsm/kg H2O) | 290 (12) | 288 (11) | 290 (8) | 302 (10)* | 290 (3) |
| Urea (mg/dl) | 95 (48–120)* | 32 (19–53) | 33 (48–120) | 137 (111–159)* | 34 (25–39) |
| Lactate (mmol/l) | 1.02 (0.8–1.13) | 0.84 (0.6–1.0) | — | — | — |
| HbA1C/ (%) | — | — | — | 8.6 (7.3–9.1) | — |
CRP, C reactive protein; Hb, haemoglobin; MCV, mean corpuscular volume; Na, sodium concentration; Osm, calculated osmolality RBC, red blood cell; SAPS II, Simplified Acute Physiologic Score; WBC, white blood cell; —, not relevant.
Values are mean (SD) or median (25–75th centiles).
*p <0.05 versus control.
†p <0.05 versus non‐septic.
Blood analyses
As expected, haemoglobin concentration and RBC count were significantly lower in critically ill patients than in patients with diabetes and healthy volunteers. Patients with terminal renal failure were also more anaemic (despite erythropoietin treatment) than the patients with diabetes and healthy volunteers (table 1). Mean corpuscular volume was higher in patients with renal failure than in the other groups. Markers of inflammation (WBC, C reactive protein) were higher in patients in ICU. Blood sugar concentration was higher in patients in ICU and those with diabetes than in patients with terminal renal failure and healthy volunteers. Calculated osmolality was higher in patients with renal failure (owing to an increased urea concentration; table 1).
Analysis of RBC shape
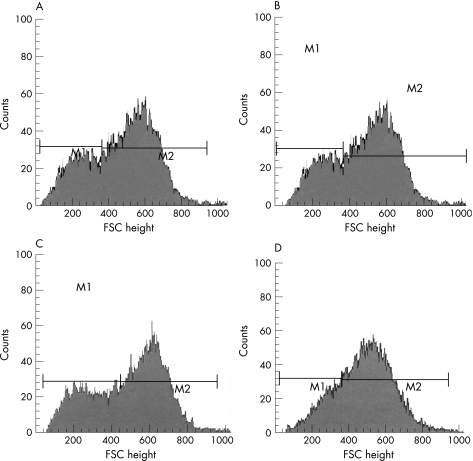
The spherical index (M2:M1 ratio) was significantly lower in all patient populations than in volunteers (table 2). The PCD was significantly reduced in all diseases, corresponding to a right shift in the R1 gate (right shift of the median channel in the FSC histogram); both events reflect the spherical nature of RBC and FSC symmetry (table 2, fig 2).
Table 2 Flow cytometry results.
| RBC shape | Correlation | p Value | ||
|---|---|---|---|---|
| M2:M1 | PCD | |||
| Septic (n = 19) | 2.28 (0.37)* | −0.58 (0.22)* | r = −0.78 | <0.001 |
| Acute inflammatory state (n = 21) | 2.35 (0.42)* | −0.65 (0.29)* | r = −0.34 | 0.13 |
| Diabetic (n = 18) | 2.27 (0.38)* | −0.59 (0.23)* | r = −0.78 | <0.001 |
| Terminal renal failure (n = 28) | 2.30 (0.20)* | −0.56 (0.14)* | r = −0.87 | <0.001 |
| Healthy volunteers (n = 17) | 2.72 (0.47) | −0.89 (0.120 | r = −0.74 | <0.001 |
RBC, red blood cell.
Values are mean (SD).
*p<0.05 versus healthy volunteers.
Figure 2 Example of a red blood cell analysis in a patient with diabetes (A), a patient with terminal renal failure (B), a patient with acute inflammatory state (C) and a patient with sepsis (D). M1 and M2 are medians. FSC, forward light scatter channel.
The spherical index (M2:M1) was strongly correlated with the PCD except for the patients without sepsis in the ICU (table 2).
Correlation between sphericity, blood sugar and HbA1C
In patients with diabetes, there was no significant correlation between the PCD or the spherical index and the blood sugar (r = −0.05; p = 0.83 and r = −0.04; p = 0.87, respectively), between the PCD and HbA1C or the spherical index and HbA1C (r = −0.24, p = 0.33 and r = −0.24; p = 0.32, respectively).
Multivariate analysis
The independent predictors of the PCD were terminal renal failure, diabetes mellitus, sepsis and acute inflammatory state (all p<0.001), and the RBC count (p = 0.034; table 3).
