Abstract
Lung cancer is the leading cause of cancer death worldwide. The disease is particularly difficult to detect, and patients often present at an advanced stage. Current treatments have limited effectiveness, and unfortunately, the prognosis remains poor. Recent insights into the molecular pathogenesis and biologic behavior of lung cancer have led to the development of rationally designed methods of early detection, prevention, and treatment of this disease. This article will review the important clinical implications of these advances, with a focus on new molecularly targeted therapies currently in development.
Global cancer statistics indicate that lung cancer is responsible for over 1 million deaths each year (1). Approximately 80%–85% of all lung cancers are non–small cell lung cancer (NSCLC), which include squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma. Small cell lung cancer (SCLC) represents approximately 15%–20% of cases, and the incidence of SCLC has been decreasing over the last several years in the United States (2). Current treatment options include surgical resection, platinum-based doublet chemotherapy (a combination of two drugs that includes carboplatin or cisplatin as the backbone), and radiation therapy alone or in combination. Unfortunately, despite these therapies, the disease is rarely curable and prognosis is poor, with an overall 5-year survival rate of only 15% (3). This article will review recent advances and future perspectives in the development of novel strategies for the treatment of lung cancer, with an emphasis on how these can be derived from our understanding of the molecular pathogenesis of the disease.
Historical perspective
In the mid-1800s, lung cancer was a rare disease, representing only 1% of all cancers seen at autopsy. By the early 1900s, the incidence of malignant lung tumors had begun to rise, and although most lung cancers occurred in men, a steady increase in women was observed beginning in the 1960s. While the link between cigarette smoking and lung cancer was suspected by clinicians in the 1930s, the cause of the dramatic increase was not well established until landmark epidemiologic studies in the 1950s provided evidence for a strong causal association between smoking and lung cancer (4). Surgical therapy for lung cancer began in the 1930s, with the first successful pneumonectomy for lung cancer reported by Evarts Graham in 1933 (5). The prognostic significance of nodal metastases was subsequently recognized, and surgical mediastinal lymph node sampling became an important aspect of staging. Current surgical approaches use lobectomy or, if necessary, pneumonectomy for curative treatment, while wedge resections are reserved for patients with severely impaired lung function. Likewise, preoperative evaluation and postoperative care have improved, resulting in operative mortality rates of less than 5%. With the invention of megavoltage linear accelerators, radical radiotherapy for potential cure of lung cancer was introduced in the 1950s. There have been significant technologic developments in radiation therapy planning and delivery such that currently, approximately 15% of patients with early-stage disease can be cured with radiotherapy alone. Nitrogen mustard was the first chemotherapy used for the treatment of lung cancer in the 1940s. Pilot studies from the late 1970s and early 1980s identified other drugs with activity against lung cancer, particularly drug combinations using cisplatin with a vinca alkaloid or etoposide (6). Over the next two decades, several prospective, randomized studies were conducted to evaluate various chemotherapy regimens (6, 7). These trials concluded that platinum-based doublet chemotherapy provides a modest survival benefit when compared with no chemotherapy and also provides palliation of symptoms and improves quality of life.
New strategies for lung cancer management
Despite advances in cytotoxic chemotherapy and combined modality treatments for lung cancer, the prognosis remains poor, as patients are often diagnosed with metastatic disease and current systemic therapies have limited effectiveness. Major advances in the understanding of the molecular pathogenesis of lung cancer have led to new strategies for early detection, diagnosis, staging, and therapy that hold promise for improving lung cancer outcomes.
Risk assessment, early detection, and prevention
Although it is well established that tobacco smoke causes lung cancer, not all smokers develop lung cancer, and genetic factors are thought to play a role in lung cancer susceptibility. Epidemiologic studies have found an approximate 2-fold increased risk attributable to a family history of lung cancer after controlling for tobacco smoke exposure (8). Racial differences in familial risk have also been observed, and first-degree relatives of black individuals with lung cancer are at a 2-fold increased risk of lung cancer compared with their white counterparts (9). A large linkage analysis of 52 pedigrees by the Genetic Epidemiology Lung Cancer Consortium (GELCC) has recently identified a major susceptibility locus for inherited lung cancer on chromosome 6q23–25 (10), and the search for a susceptibility gene in this region is ongoing. Observational studies have also suggested that there may be racial and sex differences in the smoking-related risk of lung cancer, with black smokers, native Hawaiian smokers, and female smokers having a greater risk than other populations, although findings have not been conclusive (11, 12). While these differences in lung cancer risk have largely been attributed to variations in smoking patterns and carcinogen exposure, molecular epidemiologic studies are underway to further identify hormonal and genetic factors that may explain the observed differences. Results from these studies will potentially identify high-risk groups that will most benefit from targeted preventative or therapeutic strategies.
Lung cancer is difficult to detect and is often diagnosed at a late stage, only when symptoms are clinically evident. Recent studies in spiral CT imaging have shown that early detection is possible and that surgical resection of such lesions is associated with the potential for a high cure rate (13). However, the role of population-based CT screening in actually improving survival remains controversial, and the results of the large (50,000-person) prospective, randomized National Lung Cancer Screening Trial (NLST) are awaited.
The identification of molecular biomarkers for lung cancer in sputum and blood is an area of active research, offering potentially useful tests to complement radiologic imaging and bronchoscopy for screening and early diagnosis. Recent studies have identified numerous genetic and epigenetic alterations in lung tumors (>20 per tumor), including DNA sequence alterations, copy number changes, and aberrant promoter hypermethylation (14). Several of these genetic abnormalities are also found in premalignant and histologically normal bronchial epithelium, suggesting that lung cancers develop from normal cells through a multistep process involving successive genetic and epigenetic changes. For example, greater than 90% of lung cancers have a deletion at chromosome region 3p21.3, which includes the RASSF1A, FUS1, SEMA3B, and SEMA3F tumor suppressor genes (15). Inactivation by promoter hypermethylation has been reported in more than 40 tumor suppressor genes in lung cancer, including p16INK4a, MGMT, DAPK, RASSF1A, FHIT, and TIMP3 (16). EGFR mutations are also found in histologically normal epithelium of patients whose tumors contain the same mutation, indicating that the mutations occur early in lung cancer pathogenesis (17). Studies have shown that common molecular alterations found in lung tumors are also detectable in sputum prior to lung cancer diagnosis, including 3p allele loss (15) and hypermethylation of p16INK4a and MGMT (18, 19). A recent report found that the presence of deletions in both FHIT and HYAL2 tumor suppressor genes in sputum from patients with stage I NSCLC was able to diagnose lung cancer with greater sensitivity than sputum cytology (20). Emerging genomic and proteomic technologies have also led to the discovery of biomarkers detectable in blood that have potential clinical applications (see “Individualizing prognosis and therapy”). For example, alterations of DNA sequences, such as microsatellite instability (MSI) or loss of heterozygosity (LOH), have been identified in blood samples from patients with early-stage NSCLC but not in normal controls (21). Prospective studies are clearly warranted to evaluate the clinical utility of molecular biomarkers in sputum and blood for screening and early diagnosis. Furthermore, the integration of screening tests into clinical practice will identify patients at high risk for developing lung cancer (based on abnormal findings by CT imaging, bronchoscopy, and/or biomarker profiling), and rational strategies for targeted prevention and monitoring of these individuals will need to be developed and implemented.
