Abstract
The TRAP (Thyroid hormone receptor associated proteins)/Mediator complex serves as a transcriptional coactivator. In Drosophila, Kohtalo (Kto) and Skuld (Skd), homologs of TRAP subunits, TRAP230 and TRAP240, respectively, are necessary for eye development. However, the transcriptional activators that require Kto and Skd have not been identified. Here we provide evidence that Kto and Skd are essential for the function of transcription factor Atonal (Ato) in spatial patterning of proneural clusters in the morphogenetic furrow. In the absence of Kto/Skd, Ato fails to induce its inhibitory target events such as EGFR signaling and Scabrous expression that result in ectopic Ato expression in the space between proneural groups. Kto/Skd are also required for positive Ato functions to induce Ato targets such as Ato itself and Senseless within the proneural clusters. We also show that Skd forms a protein complex with Ato in vivo. These data suggest that Kto/Skd act as essential coactivators for Ato expression during early retinal neurogenesis.
Keywords: Atonal, TRAP coactivator, neurogenesis, Drosophila eye
Introduction
One of the most interesting questions in neural development is how neurons are generated from undifferentiated neural epithelium. Different members of the neural basic helix-loop-helix (bHLH) proteins are expressed in spatially restricted domains of the developing nervous system and play key roles in the generation of different types of neurons during vertebrate and invertebrate neurogenesis (Dambly-Chaudiere and Vervoort, 1998; Guillemot, 1995; Guillemot et al., 1993; Lee, 1997; Turner and Weintraub, 1994). The Drosophila nervous system has been extensively used as a model to study the genetic mechanism of neurogenesis. During early neurogenesis, a group of proneural cells are selected from undifferentiated cells and acquire competence to become neuronal cells. From each proneural group, one cell is specified as a neuron. The neural bHLH proteins play important roles in the selection of the proneural groups and the founder neurons (Brunet and Ghysen, 1999).
In Drosophila eye development, the bHLH protein Atonal (Ato) plays an essential proneural function for the formation of photoreceptor neurons (Jarman et al., 1994; Jarman et al., 1995). The adult compound eye consists of approximately 800 ommatidia or unit eyes. Each ommatidium contains eight photoreceptor (R1-R8) neurons (Wolff, 1993). Generation of these photoreceptors begins from the posterior margin of the eye imaginal disc, and a wave of differentiation marked by the morphogenetic furrow (hereafter ‘furrow’) progresses anteriorly, giving rise to the columns of photoreceptor clusters with regular spacing (Ready et al., 1976; Wolff, 1993). Posterior to the furrow, R8 founder neurons are first specified and subsequently recruit seven additional photoreceptors to form photoreceptor clusters (Wolff, 1993). Therefore, the correct selection of the R8 founder cells is a critical step during retinal neurogenesis.
Neural patterning in the eye disc requires not only the expression of the proneural gene ato but also the negative regulators of ato to restrict the Ato expression to single R8 cells from each proneural groups. Dynamic patterns of Ato expression in the furrow region can be divided into four steps in a simplified model (Frankfort and Mardon, 2002). First, Ato expression is initiated as a stripe pattern along the anterior edge of the furrow (stage 1). Just posterior to the stripe, Ato expression is enriched in the proneural clusters (stage 2) and the R8 equivalence groups (stage 3). Finally, Ato expression is restricted to single R8 founder cells (stage 4). It is important to note that Ato plays both positive and negative roles to control its own expression. As a positive factor, Ato activates its own and Senseless (Sens) expression in the proneural clusters. On the other hand, as a negative factor, Ato indirectly represses its own expression in the uncommitted cells surrounding the proneural clusters by inducing negative regulators of Ato expression such as EGFR signaling and Scabrous (Sca) expression.
In addition to the role of transcriptional activators such as Ato, there is evidence that coactivators may play a role in neurogenesis. Homologs of TRAP coactivator subunits, TRAP230 (Kohtalo [Kto]) or TRAP240 (Skuld [Skd]), have been shown to be involved in the correct repression of Ato expression posterior to the furrow, as loss of these subunits results in ectopic Ato expression (Treisman, 2001). However, the basis for the ectopic Ato expression in the absence of Kto/Skd has not been clearly understood. Interestingly, loss of the homeodomain protein Bar causes similar ectopic Ato expression (Lim and Choi, 2003), as in kto or skd mutant clones. Thus, it is possible that Kto/Skd may act as coactivators for a transcription factor that regulates Bar expression. A candidate Bar regulator that is activated by Kto/Skd might be Ato because Bar expression requires Ato-dependent EGFR signaling from the proneural groups (Lim and Choi, 2004).
In this study, we tested the hypothesis that Ato requires Kto/Skd coactivators during early neurogenesis. We show that Kto and Skd are necessary for Ato to activate EGFR signaling and Sca/Rough expression, thus providing lateral inhibition of Ato expression between the proneural clusters. Kto and Skd are also required for the induction of positive Ato targets such as Ato itself and Sens in each proneural cluster. Furthermore, Skd exists in a complex with Ato in vivo. Our study suggests that Kto/Skd act as coactivators for Ato in retinal neurogenesis.
Materials and methods
Drosophila stocks
The following mutant and transgenic flies were used in this study: Df(1)B263-20 (Higashijima et al., 1992), roX63 (Heberlein and Rubin, 1991), skdT606, ktoT555 (Treisman, 2001), EGFRCO (Price et al., 1989), and 5′F:9.3 ato-lacZ (Sun et al., 1998). Other strains were obtained from the Bloomington Stock Center (Flybase; www.flybase.org).
