Abstract
Using a rabbit model of tuberculous meningitis (TBM), we compared the protective efficacy of Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination against central nervous system infection with the virulent M. tuberculosis clinical isolate HN878 and the laboratory strain H37Rv. Although BCG clearly provided protection against infection with either challenge strain, protection against disease manifestations was significantly poorer in rabbits infected with HN878. BCG was less efficient in protecting against HN878 dissemination to the liver and spleen and against HN878-induced inflammation, loss of body weight, lung and brain pathology, and signs of disease. We suggest that the efficacy of newly developed vaccines should be tested in animal models not only against challenge with M. tuberculosis H37Rv but also with different clinical isolates including the highly virulent strains of the W-Beijing family.
Keywords: tuberculous meningitis, BCG vaccination, rabbits, M. tuberculosis strains, W-Beijing strains
1. Introduction
The development of improved anti-tuberculosis (TB) vaccines is a major public health priority. The only currently available TB vaccine is the attenuated strain of Mycobacterium bovis bacillus Calmette-Guérin (BCG) [1,2]. Although relatively effective against disseminated TB and tuberculous meningitis (TBM) in children [3], BCG has variable efficacy against pulmonary TB in adults [4-6]. Researchers have tried to explain this variability in terms of genetic variations of vaccinated individuals, interference of environmental mycobacteria, differences in vaccination schedules, or even the use of different BCG strains [6-9]. However, the impact of the genetic variability of the infecting M. tuberculosis strains on vaccine efficacy has not been examined systematically. For example, strains belonging to the W-Beijing family have been implicated as more virulent in mice than other clinical isolates and laboratory strains [10,11]. This family of strains, which is distributed throughout the world, has been associated with major TB outbreaks [12,13]. Only a few comparative studies on post-vaccination protection in mice infected with more virulent strains, such as W-Beijing clinical isolates, have been reported [14-16].
In our previous studies, we established and characterized a rabbit model of TBM that mirrors human central nervous system (CNS) disease [17]. In this rabbit model, mycobacteria are inoculated directly intrathecally and the course of infection and the host response to the infection are monitored. Recently, we evaluated the protective efficacy of Mtb72F, a recombinant fusion polyprotein, against M. tuberculosis H37Rv CNS infection and disease and found that vaccination of rabbits with Mtb72F, formulated in two different adjuvant systems, AS02A or AS01B, is as protective as BCG against challenge with H37Rv [18]. Using the rabbit CNS model, we have also shown that the laboratory strain H37Rv is less virulent in the rabbit than the clinical isolates M. tuberculosis HN878 and W4 (W-Beijing genotype) [19]. Taken together, our results suggest that the rabbit model of CNS infection is a useful tool to evaluate strain virulence, as defined by disease progression and pathology, as well as to test the efficacy of new candidate vaccines. Furthermore, compared to the mouse model of TB, the rabbit model provides additional criteria for evaluation of vaccine protection beyond bacterial load and survival of the animals, such as protection against hematogenous dissemination, inflammation, and pathology.
The present study aims to determine the protective efficacy of BCG vaccination against CNS infection and disease manifestations in rabbits challenged with the laboratory strain H37Rv compared to a more virulent M. tuberculosis strain, the clinical isolate HN878.
2. Materials and Methods
2.1. Clinical Strains of M. tuberculosis
The strains used in this study: 1) M. tuberculosis HN878, kindly provided by Dr. J. M. Musser, Houston, TX [20]; 2) M. tuberculosis H37Rv, kindly provided by Dr. Robert North (TMC No.102, Trudeau Institute, Saranac Lake, NY) and 3) M. bovis BCG (Danish 1331; SSI, Copenhagen, Denmark). HN878 and H37Rv were kept frozen in aliquots until used. Before each experiment, a vial was thawed and subjected to brief ultrasonication 3 times for 10 seconds by using a water bath sonicator to break up aggregates. Aliquots from the same stocks of H37Rv and HN878 were used to infect mice and found to be virulent [21].