Diagnosis and staging
The traditional diagnosis and staging of lung cancer includes clinical and radiographic assessment, and biopsy for tissue diagnosis. Histologic subtypes are distinguished based on cellular morphology visualized under light microscopy, and histopathological diagnosis is particularly important, as treatment for SCLC differs significantly from that for NSCLC. However, morphological classification can be difficult because of heterogeneity of lung tumors. Gene expression profiling of lung tumors has identified distinct molecular subtypes that correlate with clinical outcome and may enhance our ability to predict relapse and survival and thus facilitate treatment decisions (22–24). For example, expression profiling studies have identified two subclasses of adenocarcinomas that correlate with survival (22, 24).
Molecularly targeted therapies
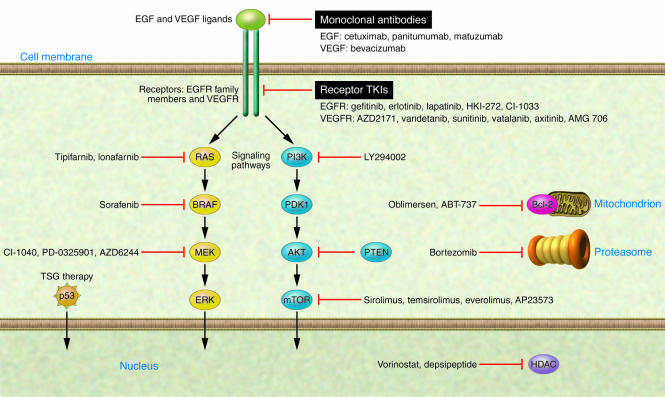
Studies in the molecular and cellular biology of lung cancer have gradually revealed the “circuit diagram” of pathways and molecules driving cells to full-fledged lung cancer. These studies include identification of genetic and epigenetic changes of specific molecules resulting in the activation of signaling pathways important in carcinogenesis. Some of these alterations involve known oncogenes and tumor suppressor genes. In searching for targeted therapies, special attention is paid to identifying single or multiple genes that the lung cancer cells absolutely require for their malignant phenotype and survival. These are often thought of as “oncogene addictions” (25). Several novel drugs specifically targeting these key components have been developed (Figure 1 and Table 1), and clinical trials thus far have yielded encouraging results.
Figure 1. Novel therapies targeting key oncogenic pathways in lung cancer.
Several of the signaling and cell physiology pathways that are abnormal in lung cancer are depicted schematically along with drugs targeting components of these abnormal pathways. These drugs specifically target important molecules and pathways involved in lung cancer cell proliferation, inhibition of apoptosis, angiogenesis, and invasion and are currently in clinical trials for lung cancer. These include agents specifically inhibiting components of EGFR and other family members (such as ERBB2/Her2) and/or VEGFR pathways (with monoclonal antibodies and receptor TKIs or with inhibitors of key downstream pathway mediators such as the RAS/RAF/MEK or PI3K/Akt/mTOR pathway). Other agents in development include tumor suppressor gene (TSG) therapies, inhibitors of antiapoptotic proteins such as Bcl-2, HDAC inhibitors targeting the multiple epigenetic changes found in lung cancer, and proteasome (Pr) inhibitors. PDK1, pyruvate dehydrogenase kinase isozyme 1; PTEN, phosphatase and tensin homolog.
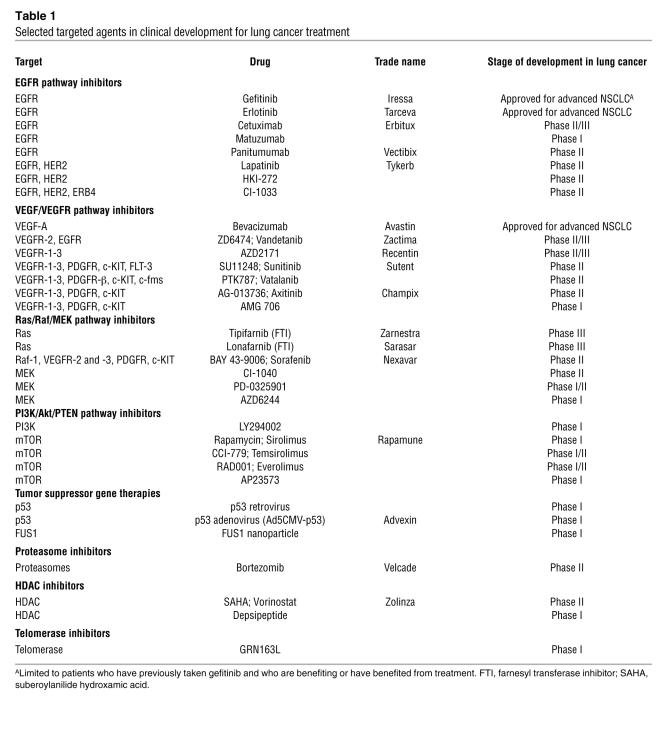
Table 1 .
Selected targeted agents in clinical development for lung cancer treatment
EGFR pathway inhibitors
The EGF pathway is frequently dysregulated in human cancers, making it an attractive target for anticancer therapy. The EGFR family of receptors consists of transmembrane tyrosine kinase (TK) receptors and includes EGFR (also known as HER1 or ERBB1), HER2 (EGFR2 or ERBB2/NEU), HER3 (EGFR3 or ERBB3), and HER4 (EGFR4 and ERBB4) (26). EGFR (erbB1) and its ligands are frequently overexpressed in NSCLC tumors (~70%) but rarely expressed in SCLCs (27). Binding of ligand to the EGFR causes dimerization of the receptor, which in turn activates the intracellular TK domain of the receptor, leading to its autophosphorylation and further activating a cascade of intracellular events leading to cell proliferation, inhibition of apoptosis, angiogenesis, and invasion, all resulting in tumor growth and spread. The various EGFR family members can heterodimerize with each other so that all of the ERB receptors expressed in a tumor cell must be known in order to understand their importance as therapeutic targets. Agents targeting EGFR include the TK inhibitors (TKIs), such as gefitinib (ZD1839; Iressa) and erlotinib (OSI-774; Tarceva), and the monoclonal antibodies cetuximab (Erbitux) and panitumumab (ABX-EGF; Vectibix).
Recently, tumor-acquired mutations in the TK domain of EGFR have been described; they are found in approximately 5%–10% of NSCLCs in the United States and are often associated with gene amplification (28, 29). Several clinicopathologic features have been found to correlate with the frequency of EGFR mutations and EGFR gene amplification, including adenocarcinoma histology, never smoking history, female sex, and East Asian ethnicity (30). Patients with all of these features have greater than 50% probability of having an EGFR TK domain mutation (30). Importantly, the presence of EGFR TK domain mutations in tumors correlates with response to EGFR TKI therapy with drugs such as gefitinib and erlotinib (28, 29). Of note, a subset of NSCLC patients with mutant EGFR do not respond to TKIs, and a “second” TK domain mutation (T790M), which is associated with acquired drug resistance, has been described (31, 32). Several patients with tumors having the T790M mutation but who have never received EGFR TKI therapy have been described, suggesting that the T790M mutation has oncogenic potential. Drugs that inhibit EGFR with the T790M mutation have been developed and are being tested in clinical trials (33).
While patients with EGFR mutations are most likely to have a dramatic response to EGFR TKI therapy, EGFR amplification and protein overexpression have been found to correlate with survival after EGFR TKI therapy, as does Akt activation (34, 35). Although the hypothesis is controversial, some studies indicate that EGFR amplification and/or protein expression are better predictors of survival after EGFR TKI therapy than are mutations (35, 36).