Generation of loss-of-function (LOF) mosaic clones
LOF clones were generated by the FLP/FRT system (Xu and Rubin, 1993). First instar larvae from the cross between yw, Df(1)B263-20 FRT18A/FM7 females and w, Ubi-GFPS65T FRT18A; hs-FLP3 males were treated for 1 hr at 37°C and incubated at room temperature until dissection. EGFRCO LOF clones were similarly generated in yw, hs-FLP/+; EGFRCO, GMR-P35 FRT42D/arm-lacZ, M(2)561 FRT42D larvae. kto or skd LOF clones were generated in yw, ey-FLP/+; ktoT555(or skdT606) FRT80B/Ubi-GFP61EF FRT80B larvae.
Immunocytochemistry
Third instar eye imaginal discs were dissected in phosphate-buffered saline (PBS) on ice, fixed in 2% paraformaldehyde-lysine-periodate fixative and stained as described (Lim and Choi, 2003). The following primary antibodies were used in this study: mouse anti-Elav (1:10; Developmental Studies Hybridoma Bank [DSHB]), mouse anti-Rough (1:200; DSHB), mouse anti-β-gal (1:250; Promega), mouse anti-dpERK (1:250; Sigma), mouse anti-GFP (1:25; Clontech Laboratories, Inc), mouse anti-Sca (1:200; DSHB), rabbit anti-Ato (1:5000) (Jarman et al., 1995), rabbit anti-BarH1 (1:100) (Higashijima et al., 1992), rabbit anti-GFP (1:2000; Molecular Probes), rabbit anti-Skd (1:5000) (Janody et al., 2003), guinea pig anti-Ato (1:1000) (Hassan et al., 2000), and guinea pig anti-Dlg (1:1000) (Lim and Choi, 2003), and guinea pig anti-Sens (1:1000) (Nolo et al., 2000). Secondary antibodies were anti-mouse-CY3, anti-mouse-fluorescein isothocyanate (FITC), anti-rabbit-CY3, anti-rabbit-FITC and anti-guinea pig-CY5 (Jackson Immunochemicals). Fluorescent images were scanned using Zeiss LSM laser-scanning confocal microscope and processed with Adobe Photoshop.
Co-immunoprecipitation (Co-IP) assays
Co-IP experiments were performed similarly as described (Lim et al., 2006). Briefly, Myc-tagged Skd and Flag-tagged Ato plasmids were transfected into HEK293T cells using Lipofectamine 2000 according to the manufacture’s instructions (Invitrogen), and cells were cultured in DMEM medium with 10% fetal bovine serum. Two days after transfection, cells were harvested and lysed with lysis buffer (0.5% NP-40, 20mM Tris-HCl [pH 8.0], 180mM NaCl, 1mM EDTA, and complete protease inhibitor cocktail [Roche]) for 15min on ice. Whole cell lysates were cleared by centrifugation for 15min at 13,000rpm at 4°C and soluble protein complexes were immunoprecipitated with anti-Flag M2-agarose beads (Sigma). Beads were then washed extensively four times with lysis buffer, and purified proteins were displayed using standard western blot analysis.
Wild-type fly embryos were collected and homogenized in homogenization buffer (20mM Hepes [pH 7.6], 70mM KCl, 2mM dithiothreitol, 0.1% NP-40, 8% glycerol, 1mM phenyl methylsulphonyl fluoride, complete protease inhibitor cocktail, EGTA and 1,10-phenanthroline). Embryonic extracts (10mg protein/ml of homogenization buffer) were incubated overnight at 4°C with anti-Ato antibody and then with Sepharose 6MB beads (Amersham). Sepharose beads were then washed extensively with the homogenization buffer. Equal amounts of precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane. Rabbit anti-Ato and rabbit anti-Skd antisera were used at 1:5,000 for immunoblot analyses.
Results
Kto and Skd are required for Bar expression in the basal undifferentiated cells behind the furrow
Ato is expressed in a stripe pattern in the most anterior region of the furrow (stage 1), in the proneural clusters (stages 2/3), and finally in the R8 founder neurons (stage 4) (Figs. 1A-C). After the transient expression in R8 cells, Ato expression is repressed in more posterior region of the eye disc. Bar is expressed in a complementary pattern to Ato expression in the basal undifferentiated cells (Fig. 1D) and is required for transcriptional repression of ato behind the furrow (Lim and Choi, 2003). Loss of kto or skd causes inappropriate expression of Ato posterior to the furrow in the eye disc where Ato is normally repressed (Treisman, 2001). This phenotype is similar to that seen in Bar mutant eye (Figs. 1E, F). Thus, ectopically expressed Ato+ cells within kto or skd loss-of-function (LOF) clones posterior to the furrow might be due to loss of Bar expression in undifferentiated cells. To test this possibility, we examined Bar expression within kto or skd mutant clones generated by the FLP/FRT method (Xu and Rubin, 1993). We found that Bar expression was strongly downregulated or absent within the kto or skd LOF clones (Figs. 1G-L). Loss of kto or skd activity showed indistinguishable phenotypes. On the contrary, loss of Bar did not affect the expression of Kto/Skd (data not shown). These results suggest that Kto and Skd are required for Bar expression, thereby responsible for inhibition of Ato expression in the region posterior to the furrow.
Fig. 1. Kto/Skd are required for Bar expression.