2.2. Vaccination of animals
Outbred New Zealand white rabbits, approximately 2.0 kg (Covance Research Products Inc. Denver, PA), were used for this study. Rabbits were vaccinated subcutaneously with 0.1 ml BCG [5×105 colony forming units (cfu)] as described [18].
2.3. Induction of Meningitis
Six weeks after BCG vaccination, a helmet of dental acrylic was attached to the skulls of non-vaccinated and vaccinated rabbits to facilitate immobilization in a stereotaxic frame as previously described [17]. Five vaccinated rabbits were infected intrathecally with 5x105 cfu of M. tuberculosis HN878; a second group of eight vaccinated rabbits was infected with the same inoculum of M. tuberculosis H37Rv. Twelve or ten non-vaccinated rabbits were infected with either HN878 or H37Rv respectively. Clinical and immunologic parameters were monitored for 8 weeks post-challenge, when the rabbits were euthanized. At this time, half of the brain and segments of the lungs, liver and spleen were collected aseptically, homogenized, and used for cfu determinations. The remaining tissues were fixed in 10% buffered formalin acetate (v/v) (Fisher Chemical, Fairlawn, NJ) for histology. The protocol for these experiments was approved by the IACUC at UMDNJ-Newark campus and PHRI, Newark, NJ.
2.4. Evaluation of Rabbit Responses
Cerebrospinal fluid (CSF) samples were analyzed for leukocyte counts (Coulter Electronics Inc., Hialeah, FL) and 0.1 ml was used for the cfu assay. The cfu were evaluated in the CSF and organ homogenates by plating undiluted and 10-fold serial dilutions onto Middlebrook 7H11 agar (Difco), as described [17]. Formalin-fixed brains were cut transversely in serial sections from rostral to caudal, representing the fore-, mid-, and hindbrains. All tissues were embedded in paraffin, sectioned, and stained with haematoxylin and eosin (H&E) and Ziehl-Neelsen for microscopic evaluation.
To evaluate the clinical course of CNS infection in rabbits, we developed the following scoring system [19]: Stage 0 - normal; 1 - hyperesthesia, head tilt, lethargy; 2 -monoparesis; 3 - hemiparesis, recumbency; 4 - quadriplegia; 5 - anorexia, CNS depression progressing to moribund state and death. Stages 4 or 5 were indication for euthanasia.
To evaluate pathological changes in the CNS and lungs of infected rabbits, we have developed a scoring system, described previously [18]: Brain and meninges: 0 – normal; 1- mild meningitis; 2 – moderate focal meningitis; 3 – moderate meningitis with vasculitis; 4 – severe meningitis and encephalitis. Lung: 0 – normal parenchyma; 1 – increased cellularity; 2 – one to two small, organized granulomas per section (approximately 1 sq. cm); 3 – more than two larger lesions per section, with central necrosis; 4 – large confluent lesions with acid fast bacilli. All histologic sections were evaluated by a single investigator blinded to the rabbit treatment.
2.5. Statistical Considerations
The independent Student t test or the Mann-Whitney test for nonparametric independent data was used for analyses. The Kruskal - Wallis test was used to determine statistical differences in the clinical manifestation scores between different groups. A P value of <0.05 was considered significant.