The EGFR TKIs gefitinib and erlotinib have been extensively studied either alone or in combination with cytotoxic chemotherapy in NSCLC. Two large randomized studies with these agents as monotherapy in previously treated advanced-stage NSCLC patients have been conducted. The results showed that compared with best supportive care, erlotinib prolonged survival by 2 months (BR.21 trial), while gefitinib failed to demonstrate a survival benefit (ISEL trial) (37, 38). The reasons for the different outcomes are unclear, although differences in drug dosing and clinical features of patients between the two trials may have contributed. Specifically, in the BR.21 trial, erlotinib was given at its maximum tolerated dose (MTD), while in the ISEL study, gefitinib was given at a dose below the MTD (250 mg/d as compared with MTD 600 mg/d). Thus, at higher doses, erlotinib may hit TK targets other than EGFR. Indeed, an important advantage of specific oncogene–driven mouse models of lung cancer is that the reversal of the target genetic changes is well defined. Examples of this are seen in reversing oncogenic KRAS or EGFR in mouse models of lung cancer leading to “cure” of these mouse tumors (39–41). In addition, the ISEL trial only included patients with progressive disease within 90 days of stopping previous chemotherapy, while the BR.21 trial did not have similar criteria. A greater proportion of patients had progressive disease in the ISEL trial as well (45% versus 28%).
Gefitinib and erlotinib have both been tested in randomized studies in combination with cytotoxic chemotherapy as first-line treatment for metastatic NSCLC. These studies demonstrated no overall survival benefit of adding either drug to chemotherapy, although a retrospective subset analysis suggested that patients who have never smoked may derive benefit with the combination (42, 43). While tumor EGFR mutation and/or amplification status are highly associated with beneficial responses to EGFR TKI therapy, all of the trials have shown that some patients without these features can also benefit. Thus, there are efforts to identify new tumor molecular profiles, interindividual germline differences (tested on a genome-wide scale with single nucleotide polymorphism analyses), or serum proteomic or genomic biomarkers that will help predict which patients would benefit from single-agent or combined therapy. Randomized clinical trials evaluating the role of first-line EGFR TKI therapy in patient populations determined to be potentially responsive according to predictive clinical and/or molecular features are ongoing.
Cetuximab, a humanized monoclonal antibody to the extracellular domain of EGFR, has also been studied in NSCLC. When administered to patients with previously treated NSCLC, its activity was similar to those of other second-line cytotoxics (44). Preliminary results of a randomized phase II study of cetuximab in combination with chemotherapy versus chemotherapy alone in the first-line treatment of patients with EGFR-expressing advanced NSCLC suggested a higher response rate with the addition of cetuximab (45), and a confirmatory phase III study is ongoing (46). Cetuximab is also being studied in combination with chemoradiation for stage III NSCLC (47) and with chemotherapy in the neoadjuvant setting in patients with resectable stage IB–IIIA NSCLC (48). Other agents targeting the EGFR pathway in clinical trials include panitumumab (targeting EGFR), lapatinib (targeting EGFR and HER2), and HK-272 (targeting EGFR and HER2) (Table 1).
Angiogenesis inhibitors
For tumors to be able to grow beyond 2 mm3, angiogenesis is essential in order to supply adequate oxygenation and nutrition to tissues (49). VEGF is the most important growth factor controlling angiogenesis in normal and tumor cells (50). The VEGF family consists of six growth factors (VEGF-A, -B, -C, -D, and -E and placental growth factor [PlGF]) and three receptors (VEGFR-1 [also known as Flt-1], VEGFR-2 [KDR/Flk-1], and VEGFR-3 [Flt-4]). The VEGF/VEGFR pathway is frequently upregulated in lung cancer (51), and VEGF overexpression is associated with tumor progression and poor prognosis (52). Several agents aimed at targeting the VEGF/VEGFR signaling network are currently under investigation. The monoclonal antibodies against VEGF and the VEGFR TKIs are among the best studied.
Bevacizumab (Avastin) is a monoclonal antibody that binds to all isoforms of VEGF-A and has been tested in clinical trials. A recent randomized study demonstrated that the addition of bevacizumab to carboplatin and paclitaxel for the first-line treatment of patients with advanced nonsquamous NSCLC provides a significant survival benefit (53), and consequently, bevacizumab has been recently approved for use in NSCLC. Pulmonary hemorrhage associated with bevacizumab treatment has been reported as an occasional but serious life-threatening side effect, highlighting the importance of patient selection and monitoring for this therapy (54). Patients with squamous cell histology are particularly at risk of bleeding and have been excluded from recent trials. Clinical trials to further assess the role of bevacizumab in combination with other therapies in lung cancer are underway. These include randomized studies of bevacizumab given with chemotherapy in the adjuvant (postoperative) or neoadjuvant (preoperative) settings for stage IB-IIIA NSCLC and a phase II study with chemotherapy in extensive-stage SCLC. A phase III trial of erlotinib with or without bevacizumab in NSCLC has been initiated given encouraging results from a phase I/II study (55).
VEGFR TKIs are small molecules that bind to the ATP pocket of the TK residues of the intracellular domain of VEGFR, thus inhibiting downstream pathways. These compounds frequently target other receptor TKs, such as EGFR and c-KIT (Table 1). ZD6474 (Zactima) is an oral, dual kinase inhibitor that targets VEGFR-2 and, to a lesser extent, EGFR. The combination of ZD6474 with docetaxel as second-line therapy for patients with advanced NSCLC improved progression-free survival as compared with docetaxel alone in a randomized phase II clinical trial (56), and a confirmatory phase III study has been initiated. AZD2171 is a highly potent oral inhibitor of VEGFR-1, -2, and -3, and a phase I trial of AZD2171 with carboplatin and paclitaxel in advanced NSCLC has demonstrated that this combination is well tolerated, with encouraging antitumor activity (57); a phase II/III trial of carboplatin and paclitaxel with AZD2171 or placebo is ongoing. Several other VEGFR TKIs have showed acceptable safety profiles in phase I trials, and results from phase II trials in lung cancer are awaited.
RAS/RAF/MEK/ERK pathway inhibitors
The RAS family of proto-oncogenes (HRAS, KRAS, and NRAS) are plasma membrane–associated G proteins that regulate key signaling pathways involved in normal cell differentiation, proliferation, and survival (58). The RAS pathway is often aberrantly activated in lung cancer due to RAS mutations, mutation of v-raf murine sarcoma viral oncogene homolog B1 (BRAF), or overexpression of growth factor receptors. Activating RAS mutations are found in 10%–15% of all NSCLCs (15%–35% of adenocarcinomas) but rarely in SCLCs (59). Although the distinct functions of HRAS, KRAS, and NRAS have yet to be established, mutations in KRAS account for approximately 90% of RAS mutations in lung cancer. KRAS mutations are found more frequently in lung cancers arising in smokers and are associated with poor survival (60). In addition, EGFR and KRAS mutations appear to be mutually exclusive in lung cancers (61), and KRAS mutations are associated with primary resistance to EGFR TKI therapy (62). A number of agents targeting different components of the RAS pathway have been developed and are under clinical investigation (58). Of these, farnesyl transferase inhibitors (FTIs) are the most studied, and two orally bioavailable FTIs (tipifarnib and lonafarnib) are being tested in combination with cytotoxic therapy in clinical trials in lung cancer (63).
BRAF is an important downstream effector of the RAS pathway and represents a rational therapeutic target. Sorafenib is an oral, dual-action, multikinase inhibitor with antiproliferative and antiangiogenic activity that is a potent inhibitor of RAF kinase and several other receptor TKs including VEGFR-2, VEGFR-3, PDGFR-β, Flt-3, and c-KIT (64). Early clinical studies of single-agent sorafenib have demonstrated that the drug is well tolerated and is particularly active as a cytostatic agent with prolonged disease stabilization. A phase II randomized discontinuation trial of sorafenib in previously treated NSCLC patients is ongoing.