Table 3 Multivariate analysis.
| Standardised regression coefficient | p Value | |
|---|---|---|
| PCD (n = 103), r2 = 0.22 | F = 5.3 | <0.001 |
| Terminal renal failure | 0.76 | <0.001 |
| Sepsis | 0.70 | <0.001 |
| Acute inflammatory state | 0.52 | <0.001 |
| Diabetes mellitus | 0.48 | <0.001 |
| RBC count | 0.26 | 0.034 |
| Spherical index (n = 103), r2 = 0.13 | F = 3.49 | 0.01 |
| Sepsis | −0.44 | <0.001 |
| Diabetes mellitus | −0.41 | 0.002 |
| Terminal renal failure | −0.37 | 0.002 |
| Acute inflammatory state | −0.34 | 0.002 |
PCD, Pearson coefficient of dissymmetry; RBC, red blood cell.
Models included underlying pathologies, age, RBC count, leucocyte count, platelets, blood sugar, C reactive protein, sodium and urea concentrations and calculated osmolality.
Independent predictors of the spherical index were terminal renal failure (p = 0.002), diabetes mellitus (p = 0.002), sepsis (p<0.001), and acute inflammatory state (p = 0.009; table 3).
Discussion
This rapid and easy flow cytometry technique demonstrated that RBCs in patients with diabetes, terminal renal failure, sepsis and acute inflammatory states have a more spherical shape than normal as evaluated either by the spherical index or by the PCD. These diseases are associated with alterations in RBC rheology,10,11,12 and these observations suggest that the deformability capacity is altered in these diseases perhaps because the RBCs are more spherical in vivo. Regrettably, only a few studies have looked at the RBC shape in these pathologies.7,10,12,21 The reason why RBCs are spherical remains unexplained and the causes are probably multifactorial (modifications of RBC membrane, reactive oxygen species and so on). Osmolality was not found to be a significant variable. In a recent study, we also observed a more spherical RBC shape in patients with sepsis than in volunteers, and these differences were not explained by different osmolalities.10 Modifications in RBC membrane composition could explain variations in RBC rigidity and alter the RBC shape,10,12,15,22,23 but these were not studied here. Further studies on haematological pathologies (membrane disorders, erythroid dysplasia in patients with myelodysplastic syndrome) are necessary to correlate the possible link between RBC protein membrane alterations and shape.24,25 Another possible use of this flow cytometry technique is the assessment of the evolution of the RBC shape after splenectomy.26
Using flow cytometry, we observed that the more spherical shape of the RBCs in patients with diabetes was not correlated with blood sugar or the severity of the disease as assessed by the HbA1C. These RBC alterations could increase the risk of peripheral ischaemia in these patients, especially if blood viscosity is increased.11 Increased adhesion of RBCs to endothelial cells in patients with diabetes was already demonstrated 20 years ago by Wautier et al27 and was correlated with the extent and the severity of diabetic microvascular alterations (especially nephropathy).28,29 The origin of the increased spherical shape is unclear. A possible explanation is a reduced phosphorylation of the spectrin responsible for the increased membrane viscosity in patients with diabetes.29 Other explanations include reduced ATP levels30 or an imbalance in the cholesterol:phospholipid ratio.31,32,33
Take‐home messages
A new flow cytometry technique is described for the rapid assessment of red blood cell (RBC) shape in different pathologies that are associated with alterations in RBC rheology (eg, terminal renal failure, diabetes mellitus, sepsis, acute inflammatory state).
Using this technique, the RBC shape of healthy volunteers shows a bimodal distribution related to the ellipsoid biconcave form. In many disease states, RBCs are characterised by a more spherical shape.
This technique could facilitate the investigation of abnormalities of RBC rheology and be used to monitor RBC shape in various disease states.
RBCs are also more spherical in patients with terminal renal failure requiring haemodialysis, and this alteration may contribute to the mechanical fragility of RBC membranes and to the impaired RBC deformability in these patients.12,34,35 A possible explanation is that patients with terminal renal failure have reduced serum antioxidant activity, and so may be more susceptible to free radical‐induced injury.36 In our study, we could not exclude a possible effect of erythropoietin treatment on RBC shape as described by others,37,38,39 but it would have been unethical to discontinue this treatment.
Our study confirmed that RBCs from critically ill patients with an acute inflammatory reaction (postoperative state, intracerebral haemorrhage, chronic obstructive pulmonary disease, drug intoxication or epilepsy) were more spherical than those from healthy volunteers.10 These RBC alterations may contribute to impaired cellular oxygen supply.7,40 Increased RBC aggregability and decreased RBC deformability have been described in patients with sepsis.7,40 These modifications of RBC rheology probably contribute to the microcirculatory disturbances recently observed in these patients.41 This patient population with some inflammatory response has alterations in RBC shape and RBC membrane intermediate between patients with sepsis and volunteers.10,42
Disease was the major factor associated with these alterations but our study cannot identify the individual factors that may be responsible in each disease state. We recommend the measurement of the PCD rather than the spherical index to estimate RBC shape in order to limit manual error.