3. Results
3.1. Effect of BCG vaccination on M. tuberculosis CNS infection and dissemination
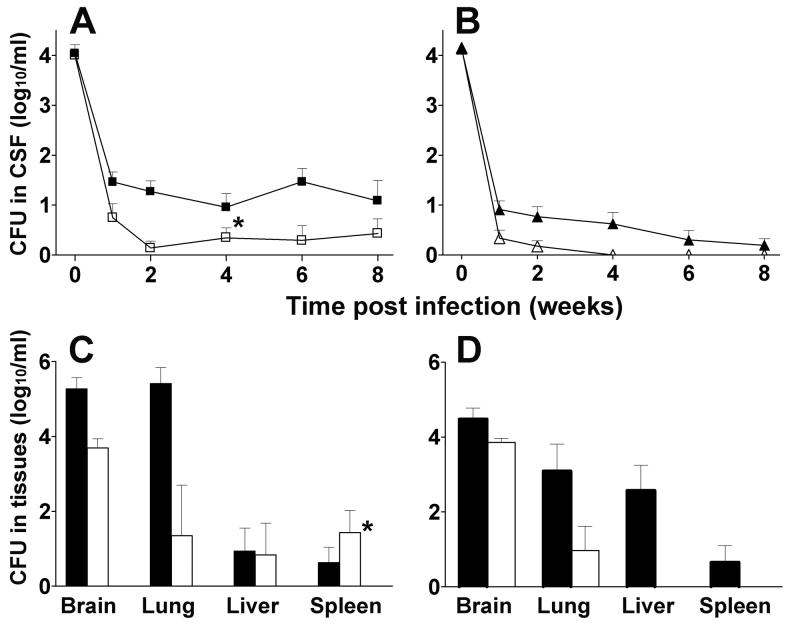
We evaluated the protective efficacy of vaccination with BCG in rabbits challenged with either M. tuberculosis HN878 or H37Rv. At 2 hours post-challenge, the number of cfu detected in the CSF of animals from all experimental groups was approximately 4 log10 (Fig. 1A and B). In non-vaccinated rabbits infected with HN878, the bacillary load in the CSF persisted at a higher level (>1.5 log10) throughout the experiment compared to the bacillary load in rabbits infected with H37Rv (Fig. 1A and B). In BCG-vaccinated rabbits infected with HN878, the bacillary load in the CSF was significantly reduced (P=0.04 by Mann-Whitney); however, small numbers of viable bacilli remained in the CSF up to 8 weeks. In comparison, BCG vaccination followed by H37Rv challenge, resulted in a significant (P=0.006) reduction in the bacillary load compared to non-vaccinated animals, reaching undetectable levels by 4 weeks post-challenge. At this time, the bacillary load in the CSF was significantly higher in vaccinated HN878-infected rabbits compared to vaccinated H37Rv-infected rabbits (P=0.02) (Fig. 1A and B).
Fig. 1.
Effect of BCG vaccination on M. tuberculosis CNS infection and dissemination. A. Number of CFU of M. tuberculosis HN878 in the CSF. Control non-vaccinated rabbits (black squares) (n=12), rabbits vaccinated with BCG (open squares) (n=5). B. Number of CFU of M. tuberculosis H37Rv in the CSF. Control non-vaccinated rabbits (black triangles) (n=10), rabbits vaccinated with BCG (open triangles) (n=8). Bacillary clearance was significantly accelerated in BCG-vaccinated rabbits challenged with H37Rv compared to challenge with HN878 (*P=0.02). C. CFU in tissues 8 weeks post-infection with HN878. Control nonvaccinated rabbits (black bars) (n=7), BCG-vaccinated rabbits (white bars) (n=5). D. CFU in tissues 8 weeks post-infection with H37Rv. Control non-vaccinated rabbits (black bars) (n=10), BCG-vaccinated rabbits (white bars) (n=8). Significant differences in dissemination of H37Rv vs HN878 to the liver and spleen of BCG-vaccinated rabbits were observed (*P=0.01). Values are mean ± SEM.
At 8 weeks post-challenge, rabbits were euthanized and bacillary spread to other organs was evaluated. BCG vaccination of HN878-infected animals induced a significant reduction in the CFU observed in the brains and lungs (Fig. 1C) (P=0.003 and P=0.005, respectively). Vaccination of rabbits with BCG did not protect against dissemination and/or growth of HN878 in liver and spleen. As previously noted [19], infection of control rabbits with H37Rv resulted in lower CFU in brains and lungs compared to infection with HN878. BCG-vaccination in animals infected with H37Rv was associated with somewhat reduced bacillary loads in the lungs (Fig. 1D). In BCG-vaccinated animals, H37Rv did not disseminate to the liver or spleen. Thus, at 8 weeks post-challenge, a significant difference in CFU in the spleen was noted between animals challenged with H37Rv versus HN878 (P=0.01) (Fig. 1C and D). These results suggest that protection by BCG against bacillary dissemination from the CNS to liver and spleen correlates inversely with the virulence of the infecting M. tuberculosis strain.