Inhibitors of MEK, which target further downstream along the RAS/RAF pathway, have recently been developed (CI-1040, PD-0325901, and AZD6244). Preclinical and early clinical studies with these agents have shown promising antitumor activity in the treatment of NSCLC, with prolonged disease stabilization reported in patients with NSCLC in phase I trials (65). Phase II studies with MEK inhibitors are underway in NSCLC.
PI3K/Akt/PTEN pathway inhibitors
PI3Ks regulate several cellular processes, including cell proliferation, growth, apoptosis, and cytoskeletal rearrangement (66). The PI3K signaling pathway is frequently activated in human cancers through a sequence of events involving activation of upstream receptor TKs (including EGFR and PDGFR) and/or mutations in PIK3CA, which encodes the catalytic subunit of PI3K (66). Akt is a downstream effector of PI3Ks and is constitutively activated in NSCLCs (67). While PIK3CA mutations occur in only approximately 4% of NSCLC tumors (68, 69), expression of PTEN protein, which inhibits the PI3K/Akt pathway, is frequently reduced or lost in lung cancers, representing an alternate mechanism of activating this pathway.
Preclinical studies of LY294002, a PI3K inhibitor, have indicated that the agent enhances sensitivity of NSCLC cells to chemotherapy and radiation, and phase I trials of this agent are underway (67). Several inhibitors of the mammalian target of rapamycin (mTOR), a downstream target of PI3K signaling, have also been developed. These include rapamycin and its analogs temsirolimus (CCI-779), everolimus (RAD001), and AP23573. These agents have shown promising antitumor activity in early clinical trials.
Tumor suppressor gene therapy
The p53 tumor suppressor is a key cellular gatekeeper that is activated by multiple stress signals such as DNA damage, oncogenes, and hypoxia, leading to the expression of downstream genes involved in cell-cycle arrest, allowing DNA repair or initiation of apoptosis. p53 is frequently inactivated by mutation in lung cancer (50% of NSCLCs and 90% of SCLCs) (70, 71). Restoration of p53 function in lung cancer cells with mutant or deleted p53 leads to apoptosis of tumor cells (72), and these findings have led to the development of pharmacological methods of reactivating p53, such as by gene replacement therapy. Clinical trials of p53 gene therapy with a retroviral p53 expression vector in patients with NSCLC have demonstrated that gene therapy is safe and feasible, with some evidence of antitumor activity (73). A p53 adenoviral vector (INGN 201; Ad5CMV-p53, Advexin) has also been studied in patients with lung cancer, and preliminary results suggest that it is well tolerated and effective (74). FUS1 is a novel tumor suppressor gene located on chromosome 3p21.3, a region that is commonly deleted in lung cancer. Frequent loss of FUS1 protein expression or deficiency of posttranslational modification of the protein has been reported in the majority of NSCLCs and SCLCs, and exogenous overexpression of FUS1 in 3p21.3-deficient lung cancer cells results in the inhibition of tumor cell proliferation and apoptosis (75, 76). FUS1 gene therapy using a nanoparticle delivery system has been designed, and early results from a phase I study in lung cancer patients indicate that FUS1 nanoparticles can be safely administered (77). Further trials are awaited to determine the clinical benefit of these gene therapies in lung cancer.
Inhibitors of DNA methylation and histone deacetylase
Hypermethylation of promoter regions of tumor suppressor genes represents an epigenetic mechanism of gene silencing that plays an important role in the initiation and progression of human cancer (78) and thus represents an attractive target for therapy. An inhibitor of DNA methylation, 5-azacitidine (Vidaza), has been developed and prolongs survival in patients with myelodysplastic syndrome (79), although its efficacy in lung cancer is unknown. Histone deacetylases (HDACs) reversibly modify histones and repress transcription of genes involved in cell proliferation by restricting transcription factor access to DNA. HDAC inhibitors can reverse gene silencing and exert an antiproliferative effect by upregulating tumor suppressor gene expression. Several HDAC inhibitors, including suberoylanilide hydroxamic acid (SAHA), valproic acid, and depsipeptide, are either in or being considered for clinical trials in lung cancer.
Enhancing apoptosis
The ability to evade apoptosis is an important feature of cancer cells (80). Bcl-2 is a key anti-apoptotic protein that is overexpressed in 10%–35% of NSCLCs and 75%–95% of SCLCs (59) and preclinical studies have shown that oblimersen sodium (Genasense), an antisense oligonucleotide targeting Bcl-2, confers resistance to cytotoxic chemotherapy, radiotherapy, and monoclonal antibodies (81). Randomized phase II trials of oblimersen in combination with chemotherapy are underway in SCLC and NSCLC (81). A potent small molecule inhibitor of the antiapoptotic proteins Bcl-2, Bcl-XL, and Bcl-w has also been developed (ABT-737) and demonstrates single-agent preclinical activity against both NSCLC and SCLC (82).
Proteasome inhibitors
The ubiquitin-proteasome system plays a central role in protein homeostasis by regulating the degradation of proteins involved in the cell cycle, DNA transcription and repair, apoptosis, angiogenesis, and cell growth. Bortezomib (Velcade), a proteasome inhibitor, has demonstrated cytotoxic activity as a single agent or in combination with chemotherapy in preclinical lung cancer cell line studies (83). A recent trial of bortezomib given with first-line chemotherapy for advanced NSCLC reported encouraging survival results for patients treated with the combination (84). In addition, a randomized phase II study of bortezomib alone versus bortezomib plus docetaxel demonstrated modest activity of both therapies that was comparable to that of other second-line treatments for NSCLC (85). Further studies in lung cancer with bortezomib in combination with chemotherapy are anticipated.
Individualizing prognosis and therapy
The selection of appropriate treatment for individual patients remains a challenge in clinical oncology, particularly in the advanced disease setting, given the limited benefit and significant toxicities of currently available therapies. An exciting area of investigation is the use of predictive and prognostic molecular markers from each patient’s tumor, blood, or germline DNA to refine clinical decision making. Significant technologic advances in genomics and proteomics have provided insights into the molecular basis of lung cancer, and studies have been both small scale, involving analyses of single or limited numbers of biomarkers, and large scale, involving analyses of expression profiles of thousands of genes or proteins in lung tumors. In a large study of early-stage NSCLC patients treated with adjuvant chemotherapy, absence of protein expression of a marker for the repair of cisplatin-induced DNA adducts (excision-repair cross-complementation group 1 [ERCC1]) was found to correlate with benefit from cisplatin-based chemotherapy (86). The potential clinical application of tumor ERCC1 status has recently been evaluated in a phase III study randomizing advanced NSCLC patients to customized or noncustomized therapy according to ERCC1 mRNA levels (87). With this approach, patients in the noncustomized (control) arm received cisplatin plus docetaxel, while patients in the customized (genotypic) arm with low ERCC1 mRNA levels received cisplatin plus docetaxel and those with high ERCC1 mRNA levels received gemcitabine plus docetaxel. As hypothesized, patients in the customized therapy arm had a significantly higher response rate to chemotherapy as compared with those in the control arm. The results of this study provide support for further prospective studies of predictive molecular markers for individualized therapy in lung cancer.
Genome- and proteome-wide approaches to identifying unique gene or protein expression signatures in human cancers have been developed, and possible applications of these technologies include: (a) finding markers for early detection; (b) classifying subtypes of cancers; (c) predicting prognosis; (d) predicting response to treatment; and (e) identifying potential targets for cancer therapeutics.