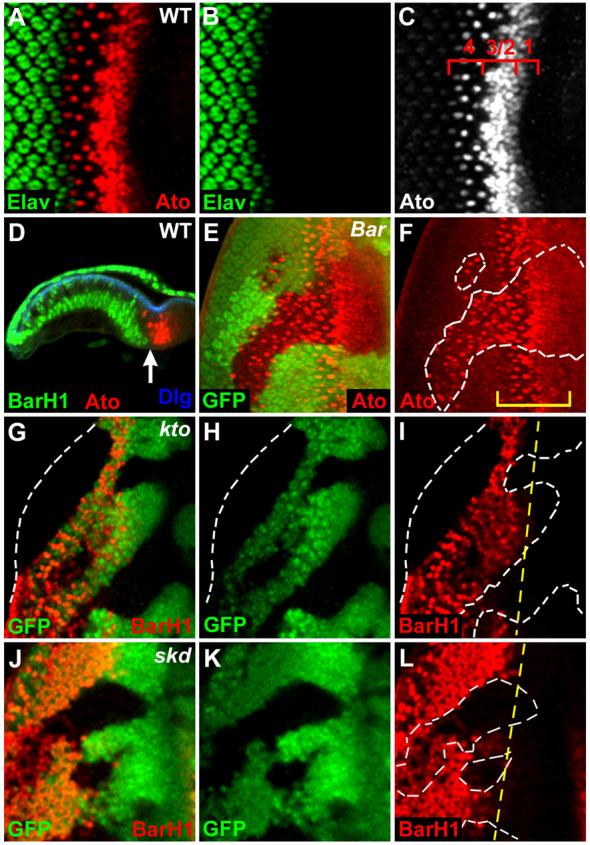
(A-C) Wild-type third instar eye disc. Four different stages of Ato expression near the furrow are numbered (C, 1 through 4).
(D) Complementary expression pattern of Bar and Ato in the longitudinal confocal section of eye disc. Dlg (Discs large) is used as a cell membrane marker. The arrow marks the furrow.
(E, F) Bar is required for Ato repression in the basal undifferentiated cells posterior to the furrow. Ato expression is strongly elevated within the Bar loss-of-function (LOF) clones marked by the absence of GFP staining (white dashed lines). A bracket marks the domain of Ato expression in wild-type cells.
(G-L) Loss of Bar expression in kto (G-I) and skd (J-L) LOF clones. The kto or skd LOF clones are identified by the absence of GFP staining (white dashed lines). The yellow dashed line in (I, L) marks the normal anterior boundary of Bar expression.
In this and all subsequent figures, antibodies used for staining are as indicated in each panel with matched colors. LOF mutant clones are identified by the absence of clonal marker staining (GFP or LacZ) and marked by white dashed lines. Posterior is to the left and dorsal is up in all discs unless mentioned otherwise.
Kto and Skd are required for spacing of proneural clusters
Loss of kto or skd results in ectopic expression of Ato in the region posterior to the furrow where Bar is expressed. However, the effects of kto or skd LOF clones within the furrow where Bar is not expressed have not been characterized in detail. It has been shown that Bar expression is induced by Ato-dependent signaling during furrow progression (Lim and Choi, 2004). This raises a possibility that Kto and Skd may be involved in the activation of Ato-dependent proneural signaling events within and near the furrow. To test this possibility, we examined the effects of kto/skd mutations within and near the furrow (Fig. 2). Loss of Kto or Skd significantly reduced the level of the ato stripe labeled by 3′F:5.8-lacZ reporter, and Ato expression was slightly delayed (Figs. 2A-C). In addition, interommatidial space between proneural clusters was filled with ectopic Ato+ cells, resulting in the fused clusters (defects in the stages 2/3; Fig. 2F, red dotted circle). However, in the following step (stage 4), many Ato+ cells in kto or skd mutant clones were singled out as individual R8 cells (Fig. 2F, white arrow), although occasionally more than one Ato+ R8 founder cells were seen adjacent to each other (Fig. 2F, yellow arrows). Taken together, these data suggest that Kto and Skd are necessary for proper regulation of Ato expression at all steps of proneural processes in the furrow region, but they are most essential for lateral inhibition of Ato expression in proneural groups during stages 2/3, whereas it is only partially required for R8 selection during the stage 4.
Fig. 2. Kto and Skd are required for correct patterning of proneural clusters and R8 founder cells.
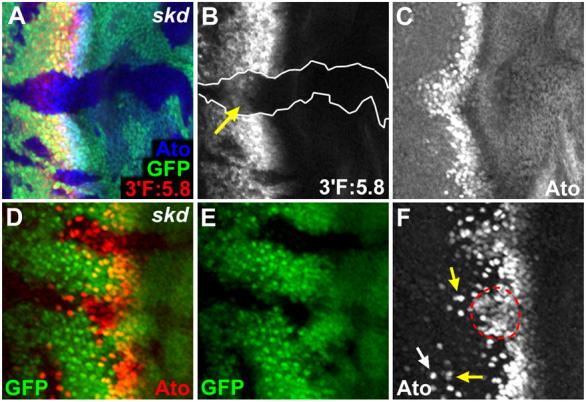
(A-C) LacZ expression driven by 3′F:5.8 ato-lacZ was significantly reduced within skd LOF clones (B, arrow), suggesting that Skd is important for the ato stripe expression. Note that Ato protein expression stained by antibody was slightly delayed within skd LOF clone (C).
(D-F) In skd LOF clones, Ato expression is expanded, resulting in fused proneural clusters with no spacing. Some ectopic Ato+ cells in the posterior region of the clone are not seen at this focal plane. More than 50 clones examined showed similar phenotypes. Loss of kto or skd function showed indistinguishable phenotypes in this and all other analysis. In (F), a white arrow marks ectopic Ato+ R8 cells within a clone away from the furrow, and yellow arrows mark ommatidial preclusters with more than one R8 cell near the furrow. Red dotted circle indicates fused proneural clusters.
Kto and Skd are required for expression of Rough and activation of EGFR signaling
Loss of kto or skd caused defects in selecting the single R8 cells per proneural clusters (Fig. 2F, yellow arrows). This phenotype is similar to that observed in the rough (ro) mutant eyes (Fig. 3B). Ro, a homeodomain protein (Heberlein and Rubin, 1991), is expressed in a mutually exclusive pattern to Ato along the furrow in the wild-type eye disc (Fig. 3A) and antagonizes Ato expression in the R2/5 precursor cells of the R8 equivalence group to specify single Ato+ R8 cells per proneural clusters (Dokucu et al., 1996; Frankfort et al., 2001). Therefore, loss of ro function affects the selection of single R8 cell from each R8 equivalence group, resulting in ommatidia with more than one R8 cell (defects in stages 3/4; Fig. 3B, arrows). Because this extra R8 phenotype of ro mutant is similar to that of kto/skd mutant clones, we tested whether Kto and Skd are required for Ro activation. As expected, Ro expression was dramatically reduced within kto or skd LOF clones (Figs. 3D, F, arrows), providing further support that Kto and Skd may be required for lateral inhibition of Ato expression by activating Ro.