The flow cytometry technique is a rapid technique, easier than conventional methods, and provides information about RBC shape in a large number of RBCs. This technique may be useful to better define rheological abnormalities in many disease states.
Acknowledgements
MP is the recipient of a grant from the Erasme Foundation.
Abbreviations
FSCs - forward light scatter channels
HbA1c - haemoglobin subtype 1c
ICU - intensive care unit
PCD - Pearson coefficient of dissymmetry
RBC - red blood cell
WBC - white blood cell
Footnotes
Competing interests: None declared.
References
- 1.Deuticke B. Membrane lipids and proteins as a basis of red cell shape and its alterations. In: Bernhardt I, Ellory CJ, eds. Red cell membrane transport in health and disease 1st edn. Berlin, Germany: Springer‐Verlag, 200327–60.
- 2.Mohandas N, Chasis J A, Shohet S B. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol 198320225–242. [PubMed] [Google Scholar]
- 3.Lux S E. Dissecting the red cell membrane skeleton. Nature 1979281426–429. [DOI] [PubMed] [Google Scholar]
- 4.Mohandas N, Chasis J A. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol 199330171–192. [PubMed] [Google Scholar]
- 5.Dobbe J G G, Hardeman M R, Streekstra G J.et al Analyzing red blood cell‐deformability distributions. Blood Cells Mol Dis 200228373–384. [DOI] [PubMed] [Google Scholar]
- 6.Hardeman M R, Goedhart P T, Dobbe J G G.et al Laser‐assisted optical rotational cell analyzer (LORCA): a new instrument for measurement of various structural hemorheological parameters. Clin Hemorheol 19944605–618. [Google Scholar]
- 7.Baskurt O K, Gelmont D, Meiselman H J. Red blood cell deformability in sepsis. Am J Respir Crit Care Med 1998157421–427. [DOI] [PubMed] [Google Scholar]
- 8.Piagnerelli M, Zouaoui Boudjeltia K, Piro P.et al Effects of sample temperature on red blood cell shape in septic patients. Clin Hemorheol Microcirc 200430463–466. [PubMed] [Google Scholar]
- 9.Baskurt O K, Mat F. Importance of measurement temperature in detecting the alterations of red blood cell aggregation and deformability studied by ektacytometry: a study on experimental sepsis in rats. Clin Hemorheol Microcirc 20002343–49. [PubMed] [Google Scholar]
- 10.Piagnerelli M, Zouaoui Boudjeltia K, Brohee D.et al Alterations of red blood cell shape and sialic acid membrane content in septic patients. Crit Care Med 2003311052–1061. [DOI] [PubMed] [Google Scholar]
- 11.MacRury S M. Blood rheology in diabetes mellitus. Diabetic Med 19917285–291. [DOI] [PubMed] [Google Scholar]
- 12.Bonomini A, Sirolli V, Settefrati N.et al Increased erythrocyte phosphatidylserine exposure in chronic renal failure. J Am Soc Nephrol 1999101982–1990. [DOI] [PubMed] [Google Scholar]
- 13.Levy M M, Fink M P, Marshall J C.et al 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003311250–1256. [DOI] [PubMed] [Google Scholar]
- 14.Le Gall J R, Loirat P, Alperovitch A.et al A simplified acute physiologic score for ICU patients. Crit Care Med 198412975–977. [DOI] [PubMed] [Google Scholar]
- 15.Hasler C R, Owen G R, Brunner W.et al Echinocytosis induced by haemodialysis. Nephrol Dial Transplant 1998133132–3137. [DOI] [PubMed] [Google Scholar]
- 16.Gutowski K A, Hudson J L, Aminoff D. Flow cytometric analysis of human erythrocytes: I. Probed with lectins and immunoglobulins. Exp Gerontol 199126315–326. [DOI] [PubMed] [Google Scholar]
- 17.Rolfes‐Curl A, Ogden L L, Omann G M.et al Flow cytometric analysis of human erythrocytes: II. Possible identification of senescent RBC with fluorescently labeled wheat germ agglutinin. Exp Gerontol 199126327–345. [DOI] [PubMed] [Google Scholar]
- 18.Bitbol M. Red blood cell orientation in orbit C = 0. Biophys J 1986491055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cokelet G R, Goldsmith H L. Decreased hydrodynamic resistance in the two‐phase flow on blood through small vertical tubes at low flow rates. Circ Res 1991681–17. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel M R.Spiegel, théorie et applications de la Statistique. New York:Serie Schaum. Mc Graw‐Hill 200489–98.