3.2. Effect of BCG vaccination on the inflammatory response, body weight and pathology of brain and lungs of infected rabbits
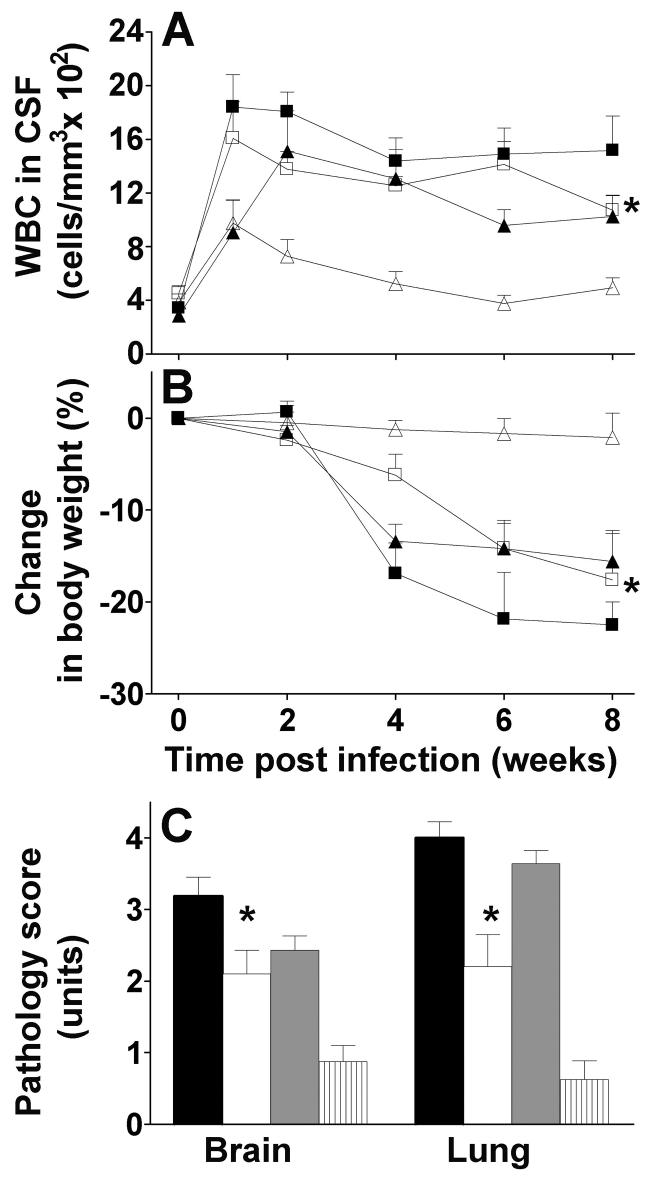
A major criterion for meningeal inflammation is leukocyte influx into the CSF. Infection of non-vaccinated rabbits with M. tuberculosis HN878 resulted in early recruitment and persistence of leukocytes in the CSF at a slightly higher level than infection with H37Rv (Fig. 2A). BCG-vaccination of HN878-infected rabbits resulted in a non-significant reduction compared with non-vaccinated animals. In contrast, BCG vaccination of H37Rv-infected rabbits resulted in a larger reduction in leukocytosis in the CSF. Thus, at 8 weeks post-challenge, a significant difference in leukocytosis in the CSF was noted between BCG vaccinated animals following challenge with HN878 versus H37Rv (P=0.02 by Mann-Whitney) (Fig. 2A).
Fig. 2.