The large datasets (with >20,000 human genes) from gene expression analyses of lung tumors have showed that microarray profiling is useful for classifying tumors and allows division of known histologic types, such as adenocarcinomas or squamous cell carcinomas, into distinct subgroups with significantly different survival rates (88–90). Clinical trials have demonstrated a survival benefit of adjuvant chemotherapy for patients with early-stage NSCLC. However, the current lung cancer staging system has limited power to predict prognosis, and better methods of identifying subgroups of patients with poor clinical outcome are needed. To accurately predict the prognosis for patients with early-stage NSCLC, a recent study showed that by using genome-wide mRNA expression profiling, gene expression signatures, when integrated with clinical data, were able to predict recurrence better than clinical prognostic factors alone (91). Another article reported a five-gene signature that was predictive for relapse-free and overall survival among patients with NSCLC (92). A major question is, Which of the many prognostic expression signatures should we use? A metaanalysis of all microarray studies associating gene expression and clinical outcome in NSCLC confirmed that gene signatures can predict survival for stage I NSCLC patients (93). In this study, the authors took microarray data from seven independent studies that had been correlated with survival time of stage I NSCLC patients. The data from five of the studies were pooled after statistical adjustments to account for differences in the arrays being performed at different times and sites, and then 64 genes were identified whose expression was associated with survival. This 64-gene signature predicted survival in two other data sets with 85% accuracy, as well as predicting survival better than stage IA and IB classification in all of the patients (93). In addition, some of these genes had predicted survival in earlier reports in lung cancer and in other diseases. Continued integration of array datasets, coupled with testing on additional patient datasets, will be needed to arrive at an approach that can be employed in general clinical practice. While it is tempting to compare these different approaches and signatures, this must be done with great caution. In fact, the integration and development of such clinically validated tumor prognostic signatures (or of signatures directing treatment) is complex and will require careful attention to biostatistical methods used, adequate sample size, development of prediction rules using a priori selected classification methods, validation in totally independent data sets, as well as proof that the signatures significantly add to available (e.g., clinical) prognostic information (94).
Several studies have also identified sets of genes in lung cancer whose expression levels are associated with response to antitumor drugs (95). In addition, gene expression signatures involving oncogenic pathways have been identified and were found to significantly correlate with the prognosis of patients and sensitivity to chemotherapeutic drugs in multiple types of cancers, including NSCLC (96). These results are encouraging; however, the multiple drug response gene sets will need to be incorporated into common signatures for individual therapies and further tested in prospective clinical trials. Of course, all of the same biostatistical concerns exist for predicting response to drugs as for predicting patient prognosis.
Although proteome-wide analyses are relatively recent and still under technical development as compared with microarray profiling, the results obtained thus far indicate that this is another potential approach for more accurate cancer staging and prediction of response to treatment and survival. Indeed, proteomic patterns distinguished different lung cancer histologic subtypes (primary lung tumors from lung metastases), classified nodal involvement, and identified groups of NSCLC patients with significantly different clinical outcomes (97). Finally, the identification of specific serum protein peptides by mass spectrometry appears to have great promise for both the early detection of lung cancer and predicting response to targeted therapy (98).
Cancer stem cells: a shift in paradigm
Recent evidence has emerged suggesting that most malignancies, if not all, may arise from a rare subpopulation of stem cell–like tumor cells, i.e., “cancer stem cells,” prompting a paradigm shift in the models of carcinogenesis with important clinical implications (99). The cancer stem cell hypothesis posits that, like normal stem cells, cancer stem cells have three distinctive properties: the ability to undergo self-renewal (i.e., to divide and give rise to another malignant stem cell), to develop into any cell in the phenotypically diverse tumor cell population, and to proliferate extensively and drive continued expansion of malignant cells (100). Current cytotoxic treatments target highly proliferating cells that, according to the cancer stem cell model, represent the majority of the tumor bulk but are incapable of self-renewal. However, the small subpopulation of cancer stem cells may be inherently resistant to the cytotoxic effects of chemotherapy and radiotherapy due to their low proliferation rate and drug transporter expression. Thus, the development of therapies specifically targeting cancer stem cells represents a strategy to completely eradicate tumors and potentially lead to cure, even in advanced-stage disease. In addition, the identification of cancer stem cell markers may be useful for early detection and provide valuable predictive and prognostic information. In support of this, an 11-gene stem cell signature in human lung cancers that was a predictor of response to therapy and outcome has been reported (101). A recent study also demonstrated that the presence of a 186-gene “invasiveness” gene signature obtained from gene expression profiling of stem cells in breast cancer was associated with a poor prognosis and increased likelihood of metastases in several tumor types, including lung cancer (102).
The first evidence for the existence of cancer stem cells in human cancers came from pioneering work in acute myelogenous leukemia (AML), where it was demonstrated that only a small subpopulation of leukemic cells sharing a similar surface marker profile with normal human primitive hematopoietic progenitors was able to regenerate human AML after transplantation in immunodeficient mice (103, 104). Similar results have subsequently been reported in studies of solid tumors, including brain, breast, prostate, and colon cancers, where a fraction of cancer cells with tumor-initiating and tumor heterogeneity–recapitulating activities have been successfully isolated (105–108).
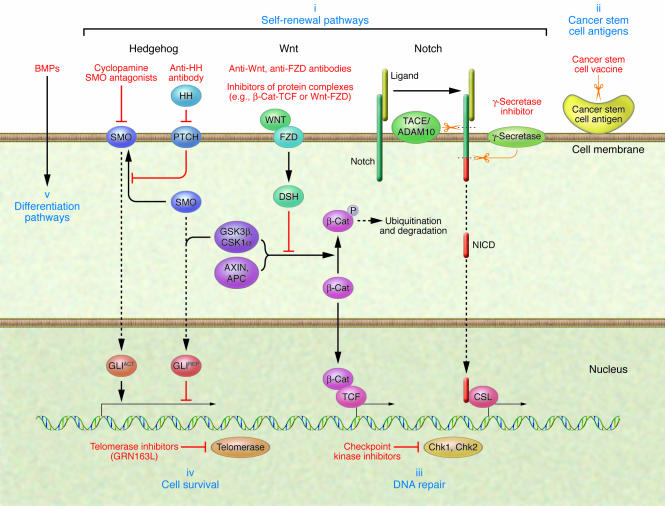
The adult lung is a complex organ whose cells normally turn over very slowly. The epithelial cells lining the airways are frequently exposed to potential toxic agents and pathogens, and they must be able to respond quickly and efficiently to cellular damage. Experimental models have identified several stem cell compartments in the normal lung with proliferative and differentiation potential (109, 110). The regenerative potential of lung stem cells has been further investigated in a mouse model of lung carcinogenesis to address the relationship between lung stem cells and tumorigenesis (111). Lung stem cells displayed an expansion upon expression of the activated KRAS oncogene, and this finding was not reproduced with other lung cell types. Induced tumorigenesis in naphthalene-treated mice conditionally expressing KRAS resulted in increased lung tumor multiplicity and size. Furthermore, the developed tumors expressed the same markers of the lung stem cell subpopulation, thus providing a strong link between stem cells and carcinogenesis in the lung. Several groups are actively characterizing and isolating stem cells in lung cancer (112), and attempts to develop selective therapies targeting cancer stem cells are ongoing (Figure 2).
Figure 2. Cancer stem cell–specific therapeutic approaches.