- 21.Turchetti V, De Matteis C, Leoncini F.et al Variations of erythrocyte morphology in different pathologies. Clin Hemorheol Microcirc 199717209–215. [PubMed] [Google Scholar]
- 22.Snyder L M, Fortier N L, Trainor J.et al Effect of hydrogen peroxide exposure on normal erythrocyte deformability, morphology, surface characteristics, and spectrine‐hemoglobin cross‐linking. J Clin Invest 1985761971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies K J A, Goldberg A L. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem 19872628220–8226. [PubMed] [Google Scholar]
- 24.McMullin M F. The molecular basis of disorders of the red cell membrane. J Clin Pathol 199952245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Della Porta M G, Malcovati L, Invernizzi R.et al Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia 200620549–555. [DOI] [PubMed] [Google Scholar]
- 26.de Haan L D, Werre J M, Ruben A M.et al Alterations in size, shape and osmotic behaviour of red cells after splenectomy : a study of their age dependence. Br J Haematol 19886971–80. [DOI] [PubMed] [Google Scholar]
- 27.Wautier J L, Paton C, Wautier M P.et al Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N Engl J Med 1981305237–242. [DOI] [PubMed] [Google Scholar]
- 28.Gambaro G, Baggio B, Cicerello E.et al Abnormal erythrocyte charge in diabetes mellitus. Link with microalbumineria.Diabetes 198837745–748. [DOI] [PubMed] [Google Scholar]
- 29.Mazzanti L, Rabini R A, Salvolini E.et al Sialic acid, diabetes, and aging: a study on the erythrocytes membrane. Metabolism 19974659–61. [DOI] [PubMed] [Google Scholar]
- 30.Birchmeier W, Singer S J. On the mechanism of ATP‐induced shape changes in human erythrocyte membranes. J Cell Biol 197773647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice‐Evans C, Chapman D. Red blood cell biomembrane structure and deformability. Scand J Clin Lab Invest 198141(Suppl 156)99–110. [DOI] [PubMed] [Google Scholar]
- 32.Bryszewska M, Watala C, Torzecka W. Changes in fluidity and composition of plasma lipids in type 1 diabetes. Br J Haematol 198662111–116. [DOI] [PubMed] [Google Scholar]
- 33.Otsuji S, Baba Y, Kamada T. Erythrocyte membrane microviscosity in diabetes. Horm Metab Res 19811197–102. [PubMed] [Google Scholar]
- 34.Docci D, Del Vecchio C, Salvi P.et al Osmotic fragility of erythrocytes, cell deformability and secondary hyperparathyroidism in uremic patients on maintenance hemodialysis. Clin Nephrol 19852366–73. [PubMed] [Google Scholar]
- 35.Komidori K, Kamada T, Yamashita T.et al Erythrocyte membrane fluidity decreased in uremic hemodialysed patients. Nephron 198540185–188. [DOI] [PubMed] [Google Scholar]
- 36.Maher E R, Wickens D G, Griffin J F A.et al Increased free‐radical activity during haemodialysis. Nephrol Dial Transplant 19872169–171. [PubMed] [Google Scholar]
- 37.Bohler T, Leo A, Linderkamp O.et al Haemorheological changes in uraemic children in response to erythropoietin treatment. Nephrol Dial Transplant 19938140–145. [PubMed] [Google Scholar]
- 38.Dobbe J G G, Streekstra G J, Hardeman M R.et al Measurement of the distribution of red blood cell deformability using an automated rheoscope. Cytometry (Clin Cytometry) 200250313–325. [DOI] [PubMed] [Google Scholar]
- 39.Bor‐Kucukatay M, Yalcin O, Meiselman H J.et al Erythropoietin‐induced rheological changes of rat erythrocytes. Br J Haematol 200011082–88. [DOI] [PubMed] [Google Scholar]
- 40.Powell R J, Machiedo G W, Rush B F. Decreased red blood cell deformability and impaired oxygen utilization during human sepsis. Am Surg 19935965–68. [PubMed] [Google Scholar]
- 41.De Backer D, Creteur J, Preiser J C.et al Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 200216698–104. [DOI] [PubMed] [Google Scholar]
- 42.Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. N Engl J Med 1999340448–454. [DOI] [PubMed] [Google Scholar]