Effect of BCG vaccination on the inflammatory response, body weight and pathology score of brain and lungs of infected rabbits. A. White blood cell (WBC) density in CSF. Control non-vaccinated rabbits infected with HN878 (black squares) (n=12), BCG-vaccinated rabbits infected with HN878 (open squares) (n=5), control non-vaccinated rabbits infected with H37Rv (black triangles) (n=8), BCG-vaccinated rabbits infected with H37Rv (open triangles) (n=8). BCG protection against leukocytosis was more pronounced in animals challenged with H37Rv vs. animals challenged with HN878 (*P=0.02, Mann-Whitney test). B. Changes in body weight. Control non-vaccinated rabbits infected with HN878 (black squares) (n=12), BCG-vaccinated rabbits infected with HN878 (open squares) (n=5), control non-vaccinated rabbits infected with H37Rv (black triangles) (n=10), BCG-vaccinated rabbits infected with H37Rv (open triangles) (n=8). BCG-vaccinated rabbits infected with H37Rv lost less body weight compared to BCG vaccinated rabbits infected with HN878 (*P=0.01-0.001). C. Severity of pathology in the brain and lungs. Control non-vaccinated rabbits infected with HN878 (black bars) (n=8), BCG-vaccinated rabbits infected with HN878 (white bars) (n=5), control non-vaccinated rabbits infected with H37Rv (gray bars) (n=8), BCG-vaccinated rabbits infected with H37Rv (striped bars) (n=8). Significantly better BCG-induced protection against pathology was observed following challenge with H37Rv compared to challenge with HN878 in the brain and lungs (*P=0.009 and *P=0.007 respectively). Values are mean ± SEM.
Weight-loss, a manifestation of TB disease, was most extensive in non-vaccinated animals infected with HN878, where animals lost more than 20% of their body weight over the 8 weeks of infection. Non-vaccinated rabbits infected with H37Rv lost less weight (Fig. 2B). BCG-induced protection from weight-loss was significantly better in the animals infected with H37Rv compared to those infected with HN878 (P= 0.01-0.001 for different time points) (Fig. 2B).
Non-vaccinated rabbits infected with HN878 developed severe meningitis with large numbers of inflammatory cells in the sub-arachnoid space (SAS), thickening of the leptomeninges and prominent necrotizing vasculitis. Encephalitis and vasculitis within the brain parenchyma (neuropil) were also observed (not shown). The brain pathology score was 3.2 from a maximum of 4 (Fig. 2C). BCG-vaccinated HN878-challenged rabbits showed significantly (P=0.02) reduced, moderate focal inflammation and partial vasculitis of the meninges with score 2.1 (Fig. 2C and 3A). Brain pathology was also extensive in response to H37Rv infection. Prior BCG vaccination of rabbits infected with H37Rv resulted in significantly (P<0.001) attenuated meningeal inflammation, with few inflammatory cells in the SAS and no vasculitis (score 0.88) (Fig. 2C and 3B). Residual pathology in the brain was significantly less or lower in BCG-vaccinated H37Rv-infected animals compared with BCGvaccinated HN878-infected animals (P=0.009) (Fig. 3A and B).
Fig. 3.
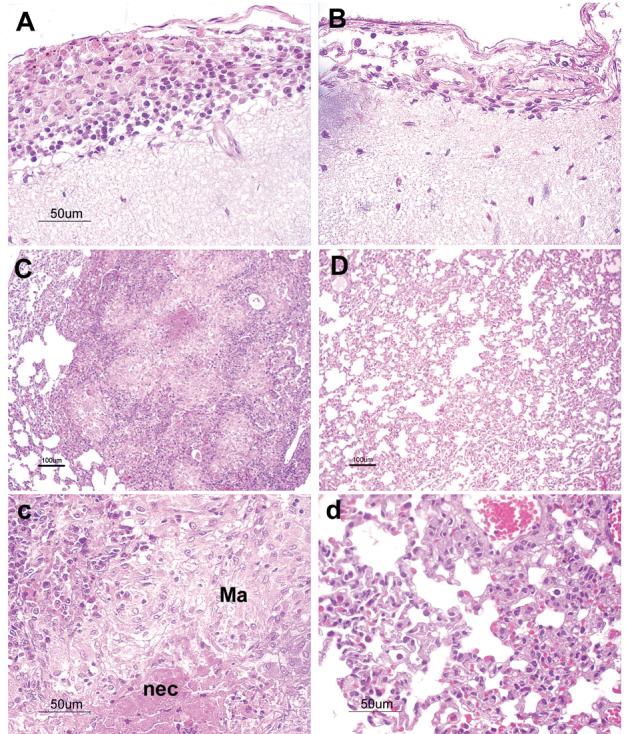
Histopathology of the meninges and brain (A-B) and the lung (C-d). A. BCG-vaccinated rabbits infected intrathecally with M. tuberculosis HN878, at 8 weeks post-infection. Distension of the sub-arachnoid space by a significant number of inflammatory cells (lymphocytes and macrophages) is noted. B. BCG-vaccinated rabbits infected with M. tuberculosis H37Rv. Very few lymphocytes are seen in the meninges. C and c. Lung of vaccinated rabbit infected with HN878. Large granulomas with central necrosis (nec) and lots of activated macrophages (Ma) and lymphocytes were seen. D and d. Lung of vaccinated rabbit challenged with H37Rv. Only increased cellularity with no granuloma formation was noted. Sections are stained with haematoxylin and eosin. Magnification, x10 (for panels C and D) and x40 (for panels A, B, c and d).