Fig. 3. Kto and Skd are required for Ro expression.
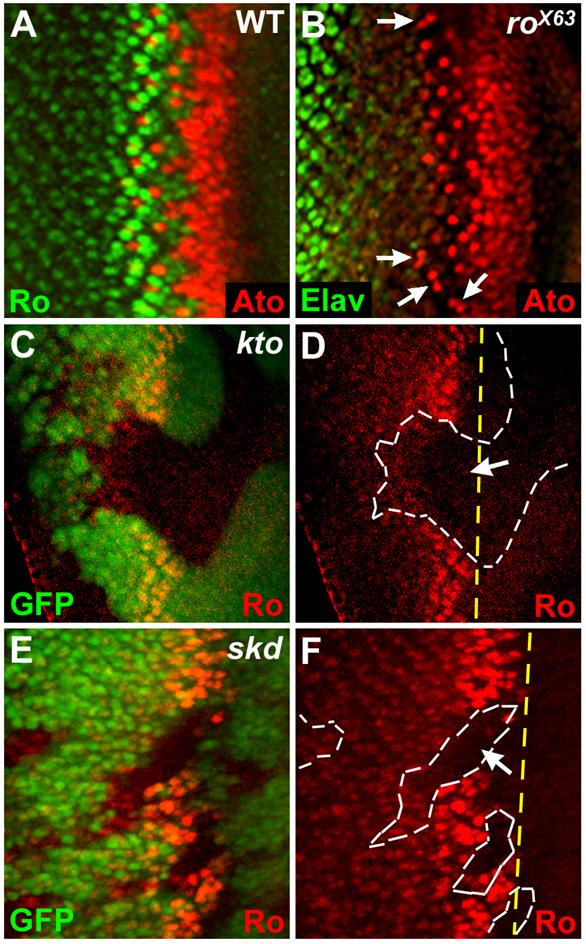
(A) Complementary expression pattern of Ro and Ato along the furrow.
(B) Ro is required for specifying single R8 cells per proneural clusters.
(C-F) Loss of Ro expression in kto (C, D) and skd (E, F) LOF clones. Yellow dashed lines in (D, F) mark the normal anterior boundary of Ro expression.
Ato expression in the proneural clusters induces EGFR signaling and Sca expression, which then laterally inhibit Ato expression in the surrounding cells (Frankfort and Mardon, 2002). Since EGFR signaling is required for the expression of Ro (Dominguez et al., 1998) and Bar (Lim and Choi, 2004) near the furrow, we tested whether Kto or Skd might be involved in the induction of EGFR signaling and Sca expression from proneural clusters, thereby repressing Ato expression around the clusters. dpERK (dual phosphorylated extracellular signal regulated kinase) activation was absent or dramatically reduced in the proneural clusters within the skd LOF clones (Figs. 4A, B, yellow arrows), suggesting that Skd is required for the activation of EGFR signaling. In contrast, loss of EGFR in the eye disc did not affect Skd expression (Figs. 4C, D). Next, we tested whether Kto or Skd is also required for Sca expression in the proneural clusters (Figs. 4E, F). Sca expression was absent in the proneural clusters within the kto LOF clones (Fig. 4F, yellow arrows). These results indicate that Kto and Skd are essential for EGFR activation and Sca expression within the proneural clusters to form regularly spaced proneural groups. Since delayed occurrence of spaced proneural clusters and phospho-ERK/Ro staining were not observed in the posterior region of kto/skd LOF clones, it is unlikely that kto/skd LOF phenotypes are simply due to delayed Ato expression/activation.
Fig. 4. Kto and Skd are essential for EGFR activation and Sca expression.
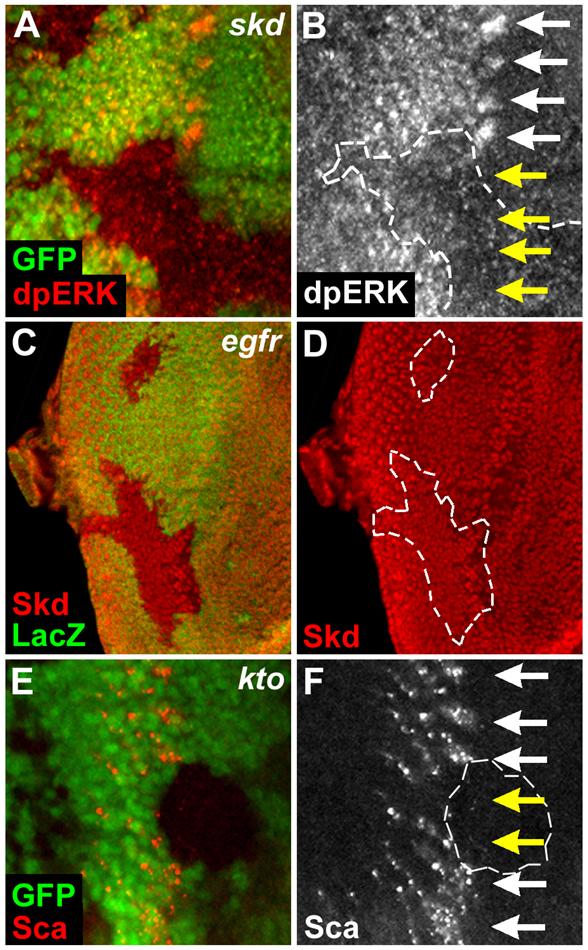
(A, B) EGFR is not activated within skd LOF clones. White and yellow arrows mark the dpERK activation within the proneural clusters in the wild-type or the skd mutant regions, respectively.