Hedgehog (HH), Notch, and Wnt signaling are key stem cell self-renewal pathways that are deregulated in lung cancer and thus represent potential therapeutic targets (i). Agents inhibiting the hedgehog pathway include monoclonal antibodies against HH ligand and cyclopamine, which is a small molecule inhibitor of smoothened (SMO). Monoclonal antibodies against Wnt ligand and frizzled (FZD) receptor and inhibitors of protein complexes mediating Wnt signaling, such as Wnt-FZD or β-catenin–transcription factor (β-Cat-TCF), are examples of ways of targeting the Wnt pathway. Strategies of blocking or silencing Notch signaling can be either selective, such as the targeting of individual Notch receptors with antisense or monoclonal antibodies, or nonselective, such as the use of soluble receptor decoys that sequester Notch ligands or γ-secretase inhibitors. Solid and dashed arrows represent multiple components of these pathways that, for simplicity, are not detailed here. These components also represent potential therapeutic targets. Other methods of targeting cancer stem cells include immunotherapy-based approaches against antigens present on cancer stem cells (ii); targeting cancer stem cell mechanisms of resistance to cytotoxic therapy by inhibiting DNA repair enzymes such as the checkpoint kinases (Chk1, Chk2) (iii); targeting stem cell–specific survival mechanisms with telomerase inhibitors (GRN163L) (iv); and inducing stem cell differentiation with soluble factors such as bone morphogenetic proteins (BMPs) (v). GLI, glioma-associated oncogene; GLIACT, active form of GLI; GLIREP, repressor form of GLI; NICD, Notch intracellular domain; CSL, CBF1, suppressor of hairless, Lag-1; TACE, TNF-α–converting enzyme; ADAM10, a disintegrin and metalloprotease domain 10; PTCH, patched homolog; GSK3β, glycogen synthase kinase 3β; CSK1α, cyclin-suppressing kinase 1; DSH, dishevelled.
Targeting self-renewal pathways
Stem cell self-renewal is a tightly regulated process, and there is growing evidence suggesting that developmental pathways, such as Wnt, Notch, and hedgehog, play a crucial role in the fate determination of normal stem cells (113–116). It has been postulated that deregulation and reactivation of these signaling pathways cause defects in the normal control of symmetric and asymmetric division and lead to stem cell expansion and malignant transformation, representing an early event in carcinogenesis (117). Accordingly, alterations in Wnt, Notch, and hedgehog pathways have been reported in several tumor types, including lung cancer (118–121). Wnt, Notch, and hedgehog are essential for embryonic cell fate determination and differentiation and play an important role in pulmonary organogenesis. Hedgehog signaling is required for lung branching morphogenesis and epithelial-mesenchymal interactions (122, 123) and controls differentiation of neuroendocrine precursor cells during lung embryogenesis and acute injury repair. Recent preclinical studies indicate that pharmacologically targeting these pathways is feasible, representing potential therapeutic targets. For example, the hedgehog pathway is reactivated in SCLC, and treatment of SCLC cells with the plant-derived inhibitor cyclopamine targeted against smoothened protein (Smo), a mediator of the hedgehog pathway, reduced tumor growth in vitro and in mouse xenografts (124). An inhibitor of γ-secretase, an enzyme required for Notch signaling, has also been shown to have preclinical activity against Notch-1–overexpressing cancers (125).
Telomerase inhibitors
There is growing evidence that the enzyme telomerase is upregulated in cancer stem cells and telomerase inhibitors can potentially target both cancer stem cells and more mature cancer cells (126). Telomeres are repetitive sequences localized at the end of mammalian chromosomes that protect from degradation and loss of essential genes (126). With each cell division, telomeres progressively shorten, which limits the life span of somatic cells. Telomere shortening and subsequent cell death can be overcome by telomerase, which stabilizes telomere length by adding DNA sequence repeats onto the telomeric ends of chromosomes. Human telomerase contains two essential components, a telomerase reverse transcriptase (hTERT) catalytic subunit and a functional telomerase RNA (hTR, also known as TERC) component. Activation of telomerase is thought to play a role in the immortalization of cells as an early step in tumorigenesis. Telomerase is almost universally expressed in human tumors, whereas telomerase activity is reduced or absent in normal tissue. Detectable telomerase levels are found in approximately 80% of NSCLCs and 100% of SCLCs (127). Thus, telomerase represents an attractive therapeutic target for lung cancer, and several agents targeting telomerase have been developed. GRN163L is a novel telomerase antagonist that targets the RNA template region of hTR. Preclinical studies have demonstrated that GRN163L inhibits anchorage-independent growth and in vivo xenograft tumor growth of lung cancer cells (128), and phase I studies with this agent are ongoing. Several other treatments targeting telomerase are currently in development, and these include immunotherapy (vaccines), gene therapy (telomerase oncolytic virus therapy), and reverse transcriptase inhibitors (126).
Other cancer stem cell–targeted approaches
In addition to targeting key self-renewal and survival pathways, recent evidence in glioblastomas indicates that the cancer stem cell population is resistant to radiation therapy, as such cells are more efficient at inducing repair of damaged DNA as compared with the bulk of the tumor cells (129). A strategy to circumvent cancer stem cell resistance to cytotoxic therapy would be to pharmacologically inhibit checkpoint kinases that control the cell cycle to allow DNA repair (e.g., Chk1, Chk2) (129). Other studies have demonstrated the possibility of inducing stem cell differentiation with soluble factors such as bone morphogenetic proteins as a potential therapeutic approach (130). Last, if there are antigens selectively expressed on cancer stem cells (such as cancer-testis antigens), these could also be targets of immunotherapy-based therapies.
Future directions
Intense research efforts in translational oncology over the last decade have provided significant insights into the molecular basis of carcinogenesis and biologic behavior of human cancers. Based on this knowledge, new strategies for screening, early detection, prevention, staging, and therapy have been developed and show promise for improving the management of lung cancer. However, the clinical application of these novel approaches awaits further research in several important areas. First, a priority will be to develop methods for early detection of lung cancers, and this will likely involve the combination of spiral CT imaging with genetic epidemiology, serum proteomics, and sputum or blood biomarkers. Second, given the heterogeneous clinical behavior of lung cancers, more individualized treatment strategies will need to be developed. The recent discovery of the correlation of EGFR mutations and response to EGFR TKI therapy has demonstrated that molecular typing of tumors to guide therapy selection for lung cancer is possible. Prospective trials will need to validate the feasibility and efficacy of selecting therapy based on tumor molecular profiles. Third, numerous molecularly targeted agents are in clinical development, and future studies will need to identify molecular and clinical tools to guide the use of these agents, particularly in combination with current therapies such as surgery, radiation, cytotoxic chemotherapy, and other targeted therapies. For example, these may include biomarkers that indicate whether drugs have reached their target(s) and resulted in the expected changes by identifying molecular alterations in tumor samples or detectable in circulating cells in the blood. Molecular imaging techniques also represent noninvasive methods of assessing tumor response to therapies based on the targeted molecular pathway(s) that may facilitate clinical management. Last, a major focus will be the development of therapies specifically targeting cancer stem cells, and future discoveries in this field will have profound implications for the potential cure of this challenging disease.
Acknowledgments
Support was provided by the University of Texas Lung Cancer SPORE (NCI P50CA75907), the Gillson Longenbaugh Foundation, the Canadian Association of Medical Oncologists, and the Canadian Institutes of Health Research. We thank Mitsuo Sato and Adi Gazdar for their help with the preparation of the manuscript.
Footnotes
Nonstandard abbreviations used: ERCC1, excision-repair cross-complementation group 1; HDAC, histone deacetylase; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer; TK, tyrosine kinase; TKI, TK inhibitor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2740–2750 (2007). doi:10.1172/JCI31809.