Large confluent granulomas with big areas of central necrosis and single acid fast bacilli were observed in the lungs of non-vaccinated rabbits infected with HN878 (lung pathology score of 4 from a maximum of 4) (Fig. 2C). BCG-vaccinated HN878-infected rabbits showed significantly (P=0.004) attenuated pathology in the lungs (score of 2.2), with few, well-organized granulomas and no acid fast bacilli (Fig. 3C and c). In these granulomas, macrophages appeared epithelioid (activated) and surrounded by lymphocytes (Fig. 3c); large areas of the lung parenchyma were normal, i.e. uninvolved in the pathologic process. Significant (P<0.001) protection against lung pathology was also obtained by BCG vaccination in rabbits infected with H37Rv (score 0.6 vs. 3.6), (Fig 2C, 3D and d). Only increased cellularity with no granuloma formation was observed in the lungs of these animals. Residual pathology in the lungs was significantly less in BCG-vaccinated H37Rv challenged rabbits compared to BCG-vaccinated animals infected with HN878 (P=0.007) (Fig. 2C). Thus, vaccination of rabbits with BCG resulted in reduction in inflammation and pathology of brain and lungs compared to non-vaccinated rabbits. This protective effect was significantly more pronounced in rabbits challenged with H37Rv.
The inflammatory response correlated with the clinical manifestations of TBM. None of the H37Rv infected animals showed neurological signs (Table 1). In contrast, rabbits infected with HN878 demonstrated significant signs of disease from 3 weeks post-challenge, including loss of coordination, pareses and paralysis of the limbs and death. BCG-vaccinated HN878-infected animals had less neurologic signs and a milder course of disease (P=0.001 by Kruskal - Wallis test). By the end of the experiment, a significant difference in disease manifestations was seen in BCG-vaccinated H37Rv-infected animals compared to BCG vaccinated HN878-infected rabbits (P=0.01) (Table 1).
Table 1.
Clinical score in BCG-vaccinated and unvaccinated control rabbits (units)
| Time post Infection (weeks) |
Control HN878 |
BCG/HN878 | Control H37Rv |
BCG/H37Rv |
|---|---|---|---|---|
| 2 | 0 | 0 | 0 | 0 |
| 4 | 0.6 ± 1.0 | 0 | 0 | 0 |
| 6 | 1.0 ± 1.5 | 0.4 ± 0.5 | 0 | 0 |
| 8 | 2.7 ± 2.6 | 0.7 ± 0.6a | 0 | 0 |
P=0.01 vs. BCG/H37Rv
4. Discussion
This study demonstrates that BCG vaccination of rabbits protects against CNS challenge with both M. tuberculosis H37Rv and HN878. However, the level of residual infection and disease manifestations are more severe following challenge of BCG-vaccinated animals with HN878. BCG-induced immunity was less efficient in overcoming HN878 dissemination to the liver and spleen, CSF inflammation, loss of body weight and pathology. We have previously shown that in the rabbit, M. tuberculosis HN878 and H37Rv are differentially virulent, as defined by severity of disease and/or higher bacillary load in infected tissues [19]. In this study too, infection with HN878 resulted in a persistent bacillary load in the CSF and higher cfu numbers in the brain and lungs as compared to H37Rv infection; a higher propensity for prolonged inflammation was also noted. Rabbits infected with HN878 developed severe progressive meningitis with limb paralyses by 3 weeks, whereas animals infected with H37Rv showed no neurological signs. Thus vaccine-induced protective immunity against TB disease is poorer when the rabbits are challenged with more virulent M. tuberculosis strains. Our observations confirm and expand previous reports of studies carried out in the mouse TB infection model [11,14-16]. In murine studies, BCG conferred only low to absent protection against M. tuberculosis W-Beijing strains. While our rabbit model of TBM may not fully reflect the natural history of CNS infection in humans, this model resembles human TBM clinically and histo-pathologically and provides additional information on the protective efficacy of vaccines against the disease and pathology of CNS TB infection.