(C, D) EGFR is not required for Skd expression. egfrCO LOF clones are identified by the absence of LacZ staining and marked by white lines.
(E, F) Sca is not expressed in the proneural clusters within the kto mutant cells. White and yellow arrows mark the Sca expression within the proneural clusters in the wild-type or the kto mutant regions, respectively.
Ato function requires Kto and Skd in the proneural clusters
Kto and Skd are required for expression of negative regulators of Ato. Since Kto and Skd are the components of the TRAP complex, they are expected to function for a transcriptional factor(s) to activate the expression of target genes. Therefore, an important question is which activators require Kto/Skd as coactivators. Ato is an ideal activator candidate, as Ato and Kto/Skd show similar functions (Baonza et al., 2001; Chen and Chien, 1999; Frankfort and Mardon, 2002; Kumar et al., 1998; Lesokhin et al., 1999; Lim and Choi, 2004; Spencer et al., 1998; Wasserman et al., 2000). Both Ato and Kto/Skd are required for (i) correct selection of R8 founder neurons, (ii) activation of EGFR signaling for ato repression in the interommatidial space, and (iii) the expression of Bar and Ro (Figs. 1-4).
To test whether Kto/Skd function as coactivators for Ato, we examined the expression of several Ato target genes within kto or skd mutant cells (Fig. 5). Ato is autoregulated within the proneural clusters and R8 founder cells through the 9.3 kb of ato 5′ enhancer (5′F:9.3) (Figs. 5B-D) (Sun et al., 1998). Expression of this 5′F:9.3 ato-lacZ reporter strictly depends on Ato because the reporter expression is completely lost in ato1 mutant that expresses non-functional Ato protein deficient in DNA binding. Another potential direct target of Ato is the sens gene (Jafar-Nejad et al., 2003), which is expressed in the proneural clusters and all R8 photoreceptor cells (Fig. 5E) (Frankfort et al., 2001; Nolo et al., 2000). Both 5′:9.3 ato-lacZ and Sens showed a precise colocalization in the proneural clusters and R8 cells (Figs. 5F-H).
Fig. 5. Kto/Skd are required for Ato activation.
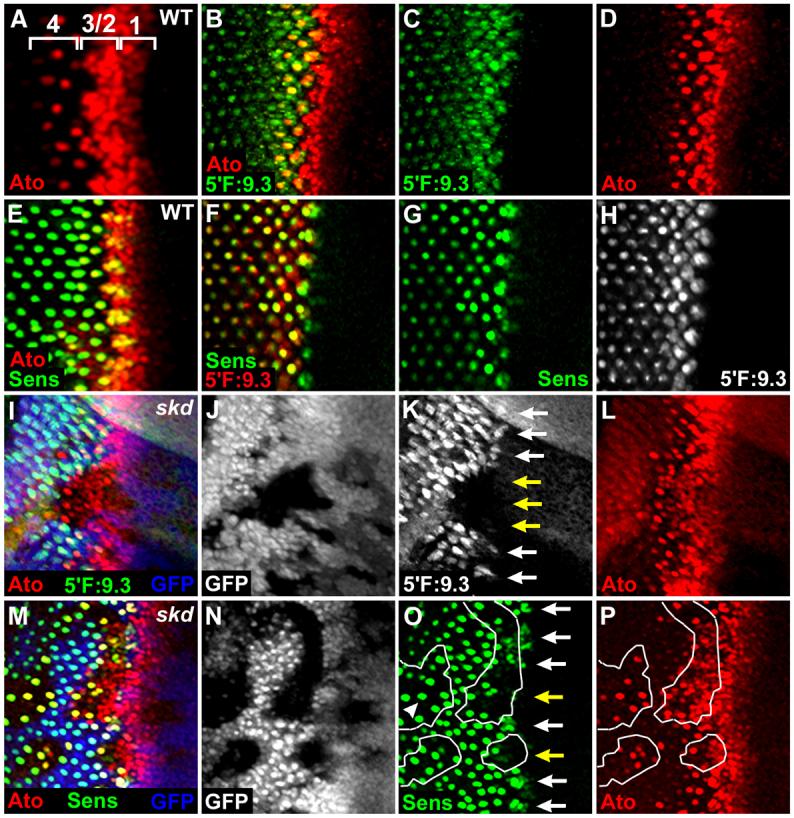
(A) In wild-type third instar eye disc, four different stages of Ato expression near the furrow are numbered (1 through 4).
(B-D) The eye disc of 5′F:9.3 ato-lacZ transgenic fly. The β-gal expression is detected in the proneural clusters and R8 cells behind the furrow.
(E) Sens is expressed in the proneural clusters and R8 cells.
(F-H) β-gal driven by 5′F:9.3 ato-lacZ is coexpressed with Sens in the proneural clusters and R8 cells.
(I-L) β-gal expression driven by 5′F:9.3 ato-lacZ is absent in the proneural clusters (K, yellow arrows) although Ato is highly expressed (I, L) within the skd LOF clones. Similar phenotypes were observed in more than 50 clones examined.
(M-P) Sens is not expressed in the proneural clusters within the skd LOF clones (O, yellow arrows). However, Sens is expressed in the singled-out Ato+ R8 cells within the skd LOF clones (O, arrowhead).
