Abstract
Purpose
In tree shrews, visual form deprivation produces increased axial elongation of the deprived eye and a myopic shift in refractive state. A change in scleral extensibility (creep rate) is closely associated with the change in axial elongation rate. These effects may be due to scleral tissue remodeling produced by a change in scleral gene expression. In this study, the authors investigated the time course of changes in scleral mRNA levels for selected proteins during the development of form deprivation myopia and during recovery, to determine which, if any, are temporally associated with changes in scleral extensibility and axial elongation rate.
Methods
Competitive RT-PCR was used to measure the levels of mRNA for structural proteins (collagen [α1(I) chain], decorin core protein), degradative enzymes (MMP-2 [gelatinase-A], MMP-3 [stromelysin-1]), and a tissue inhibitor of metalloproteinase (TIMP-1) in the scleras of tree shrews that had been subjected to 1, 2, 4, or 11 days of monocular form deprivation (MD) or 11 days of MD followed by 2 or 4 days of recovery produced by removal of the MD. Four groups of normal animals provided age-matched normal data.
Results
Compared with the control eyes, deprived-eye MMP-2 mRNA levels were higher and MMP-3 levels were lower after 4 days of MD. Deprived-eye collagen mRNA levels were lower than control eye levels after 11 days of MD. The differential effects produced by MD were absent after 2 days of recovery and generally were reversed after 4 days. Decorin mRNA levels in the deprived and control eyes were not significantly different during either MD or recovery. During MD, mRNA levels for collagen, MMP-3, and TIMP-1 decreased in both the deprived and control eyes, compared with age-matched normal eyes. The binocular changes in collagen and TIMP-1 mRNA levels and the differential changes in MMP-2 and MMP-3 levels were detected at least as early as axial, refractive, and creep rate changes.
Conclusions
The up- and downregulation of the specific mRNAs studied, on a time course similar to that for physical changes in the sclera, suggests that modulation of gene expression by the visual environment may produce scleral remodeling and changes in scleral creep rate during the development of form deprivation myopia and recovery.
Current evidence indicates that a visually guided emmetropization mechanism fine tunes the growth of the juvenile eye to match the eye’s axial length to its optical power.1,2 The eyes of avians3–6 and mammals7–11 can be induced to increase their axial elongation rate (producing myopia) by placement of a diffuser (monocular form deprivation [MD]) or a minus power lens in front of the eye. Removing the treatment causes slowed axial elongation rate (producing refractive recovery). These manipulations of the visual environment induce tissue remodeling in the sclera.12–18 In tree shrews, which, similar to other eutherian mammals, have an all-fibrous sclera,19 the tissue remodeling does not appear to modulate growth per se, but rather, alters the extensibility of the sclera (measured as change in creep rate).20,21 In a previous study,20 it was found that changes in scleral creep rate developed rapidly after the onset of MD and were closely associated with changes in axial elongation rate, suggesting that modulation of scleral extensibility may control axial elongation. Precisely controlled scleral tissue remodeling may be responsible for the changes in scleral extensibility and, hence, the changes in the axial elongation rate.
Tissue remodeling is a complex process that involves both synthesis and degradation of the extracellular matrix (ECM). A number of proteins are involved, including structural components such as collagen and proteoglycans22; enzymes, such as the matrix metalloproteinases (MMPs), that are known to degrade ECM proteins23; and tissue inhibitors of metalloproteinases (TIMPs) that bind to and inhibit the activity of the MMPs. In tree shrews, scleral remodeling in eyes with induced myopia is characterized by decreased levels of type I collagen,18 decreased levels of sulfated and unsulfated glycosaminoglycans (GAGs),14,17,24,25 and increased levels of gelatinase-A (MMP-2).26
In a previous study in tree shrews,27 we found that mRNA levels for MMP-2, collagen α1(I), and TIMP-1 were altered by 11 days of form deprivation and 4 days of recovery. This finding suggests that modulation of scleral gene expression may control scleral tissue remodeling and scleral extensibility. If a change in gene expression triggers the scleral tissue remodeling that changes scleral creep rate, then mRNA changes should be temporally associated with changes in creep rate. In the present study, we tested this hypothesis by examining the time course for changes in mRNA levels during MD and recovery and comparing it with the time course of changes in creep rate from a previous study.20
Methods
Experimental Groups and Ocular Measurements
Ten groups of tree shrews (Tupaia glis belangeri), with five animals in each group, were included in the study. Four MD groups: 1, 2, 4, or 11 days of MD; two MD/recovery groups: 2 or 4 days of recovery after 11 days of MD; and four normal groups: 24, 28, 35, and 39 days after natural eyelid opening (days of visual experience [VE]). The 11-day MD, 4-day MD/recovery, and 39-day normal data have been reported.27 They are included in the current report, because they represent end points for comparison with shorter periods of MD and MD/recovery. Starting at 21 ± 1 days of VE, the animals in the six deprived groups were anesthetized and received a pedestal that held a goggle frame.28 To produce MD, the goggle frame held a translucent diffuser randomly placed over either the right or left eye. The animals were allowed 3 days to recover from the minor surgical procedure required to install the goggle pedestal before visual treatment was begun by clipping the goggle frame to the pedestal. Recovery was initiated by removing the goggle frame. To control for any systemic effect from the surgical procedure, the animals in the 24, 28, and 35 days of VE normal groups also received a pedestal at 21 days of VE, but did not wear a goggle frame.