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R., et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A., et al. Cancer statistics, 2006. CA Cancer J. Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Doll R., Hill A.B. The mortality of doctors in relation to their smoking habits; a preliminary report. Br. Med. J. 1954;1:1451–1455. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham E.A., Singer J.J. Landmark article Oct 28, 1933. Successful removal of an entire lung for carcinoma of the bronchus. By Evarts A. Graham and J.J. Singer. JAMA. 1984;251:257–260. doi: 10.1001/jama.251.2.257. [DOI] [PubMed] [Google Scholar]
- 6.Johnson D.H. Evolution of cisplatin-based chemotherapy in non-small cell lung cancer: a historical perspective and the eastern cooperative oncology group experience. Chest. 2000;117(4 Suppl. 1):133S–137S. doi: 10.1378/chest.117.4_suppl_1.133s. [DOI] [PubMed] [Google Scholar]
- 7.Schiller J.H., et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 8.Matakidou A., Eisen T., Houlston R.S. Systematic review of the relationship between family history and lung cancer risk. Br. J. Cancer. 2005;93:825–833. doi: 10.1038/sj.bjc.6602769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cote M.L., Kardia S.L., Wenzlaff A.S., Ruckdeschel J.C., Schwartz A.G. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA. 2005;293:3036–3042. doi: 10.1001/jama.293.24.3036. [DOI] [PubMed] [Google Scholar]
- 10.Bailey-Wilson J.E., et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am. J. Hum. Genet. 2004;75:460–474. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haiman C.A., et al. Ethnic and racial differences in the smoking-related risk of lung cancer. . N. Engl. J. Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 12.Henschke C.I., Yip R., Miettinen O.S. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296:180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 13.Henschke C.I., et al. Survival of patients with stage I lung cancer detected on CT screening. . N. Engl. J. Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 14.Sato M., Shames D.S., Gazdar A.F., Minna J.D. A translational view of the molecular pathogenesis of lung cancer. J. Thorac. Oncol. 2007;2:327–343. doi: 10.1097/01.JTO.0000263718.69320.4c. [DOI] [PubMed] [Google Scholar]
- 15.Wistuba I.I., et al. High resolution chromosome 3p allelotyping of human lung cancer and preneoplastic/preinvasive bronchial epithelium reveals multiple, discontinuous sites of 3p allele loss and three regions of frequent breakpoints. Cancer Res. 2000;60:1949–1960. [PubMed] [Google Scholar]
- 16.Tsou J.A., Hagen J.A., Carpenter C.L., Laird-Offringa I.A. DNA methylation analysis: a powerful new tool for lung cancer diagnosis. Oncogene. 2002;21:5450–5461. doi: 10.1038/sj.onc.1205605. [DOI] [PubMed] [Google Scholar]
- 17.Tang X., et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65:7568–7572. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 18.Palmisano W.A., et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–5958. [PubMed] [Google Scholar]
- 19.Belinsky S.A., et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- 20.Li R., et al. Genetic deletions in sputum as diagnostic markers for early detection of stage I non-small cell lung cancer. Clin. Cancer Res. 2007;13:482–487. doi: 10.1158/1078-0432.CCR-06-1593. [DOI] [PubMed] [Google Scholar]
- 21.Sozzi G., et al. Detection of microsatellite alterations in plasma DNA of non-small cell lung cancer patients: a prospect for early diagnosis. Clin. Cancer Res. 1999;5:2689–2692. [PubMed] [Google Scholar]
- 22.Bhattacharjee A., et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber M.E., et al. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyerson M., Franklin W.A., Kelley M.J. Molecular classification and molecular genetics of human lung cancers. Semin. Oncol. 2004;31:4–19. doi: 10.1053/j.seminoncol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein I.B., Joe A.K. Mechanisms of disease: Oncogene addiction — a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 26.Rowinsky E.K. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu. Rev. Med. 2004;55:433–457. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 27.Franklin W.A., Veve R., Hirsch F.R., Helfrich B.A., Bunn P.A., Jr. Epidermal growth factor receptor family in lung cancer and premalignancy. Semin. Oncol. 2002;29:3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- 28.Lynch T.J., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 29.Paez J.G., et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 30.Shigematsu H., et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi S., et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. . N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 32.Pao W., et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi S., et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005;65:7096–7101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- 34.Cappuzzo F., et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 2004;96:1133–1141. doi: 10.1093/jnci/djh217. [DOI] [PubMed] [Google Scholar]
- 35.Cappuzzo F., et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl. Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 36.Tsao M.S., et al. Erlotinib in lung cancer — molecular and clinical predictors of outcome. . N. Engl. J. Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd F.A., et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 38.Thatcher N., et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 39.Fisher G.H., et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Politi K., et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. . 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji H., et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 42.Herbst R.S., et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 2. . J. Clin. Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 43.Herbst R.S., et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 44.Hanna N., et al. Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J. Clin. Oncol. 2006;24:5253–5258. doi: 10.1200/JCO.2006.08.2263. [DOI] [PubMed] [Google Scholar]
- 45.Rosell R., et al. Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced non-small-cell lung cancer (NSCLC). In ASCO Annual Meeting Proceedings. July 15, 2004. . J. Clin. Oncol. 2004;22(Suppl. 14S):7012. [Google Scholar]
- 46.Von Pawel J., et al. Phase III study comparing cisplatin/vinorelbine plus cetuximab versus cisplatin/vinorelbine as first-line treatment for patients with epidermal growth factor (EGFR)-expressing advanced non-small cell lung cancer (NSCLC) (FLEX). In ASCO Annual Meeting Proceedings Part I. June 20, 2006. . J. Clin. Oncol. 2006;24(Suppl. 18S):7109. [Google Scholar]
- 47.Werner-Wasik M., et al. A phase II study of cetuximab (C225) in combination with chemoradiation (CRT) in patients (PTS) with stage IIIA/B non-small cell lung cancer (NSCLC): an interim overall toxicity report of the RTOG 0324 trial. In ASCO Annual Meeting Proceedings. June 1, 2005. . J. Clin. Oncol. 2005;23(Suppl. 16S):7135. [Google Scholar]
- 48.Coate L.E., et al. Phase II pilot study of neoadjuvant cetuximab in combination with cisplatin and gemcitabine in patients with resectable IB-IIIA non small cell lung cancer. In ASCO Annual Meeting Proceedings Part I. June 20, 2006. . J. Clin. Oncol. 2006;24(Suppl. 18S):17107. [Google Scholar]
- 49.Folkman J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 51.Fontanini G., et al. Expression of vascular endothelial growth factor mRNA in non-small-cell lung carcinomas. Br. J. Cancer. 1999;79:363–369. doi: 10.1038/sj.bjc.6690058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontanini G., et al. A high vascular count and overexpression of vascular endothelial growth factor are associated with unfavourable prognosis in operated small cell lung carcinoma. Br. J. Cancer. 2002;86:558–563. doi: 10.1038/sj.bjc.6600130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandler A., et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. . N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 54.Johnson D.H., et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 55.Herbst R.S., et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 2005;23:2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 56.Heymach J.V., et al. A phase II trial of ZD6474 plus docetaxel in patients with previously treated NSCLC: Follow-up results. In ASCO Annual Meeting Proceedings Part I. June 20, 2006. . J. Clin. Oncol. 2006;24(Suppl. 18S):7016. [Google Scholar]
- 57.Laurie S.A., et al. Final results of a phase I study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor receptors (VEGFR), in combination with carboplatin (C) + paclitaxel (T) in patients with advanced non-small cell lung cancer (NSCLC): A study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG). In ASCO Annual Meeting Proceedings Part I. June 20, 2006. . J. Clin. Oncol. 2006;24(Suppl. 18S):3054. doi: 10.1200/JCO.2007.14.4741. [DOI] [PubMed] [Google Scholar]
- 58.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 59.Sekido Y., Fong K.M., Minna J.D. Molecular genetics of lung cancer. Annu. Rev. Med. 2003;54:73–87. doi: 10.1146/annurev.med.54.101601.152202. [DOI] [PubMed] [Google Scholar]
- 60.Mascaux C., et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br. J. Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shigematsu H., Gazdar A.F. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int. J. Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 62.Massarelli E., et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin. Cancer Res. 2007;13:2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 63.Isobe T., Herbst R.S., Onn A. Current management of advanced non-small cell lung cancer: targeted therapy. Semin. Oncol. 2005;32:315–328. doi: 10.1053/j.seminoncol.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 64.Wilhelm S.M., et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. . Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 65.LoRusso P.M., et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J. Clin. Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 66.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. . Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 67.Brognard J., Clark A.S., Ni Y., Dennis P.A. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 68.Samuels Y., et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 69.Kawano O., et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006;54:209–215. doi: 10.1016/j.lungcan.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Hainaut P., et al. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26:205–213. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi T., et al. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246:491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi T., et al. Wild-type but not mutant p53 suppresses the growth of human lung cancer cells bearing multiple genetic lesions. Cancer Res. 1992;52:2340–2343. [PubMed] [Google Scholar]
- 73.Roth J.A., et al. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat. Med. 1996;2:985–991. doi: 10.1038/nm0996-985. [DOI] [PubMed] [Google Scholar]
- 74.Gabrilovich D.I. INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin. Biol. Ther. 2006;6:823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- 75.Ji L., et al. Expression of several genes in the human chromosome 3p21.3 homozygous deletion region by an adenovirus vector results in tumor suppressor activities in vitro and in vivo. Cancer Res. 2002;62:2715–2720. [PMC free article] [PubMed] [Google Scholar]
- 76.Kondo M., et al. Overexpression of candidate tumor suppressor gene FUS1 isolated from the 3p21.3 homozygous deletion region leads to G1 arrest and growth inhibition of lung cancer cells. Oncogene. 2001;20:6258–6262. doi: 10.1038/sj.onc.1204832. [DOI] [PubMed] [Google Scholar]
- 77.