We first examined whether Skd is required for the expression of 5′F:9.3 ato-lacZ (Figs. 5I-L). β-gal expression driven by 5′F:9.3 ato-lacZ was strongly reduced or eliminated in the proneural clusters within the skd mutant cells (Fig. 5K, yellow arrows), suggesting that Ato depends on the function of Skd coactivator to activate the transcription of its target gene, 5′F:9.3 ato-lacZ. To further confirm that Ato requires Skd, we checked Sens expression within skd mutant clones (Figs. 5M-P) and found that Sens expression was also absent or strongly downregulated in the proneural clusters within the skd mutant cells (Fig. 5O, yellow arrows). The loss of Sens expression was due to the absence of Kto/Skd rather than Ato since Ato was highly expressed within the same clone (Fig. 5P). Sca, another target of Ato, was also not detected in the proneural clusters within the kto mutant cells (Fig. 4F, yellow arrows). These data suggest that Ato requires Kto/Skd coactivators to activate expression of its target genes in the proneural clusters.
Because Kto and Skd are expressed ubiquitously in the eye disc (Figs. 4C, D), these proteins may be required for general coactivation. To our surprise, Sens was expressed in the singled-out Ato+ R8 cells within the skd mutant clone (Fig. 5O, arrowhead), although Sens expression was absent or strongly downregulated in the proneural clusters within the skd mutant clone (Fig. 5O, yellow arrows). We also observed that 5′F:9.3 ato-lacZ and Sca were expressed in the singled-out Ato+ R8 cells but not in the proneural clusters within the skd mutant clones (data not shown). These data suggest that Ato requires Kto/Skd coactivators to activate its target genes only in the less differentiated proneural clusters, but not in the more differentiated R8 founder cells.
To obtain biochemical evidence to support our genetic data that Kto/Skd are coactivators for Ato, we checked whether Ato interacts with TRAP coactivators in vivo. We performed co-immunoprecipitaion (Co-IP) assays by transfecting HEK293T cells with Myc-tagged Skd and Flag-tagged Ato. Skd co-immuoprecipitated together with Ato (Fig. 6A), suggesting that Skd and Ato form a complex in vivo. In addition, endogenous Skd and Ato can also be co-immuoprecipitated in fly embryo extract (Fig. 6B). Taken together, this data suggests that Ato can recruit Skd in vivo, further supporting that Kto/Skd function as coactivators for Ato.
Fig. 6. Skd forms a complex with Ato in vivo.
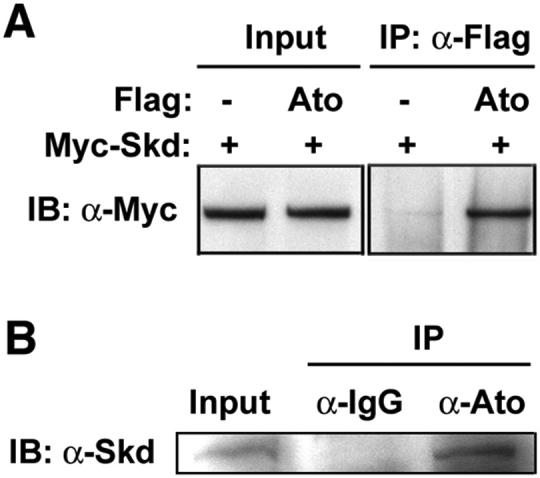
(A) Skd interacts with Ato in HEK293T cells. Co-immunoprecipitation (Co-IP) assays were performed using Myc-Skd and Flag-Ato proteins in human HEK293T cells. Left panel shows expression levels of Myc-Skd protein. Right panel shows expression of Myc-Skd protein after IP using anti-Flag M2 antibody, demonstrating binding of Myc-Skd to Flag-Ato. Flag-Ato expression was not shown.
(B) Anti-Ato antibody co-immunoprecipitated Skd protein from embryo extracts. Pre-immune serum failed to precipitate Skd.
Discussion
Kto/Skd function as transcriptional coactivators for Ato
Ato is essential for positive autoregulation of Ato and the induction of negative signals for Ato repression during early eye development. In this study, we found that kto/skd LOF mutations affect both positive and negative events regulated by Ato. Our genetic and biochemical data suggest that these dual functions of Ato depend on the activity of Kto/Skd coactivators as summarized schematically in Fig. 7. Kto/Skd are required for the expression of Ato target genes within the proneural clusters to promote or inhibit retinal neurogenesis. First, Ato-Kto/Skd activate Sca expression within the proneural clusters (Figs. 4E, F). Sca is secreted to inhibit Ato expression in uncommitted cells between proneural clusters (Baker et al., 1990; Frankfort and Mardon, 2002; Lee et al., 1996; Mlodzik et al., 1990). Second, Ato-Kto/Skd activate EGFR signaling within the proneural clusters (Figs. 4A, B), which indirectly induces Ro expression in uncommitted neighboring cells (Fig. 3) and represses Ato expression in the space between proneural clusters. The dependence of Ato function on Kto/Skd as coactivators is further supported by the presence of Ato and Kto/Skd in a protein complex in vivo (Fig. 6). Since the loss of Ato or Kto/Skd function results in the identical effects on the Ato target events near the morphogenetic furrow, it is likely that loss of Sens/Sca expression and EGFR signaling in kto/skd mutant clones is a secondary effect resulting from the failure to activate Ato. However, since Kto/Skd are components of a mediator complex that regulates the activity of many transcription factors (Treisman 2001, Janody et al., 2004), we do not exclude the possibility that Kto/Skd may act as cofactors for another transcription factor to induce Sens/Sca expression and EGFR signaling.
Fig. 7. A model of Kto/Skd-mediated Ato activation during early retinal neurogenesis.
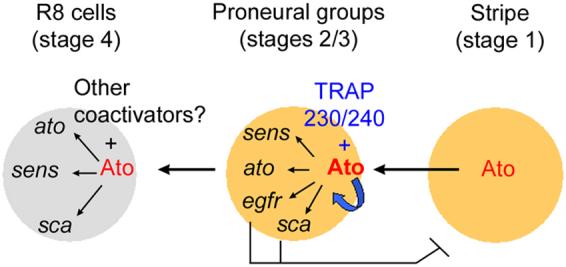
Kto (TRAP230) and Skd (TRAP240) function as coactivators for the proneural transcription factor Ato in the proneural clusters, and are therefore required for expression of Ato target genes such as Ato itself, Sens, Sca, and for activation of EGFR signaling. Ato and Sens are positively required for selection and differentiation of the R8 founder neurons. In contrast, EGFR signaling and Sca are required for generation of interommatidial space by repressing Ato expression in cells between proneural clusters. Kto/Skd may not be required for Ato activation in the R8 cells in which Ato activation may depend on other coactivators or other TRAP modules. A thick blue arrow indicates the autoregulation of ato.