At the end of the treatment period in the MD groups, the refractive state of the deprived and control eyes was measured with an autorefractor (Nidek, Gamagori, Japan)29 and axial component dimensions were measured in animals under anesthesia with A-scan ultrasonography, as previously described.30 In the MD/recovery groups, refractive state only was measured at the end of MD, whereas both refractive state and axial component dimensions were measured after recovery. Refractive state was measured in awake animals, with no ophthalmic or systemic atropine sulfate administered at any time, because of concerns that atropine can alter the effect of MD in tree shrews.31 All animals received an overdose of pentobarbital sodium, and eyes were enucleated between 10:00 and 11:30 AM. Scleras were quickly cleaned of nonscleral tissue, frozen in liquid nitrogen, and kept at −80°C until RNA was extracted. All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Association for Assessment and Accreditation of Laboratory and Animal Care (AAALAC) regulations for the use of laboratory animals.
Competitive RT-PCR
Competitive reverse transcription-polymerase chain reaction (RT-PCR) was used to quantify mRNA levels. Competitive RT-PCR involves a synthetic RNA competitor molecule that is added to the RT-PCR reaction in known copy numbers. The RNA competitor is reverse transcribed and amplified by the same primers used to reverse transcribe and amplify the native mRNA so that both are amplified at approximately the same efficiency, but, the RNA competitor has an internal deletion (~50 bases) that allows its PCR product to be distinguished from the native product by size on an electrophoretic gel. When the densities of the native product and the competitor product are equal in a gel, the number of copies of native mRNA in the RT-PCR reaction is approximately equal to the number of copies of competitor added.
In this study, we measured the levels of mRNA for the α1 chain of type I collagen (collagen α1[I]), decorin (core protein), MMP-2 (gelatinase-A), MMP-3 (stromelysin-1), and TIMP-1. Type I collagen, decorin, and MMP-2 have been implicated in other studies as being involved in scleral tissue remodeling in tree shrew. MMP-3 is known to degrade proteoglycans, and TIMP-1 is the primary TIMP that inhibits MMP-3.
Details of the procedures for the extraction of RNA, primer design, competitor RNA construction, and the performance of competitive RT-PCR have been described previously27 and are recapped briefly.
RNA Isolation
Total RNA was extracted from individual whole scleras (SV Total RNA Isolation System; Promega, Madison, WI). RNA concentration and purity were determined by spectrophotometry at 260 and 280 nm.
Primers
Tree shrew–specific primers were designed for the mRNAs of α1(I) collagen, decorin, MMP-2, MMP-3, TIMP-1, and 18s ribosomal RNA. Cloning and sequencing verified the identities of the PCR products.32 The primer sequences have been reported.27
Competitor RNA
Each competitor RNA (cRNA) for this study was made with a kit (Competitor Construction Kit; Ambion, Austin, TX) that contains modified nucleotides that render the cRNA molecules RNase resistant.
RT-PCR Procedure
Hot-start, single-tube RT-PCR reactions containing 5 ng total RNA and a known number of copies of cRNA in a total volume of 50 μL were run (GeneAmp 2400; Perkin Elmer, Norwalk, CT). The RT-PCR conditions were: buffer (20 mM Tris-acetate, 10 mM ammonium acetate sulfate, 75 mM potassium acetate, 0.05% Tween 20), 1.5 mM MgSO4, 50 mM each dNTP, 0.5 U RNAsin (Promega), 50 picomoles each primer, 5 U avian myeloblastosis virus (AMV) reverse transcriptase, and 2.5 U Thermus flavus (tfl) DNA polymerase. The RT step consisted of an initial denaturation at 60°C for 2 minutes followed by 45 minutes at 48°C. The antisense primer primed the reverse transcription. The PCR cycle parameters were 30 seconds of denaturation at 94°C, 1 minute annealing at 60°C, and 1 minute 15 seconds of extension at 72°C, with a final 5-minute extension at 72°C. From 26 to 40 PCR cycles were performed, depending on the abundance of the particular message. A master mix that contained all ingredients except cRNA, primers, and enzymes was made for each eye. Total RNA was included in each master mix, so that one 43-μL aliquot contained 5 ng total RNA. The level of each mRNA was measured in aliquots from the same master mix by adding the appropriate cRNA and primers.
Quantification Procedure
To quantify the RT-PCR products, 15-μL aliquots of each RT-PCR product were run on 2% high-resolution agarose gels, stained with ethidium bromide. The band densities were then measured with a video imaging system (Eagle Eye II Still Video System; Stratagene, La Jolla, CA). After initial RT-PCR runs to approximate the number of copies of each mRNA, three twofold dilutions of cRNA were run versus constant total RNA (5 ng) to find the cRNA copy numbers that were higher and lower than the copies of native mRNA in the sample. To calculate the cRNA copy number at which equal band densities would occur, band density versus cRNA copy number was plotted on a log–log scale for the native product and the cRNA product. The crossing point of the two lines was then determined by solving simultaneous equations.