Most TB vaccine evaluations have been carried out using the laboratory strain H37Rv as challenge [22-26]. Our present studies suggest that H37Rv may not be the most stringent test for vaccine efficacy because it is less virulent than selected clinical isolates, such as HN878. The differential response that we observe between H37Rv and HN878 challenge is not a result of attenuation of our H37Rv isolate. We have used this H37Rv strain in murine infection studies in our laboratory, where median survival times (197 days) for immune competent animals were shorter compared to animals infected with the clinical isolates CDC1551, NHN5, HN60 [21]. In addition, we have shown H37Rv to be of intermediate virulence, between CDC1551 on the one hand and HN878 and W4 on the other, in the rabbit CNS infection model [19]. Similarly, in rabbits infected by aerosol with CDC1551, Erdman or H37Rv, the latter strain showed intermediate virulence, requiring lower number of inhaled bacilli to form one grossly visible pulmonary tubercle compared to CDC1551 [27].
Recent epidemiologic studies have shown that differences in the virulence among M. tuberculosis strains are associated with the genetic background of the organisms [28],[29]. Strains of W-Beijing genotype family, which prevail in large geographic areas and significantly contribute to the current TB epidemic [12,13,30], have been associated with resistance to antituberculosis drugs in a number of studies [31,32]. The high prevalence of the Beijing genotype indicates the success of this M. tuberculosis strain type as a human pathogen [12,33]. This may be a result of certain advantages, such as acquired mechanisms to circumvent BCG-induced immunity over strains belonging to other genotypes [10,14,34].
Our present results have important implications for the evaluation of new candidate vaccines for use in control of TB. Our results provide evidence that the level of protection against M. tuberculosis infection and disease conferred by vaccination is influenced by the nature of the infecting strain. Thus, we suggest that the protective efficacy of any newly developed vaccines, targeted to be used in humans, should be tested not only against challenge with M. tuberculosis H37Rv but also against different clinical isolates infecting human populations including the widely distributed highly virulent strains of the W-Beijing family.