The loss of 5′F:9.3 ato-lacZ expression in kto/skd mutant clones suggests that Kto/Skd recruit the mediator complex to promote activation of Ato target genes. This 9.3kb promoter region is likely to contain additional binding sites for other transcription factors. However, it should be noted that 5′F:9.3 ato-lacZ expression is eliminated in ato1 mutant eye discs, suggesting that other transcription factors cannot compensate the Ato-dependent 5′F:9.3 reporter expression in the absence of Ato (Sun et al., 1998). Therefore, our conclusion that Kto/Skd are required for Ato activation may be independent of the presence of other transcription factor binding sites in the 5′F:9.3 ato regulatory region. Additional tests with minimal promoters containing only Ato binding sites may be necessary to support this prediction.
Kto/Skd are also required for expression of the anti-proneural factor Bar (Fig. 1G-L). This is consistent with the proposed function of Kto/Skd as coactivators for Ato since Ato-mediated EGFR activation in the proneural clusters is necessary for inducing nonautonomous Bar expression in the posterior region near the furrow (Lim and Choi, 2004). These findings provide an explanation for the ectopic Ato expression in kto/skd LOF clones posterior to the furrow (Treisman, 2001). Interestingly, while Ato-EGFR signaling is required for Bar expression within a limited distance from the furrow, Kto/Skd are required for Bar expression even in the region of posterior eye disc margin (Figs. 1G-L). Bar is induced not only by Ato-Kto/Skd-EGFR signals from the furrow but also by Hedgehog (Hh) signaling from the posterior margin of the eye disc (Lim and Choi, 2004). Thus, Kto/Skd may also be required for mediating Hh signaling to activate Bar expression. We have also found a decrease in expression level of 3′F:5.8-lacZ reporter in kto/skd mutant cells (Figs. 2A-C). The 3′F:5.8 ato enhancer specifies the initial stripe of ato expression independent of Ato (Sun et al., 1998) and is regulated by multiple signaling pathways including Hh, Decapentaplegic (Dpp), and Notch (N) (Borod and Heberlein, 1998; Fu and Baker, 2003; Greenwood and Struhl, 1999; Sun et al., 1998). This also suggests that Kto/Skd may be required for other transcription factors in addition to Ato and may have an additional function in the upstream of Ato to regulate the earliest stripe pattern of Ato expression.
Ato requires Kto/Skd coactivators in stage-dependent manner
An intriguing finding in our study is that Kto/Skd are selectively required for the activation of Ato target genes within the proneural clusters but not in the R8 founder cells (Figs. 5M-P). It has been shown that Ato-expressing cells fail to resolve into discrete proneural groups but results in the selection of more R8 cells (Baker et al., 1990; Frankfort and Mardon, 2002). This suggests that the formation of proneural and equivalence groups may not be an essential step for R8 selection. This observation is consistent with our result that R8 cells can be selected even in the absence of resolved proneural groups in kto/skd mutant clones near the furrow (Figs. 5M-P). Lateral inhibition for R8 spacing may occur in the absence of Sca and EGFR, possibly by another factor like N signaling that is known to be required for R8 spacing (Frankfort and Mardon, 2002).
An interesting question is how Kto/Skd are required in cell type- or stage-specific manner. It has been proposed that posttranslational modifications of transcription factors and/or coactivators can affect their interactions (Gu and Roeder, 1997; Lambert et al., 1998). For example, phosphorylation of the tumor suppressor protein p53 increases its ability to recruit CBP/p300 coactivators, which acetylate p53 to stimulate its sequence-specific DNA-binding activity (Gu and Roeder, 1997; Lambert et al., 1998). The transcriptional coactivator TRAP220 (also called PBP and DRIP205) can be phosphorylated by several kinases including protein kinase A (PKA), protein kinase C (PKC), and the mitogen-activated protein kinases (MAPKs). The activation of MAPK signal transduction pathways stimulates TRAP220 coactivator function (Misra et al., 2002). Therefore, it is possible that a component(s) of the TRAP coactivator complex, such as TRAP220 or possibly Kto/Skd, may be phosphorylated by EGFR-mediated Ras/MAPK signaling specifically within the proneural clusters to stimulate Ato-Kto/Skd interactions and thus activate the transcription of target genes. Furthermore, transcription factors and/or nuclear receptors discriminate the binding of different classes of coactivator complexes depending on their environments such as ligand binding and posttranslational modifications (Gu and Roeder, 1997; Lambert et al., 1998; Takeyama et al., 1999). It remains to be studied whether Ato in the R8 founder cells may receive different environmental cues to recruit different coactivators or other TRAP subunits to be independent from Kto/Skd.