- 78.Jones P.A., Baylin S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 79.Silverman L.R., et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J. Clin. Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 80.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 81.Herbst R.S., Frankel S.R. Oblimersen sodium (Genasense bcl-2 antisense oligonucleotide): a rational therapeutic to enhance apoptosis in therapy of lung cancer. Clin. Cancer Res. 2004;10:4245s–4248s. doi: 10.1158/1078-0432.CCR-040018. [DOI] [PubMed] [Google Scholar]
- 82.Oltersdorf T., et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;35:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 83.Adams J., et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 84.Davies A.M., et al. Bortezomib + gemcitabine (Gem)/carboplatin (Carbo) results in encouraging survival in advanced non-small cell lung cancer (NSCLC): results of a phase II Southwest Oncology Group (SWOG) trial (S0339). In ASCO Annual Meeting Proceedings Part I. June 20, 2006. . J. Clin. Oncol. 2006;24(Suppl. 18S):7017. [Google Scholar]
- 85.Fanucchi M.P., et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 86.Olaussen K.A., et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 87.Cobo M., et al. O-089 ERCC1 mRNA-based randomized phase III trial of docetaxel (doc) doublets with cisplatin (cis) or gemcitabine (gem) in stage IV non-small-cell lung cancer (NSCLC) patients (p). Lung Cancer. 2005;49:S32. [Google Scholar]
- 88.Inamura K., et al. Two subclasses of lung squamous cell carcinoma with different gene expression profiles and prognosis identified by hierarchical clustering and non-negative matrix factorization. Oncogene. 2005;24:7105–7113. doi: 10.1038/sj.onc.1208858. [DOI] [PubMed] [Google Scholar]
- 89.Beer D.G., et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. . Nat. Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 90.Ramaswamy S., Ross K.N., Lander E.S., Golub T.R. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 91.Potti A., et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N. Engl. J. Med. 2006;355:570–580. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 92.Chen H.Y., et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. . N. Engl. J. Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 93.Lu Y., et al. A gene expression signature predicts survival of patients with stage I non-small cell lung cancer. PLoS Med. 2006;3:e467. doi: 10.1371/journal.pmed.0030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michiels S., Koscielny S., Hill C. Interpretation of microarray data in cancer. Br. J. Cancer. 2007;96:1155–1158. doi: 10.1038/sj.bjc.6603673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kikuchi T., et al. Expression profiles of non-small cell lung cancers on cDNA microarrays: identification of genes for prediction of lymph-node metastasis and sensitivity to anti-cancer drugs. Oncogene. 2003;22:2192–2205. doi: 10.1038/sj.onc.1206288. [DOI] [PubMed] [Google Scholar]
- 96.Bild A.H., et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. . Nature. 2006;39:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 97.Yanagisawa K., et al. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003;362:433–439. doi: 10.1016/S0140-6736(03)14068-8. [DOI] [PubMed] [Google Scholar]
- 98.Solomon B., et al. Prediction of clinical outcome in non-small cell lung cancer (NSCLC) patients treated with gefitinib using matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) of serum. In ASCO Annual Meeting Proceedings Part I. June 20, 2006. . J. Clin. Oncol. 2006;24(Suppl. 18S):7004. [Google Scholar]
- 99.Wicha M.S., Liu S., Dontu G. Cancer stem cells: an old idea — a paradigm shift. Cancer Res. 2006;66:1883–1890; discussion 1895–1896. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 100.Jordan C.T., Guzman M.L., Noble M. Cancer stem cells. N. Engl. J. Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 101.Glinsky G.V., Berezovska O., Glinskii A.B. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J. Clin. Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu R., et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. . N. Engl. J. Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 103.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 104.Lapidot T., et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;67:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 105.Al-Hajj M., et al. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh S.K., et al. Identification of human brain tumour initiating cells. Nature. 2004;32:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 107.Patrawala L., et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 108.Ricci-Vitiani L., et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;45:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 109.Rawlins E.L., Hogan B.L. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 110.Reynolds S.D., et al. Molecular and functional properties of lung SP cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292:L972–L983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- 111.Kim C.F., et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 112.Ho M.M., Ng A.V., Lam S., Hung J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 113.Liu S., Dontu G., Wicha M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dontu G., et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taipale J., Beachy P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;11:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 116.Liu S., et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dontu G., Al-Hajj M., Abdallah W.M., Clarke M.F., Wicha M.S. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl. 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;34:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 119.Olsen C.L., Hsu P.P., Glienke J., Rubanyi G.M., Brooks A.R. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer. 2004;4:43. doi: 10.1186/1471-2407-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nickoloff B.J., Osborne B.A., Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- 121.Daniel V.C., Peacock C.D., Watkins D.N. Developmental signalling pathways in lung cancer. Respirology. 2006;11:234–240. doi: 10.1111/j.1440-1843.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 122.Pepicelli C.V., Lewis P.M., McMahon A.P. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 123.Litingtung Y., Lei L., Westphal H., Chiang C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 124.Watkins D.N., et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 125.Weijzen S., et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat. Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 126.Shay J.W., Wright W.E. Telomerase therapeutics for cancer: challenges and new directions. Nat. Rev. Drug Discov. 2006;5:577–584. doi: 10.1038/nrd2081. [DOI] [PubMed] [Google Scholar]
- 127.Hiyama K., et al. Telomerase activity in small-cell and non-small-cell lung cancers. J. Natl. Cancer Inst. 1995;87:895–902. doi: 10.1093/jnci/87.12.895. [DOI] [PubMed] [Google Scholar]
- 128.Dikmen Z.G., et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 129.Bao S., et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 130.Piccirillo S.G., et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]