Acknowledgement
We thank Dr. Andre L. Moreira for his assistance with histology. Funding for these studies was provided in part by NIH grant AI054338 (to GK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brewer TF, Colditz GA. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis. 1995;20(1):126–135. doi: 10.1093/clinids/20.1.126. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg AM. The tuberculosis epidemic. Scientific challenges and opportunities. Public Health Rep. 1998;113(2):128–136. [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Global Tuberculosis Control. World Health Organization; Geneva: 2000. [Google Scholar]
- 5.Orme IM. The search for new vaccines against tuberculosis. J Leukoc Biol. 2001;70(1):1–10. [PubMed] [Google Scholar]
- 6.Fine PE. BCG: the challenge continues. Scand J Infect Dis. 2001;33(4):243–245. doi: 10.1080/003655401300077144. [DOI] [PubMed] [Google Scholar]
- 7.Lagranderie MR, Balazuc AM, Deriaud E, Leclerc CD, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996;64(1):1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284(5419):1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 9.Mollenkopf HJ, Kursar M, Kaufmann SH. Immune response to postprimary tuberculosis in mice: Mycobacterium tuberculosis and Miycobacterium bovis bacille Calmette-Guerin induce equal protection. J Infect Dis. 2004;190(3):588–597. doi: 10.1086/422394. [DOI] [PubMed] [Google Scholar]
- 10.Manca C, Tsenova L, Bergtold A, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001;98(10):5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris SL. The pre-clinical assessment of vaccine-induced protection using different M. tuberculosis challenge strains; The Second International Conference on TB vaccines for the world; Vienna, Austria. 2006. [Google Scholar]
- 12.Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis. 2002;8(8):843–849. doi: 10.3201/eid0808.020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 2002;10(1):45–52. doi: 10.1016/s0966-842x(01)02277-6. [DOI] [PubMed] [Google Scholar]
- 14.Lopez B, Aguilar D, Orozco H, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003;133(1):30–37. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castanon-Arreola M, Lopez-Vidal Y, Espitia-Pinzon C, Hernandez-Pando R. A new vaccine against tuberculosis shows greater protection in a mouse model with progressive pulmonary tuberculosis. Tuberculosis (Edinb) 2005;85(12):115–126. doi: 10.1016/j.tube.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115(9):2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsenova L, Sokol K, Freedman VH, Kaplan G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis. 1998;177(6):1563–1572. doi: 10.1086/515327. [DOI] [PubMed] [Google Scholar]
- 18.Tsenova L, Harbacheuski R, Moreira AL, et al. Evaluation of the Mtb72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect Immun. 2006;74(4):2392–2401. doi: 10.1128/IAI.74.4.2392-2401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsenova L, Ellison E, Harbacheuski R, et al. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis. 2005;192(1):98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 20.Sreevatsan S, Pan X, Stockbauer KE, et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci U S A. 1997;94(18):9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manca C, Tsenova L, Barry CE, 3rd, et al. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162(11):6740–6746. [PubMed] [Google Scholar]
- 22.Skeiky YA, Alderson MR, Ovendale PJ, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172(12):7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 23.Brandt L, Skeiky YA, Alderson MR, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun. 2004;72(11):6622–6632. doi: 10.1128/IAI.72.11.6622-6632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orme IM, McMurray DN, Belisle JT. Tuberculosis vaccine development: recent progress. Trends Microbiol. 2001;9(3):115–118. doi: 10.1016/s0966-842x(00)01949-1. [DOI] [PubMed] [Google Scholar]
- 25.Sambandamurthy VK, Derrick SC, Hsu T, et al. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006;24(3739):6309–6320. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 26.Sampson SL, Dascher CC, Sambandamurthy VK, et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun. 2004;72(5):3031–3037. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manabe YC, Dannenberg AM, Jr., Tyagi SK, et al. Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect Immun. 2003;71(10):6004–6011. doi: 10.1128/IAI.71.10.6004-6011.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valway SE, Sanchez MP, Shinnick TF, et al. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338(10):633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 29.Bifani PJ, Mathema B, Liu Z, et al. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. Jama. 1999;282(24):2321–2327. doi: 10.1001/jama.282.24.2321. [DOI] [PubMed] [Google Scholar]
- 30.van Soolingen D, Qian L, de Haas PE, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J Clin Microbiol. 1995;33(12):3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bifani PJ, Plikaytis BB, Kapur V, et al. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. Jama. 1996;275(6):452–457. [PubMed] [Google Scholar]
- 32.Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83(13):44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 33.Abebe F, Bjune G. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille Calmette-Guerin (BCG) vaccines: is there a link? Clin Exp Immunol. 2006;145(3):389–397. doi: 10.1111/j.1365-2249.2006.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pheiffer C, Betts JC, Flynn HR, Lukey PT, van Helden P. Protein expression by a Beijing strain differs from that of another clinical isolate and Mycobacterium tuberculosis H37Rv. Microbiology. 2005;151(Pt 4):1139–1150. doi: 10.1099/mic.0.27518-0. [DOI] [PubMed] [Google Scholar]