Acknowledgments
We thank Drs. Jessica Treisman, Yuh-Nung Jan, David Cribbs, Nicholas Baker, Young-Joon Kim, Tetsuya Kojima, Kaoru Saigo, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for providing antibodies and flies. We also thank Drs. Jessica Treisman, Kyung-Ok Cho, and Sang-Chul Nam for critical reading and comments on the manuscript. Confocal microscopy was supported by a grant from the NIH to D.B. Jones. This work was supported by a grant from the NIH to K.-W. C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker NE, Mlodzik M, Rubin GM. Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990;250:1370–1377. doi: 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- Baonza A, Casci T, Freeman M. A primary role for the epidermal growth factor receptor in ommatidial spacing in the Drosophila eye. Curr Biol. 2001;11:396–404. doi: 10.1016/s0960-9822(01)00125-7. [DOI] [PubMed] [Google Scholar]
- Borod ER, Heberlein U. Mutual regulation of decapentaplegic and hedgehog during the initiation of differentiation in the Drosophila retina. Dev Biol. 1998;197:187–197. doi: 10.1006/dbio.1998.8888. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Ghysen A. Deconstructing cell determination: proneural genes and neuronal identity. Bioessays. 1999;21:313–318. doi: 10.1002/(SICI)1521-1878(199904)21:4<313::AID-BIES7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Chen CK, Chien CT. Negative regulation of atonal in proneural cluster formation of Drosophila R8 photoreceptors. Proc Natl Acad Sci U S A. 1999;96:5055–5060. doi: 10.1073/pnas.96.9.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambly-Chaudiere C, Vervoort M. The bHLH genes in neural development. Int J Dev Biol. 1998;42:269–273. [PubMed] [Google Scholar]
- Dokucu ME, Zipursky SL, Cagan RL. Atonal, rough and the resolution of proneural clusters in the developing Drosophila retina. Development. 1996;122:4139–4147. doi: 10.1242/dev.122.12.4139. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Wasserman JD, Freeman M. Multiple functions of the EGF receptor in Drosophila eye development. Curr Biol. 1998;8:1039–1048. doi: 10.1016/s0960-9822(98)70441-5. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development. 2002;129:1295–1306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Nolo R, Zhang Z, Bellen H, Mardon G. senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron. 2001;32:403–414. doi: 10.1016/s0896-6273(01)00480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Baker NE. Deciphering synergistic and redundant roles of Hedgehog, Decapentaplegic and Delta that drive the wave of differentiation in Drosophila eye development. Development. 2003;130:5229–5239. doi: 10.1242/dev.00764. [DOI] [PubMed] [Google Scholar]
- Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–5808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Analysis of the role of basic-helix-loop-helix transcription factors in the development of neural lineages in the mouse. Biol Cell. 1995;84:3–6. doi: 10.1016/0248-4900(96)81312-8. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan YN, Zoghbi HY, Bellen HJ. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Rubin GM. Star is required in a subset of photoreceptor cells in the developing Drosophila retina and displays dosage sensitive interactions with rough. Dev Biol. 1991;144:353–361. doi: 10.1016/0012-1606(91)90427-5. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Kojima T, Michiue T, Ishimaru S, Emori Y, Saigo K. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 1992;6:50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Acar M, Nolo R, Lacin H, Pan H, Parkhurst SM, Bellen HJ. Senseless acts as a binary switch during sensory organ precursor selection. Genes Dev. 2003;17:2966–2978. doi: 10.1101/gad.1122403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janody F, Martirosyan Z, Benlali A, Treisman JE. Two subunits of the Drosophila mediator complex act together to control cell affinity. Development. 2003;130:3691–3701. doi: 10.1242/dev.00607. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem. 1998;273:33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- Lee EC, Hu X, Yu SY, Baker NE. The scabrous gene encodes a secreted glycoprotein dimer and regulates proneural development in Drosophila eyes. Mol Cell Biol. 1996;16:1179–1188. doi: 10.1128/mcb.16.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- Lesokhin AM, Yu SY, Katz J, Baker NE. Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila. Dev Biol. 1999;205:129–144. doi: 10.1006/dbio.1998.9121. [DOI] [PubMed] [Google Scholar]
- Lim J, Choi KW. Bar homeodomain proteins are anti-proneural in the Drosophila eye: transcriptional repression of atonal by Bar prevents ectopic retinal neurogenesis. Development. 2003;130:5965–5974. doi: 10.1242/dev.00818. [DOI] [PubMed] [Google Scholar]
- Lim J, Choi KW. Induction and autoregulation of the anti-proneural gene Bar during retinal neurogenesis in Drosophila. Development. 2004;131:5573–5580. doi: 10.1242/dev.01426. [DOI] [PubMed] [Google Scholar]
- Lim J, Hao T, Shaw C, Patel AJ, Szabo G, Rual JF, Fisk CJ, Li N, Smolyar A, Hill DE, Barabasi AL, Vidal M, Zoghbi HY. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125:801–814. doi: 10.1016/j.cell.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Misra P, Owuor ED, Li W, Yu S, Qi C, Meyer K, Zhu YJ, Rao MS, Kong AN, Reddy JK. Phosphorylation of transcriptional coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP). Stimulation of transcriptional regulation by mitogen-activated protein kinase. J Biol Chem. 2002;277:48745–48754. doi: 10.1074/jbc.M208829200. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4:1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Price JV, Clifford RJ, Schupbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989;56:1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Spencer SA, Powell PA, Miller DT, Cagan RL. Regulation of EGF receptor signaling establishes pattern across the developing Drosophila retina. Development. 1998;125:4777–4790. doi: 10.1242/dev.125.23.4777. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jan LY, Jan YN. Transcriptional regulation of atonal during development of the Drosophila peripheral nervous system. Development. 1998;125:3731–3740. doi: 10.1242/dev.125.18.3731. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Masuhiro Y, Fuse H, Endoh H, Murayama A, Kitanaka S, Suzawa M, Yanagisawa J, Kato S. Selective interaction of vitamin D receptor with transcriptional coactivators by a vitamin D analog. Mol Cell Biol. 1999;19:1049–1055. doi: 10.1128/mcb.19.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J. Drosophila homologues of the transcriptional coactivation complex subunits TRAP240 and TRAP230 are required for identical processes in eye-antennal disc development. Development. 2001;128:603–615. doi: 10.1242/dev.128.4.603. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Wasserman JD, Urban S, Freeman M. A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling. Genes Dev. 2000;14:1651–1663. [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. “Pattern formation in the Drosophila retina.”. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]