To compensate for any difference in the quantity or the quality of total RNA in each RT-PCR reaction, the target mRNAs were normalized to the number of copies of 18s rRNA. Results were expressed as copies of target mRNA per copies of 18s. For each sclera, the RT-PCR reactions for 18s rRNA and all the target mRNAs were performed from the same master mix.
Statistical Tests
Paired t-tests were used to determine whether differences between the deprived and fellow control eyes were statistically significant. Analyses of variance (ANOVA) and least-significant difference (LSD) post hoc tests were used to test whether values changed significantly over time and to test whether differences between normal eyes and the deprived or control eyes were significant. The average normal value for a normal group was obtained by first averaging the right and left eye data for each animal in the group, and then taking the average of those data for the group. It was not possible to measure creep rate and mRNA levels in the same piece of sclera; therefore, it was not possible to make statistical comparisons between creep rate and mRNA levels in individual animals. Any correlation analysis would have to be performed with the group means of the three time points at which creep rate and mRNA levels were available: 4 days of MD, 11 days of MD, and 2 days of recovery. Correlation analysis with only three data points can be very misleading and therefore was not attempted.
Results
Data from a previous study in which scleral creep rate was measured during MD and during recovery from MD20 are shown in Figure 1. The tree shrews in that study received essentially identical visual treatment as the animals in the present study and showed similar refractive and vitreous chamber depth changes. As shown in Figure 1, the creep rate in the deprived eye was significantly elevated after 4 days of MD, the earliest that this measurement was made, and the creep rate remained high after 11 days of MD. Recovery produced a rapid decrease in creep rate in the eye that had been deprived, and after 2 days of recovery, the creep rate was significantly lower in the recovering eye than in the control eye or 35 normal eyes, and the levels remained similarly lower after 11 days of recovery. These creep rate data are useful for comparison with the mRNA data in the present study.
Figure 1.
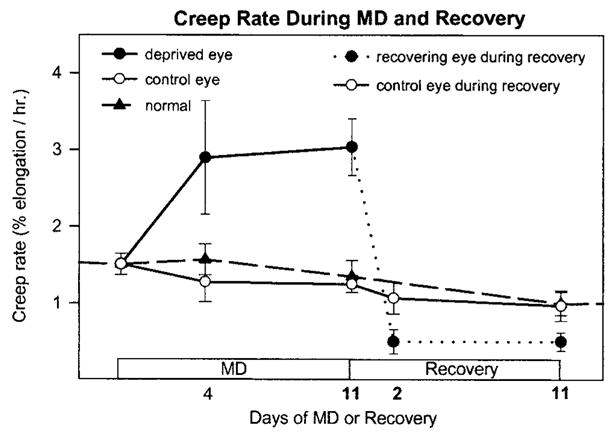
Summary of scleral extensibility (creep rate) during monocular deprivation (MD) and during recovery from MD (replotted from Siegwart and Norton20). Creep rate was increased in the deprived eyes, compared with control and normal eyes, after 4 days of MD and remained high after 11 days of MD. Two days after recovery was initiated by removing the diffuser, the creep rate in the deprived eyes (dashed line) was significantly lower than in the control eyes. Creep rate was measured in 3-mm wide strips of sclera under 1 gram of constant tension, which approximates the tension produced by normal intraocular pressure (n = 3 animals, each group). In normal animals, left and right eyes were averaged.
Refractive State and Ocular Component Dimensions
MD produced a myopic shift in refractive state and an increase in vitreous chamber depth in the deprived eye, compared with that in the control eye, that increased with length of treatment (Fig. 2). There was no evidence of a refractive or axial change after 1 day of MD. After 2 days of MD, there was an initial sign of an axial change and a significant difference in refractive state (paired t-test, P < 0.05). After 4 and 11 days of MD, there was a statistically significant difference in refractive state and vitreous chamber depth (paired t-test, P < 0.05) between the deprived and control eyes. When recovery was initiated by removing the diffuser, the myopic progression and vitreous elongation in the deprived eye stopped. After 2 and 4 days of recovery, the relative myopia and difference in vitreous chamber depth were progressively less than after 11 days of MD, similar to the pattern of recovery described previously.20 The refractive state of the control eyes did not differ from normal eyes at any point during MD and recovery. Because a primary interest of this study was to relate any changes in mRNA levels to changes in the sclera, the measurements of vitreous chamber depth were made to the anterior surface of the sclera,33 not the retina–vitreous interface, which could reflect a change in choroidal thickness without a change in the location of the anterior surface of the sclera.34–37 It appeared that changes in the location of the sclera in the deprived eyes had begun after approximately 2 days of MD and in recovering eyes, after 2 days of recovery. The vitreous chamber depth in the control eyes of the 4- and 11-day MD groups was significantly shorter than in the age-matched normal groups (28N and 35N respectively, P < 0.05, unpaired t-tests), suggesting an effect on the control eyes. There were no significant differences in refractive state or ocular component dimensions between the right and left eyes in any of the normal groups.
Figure 2.
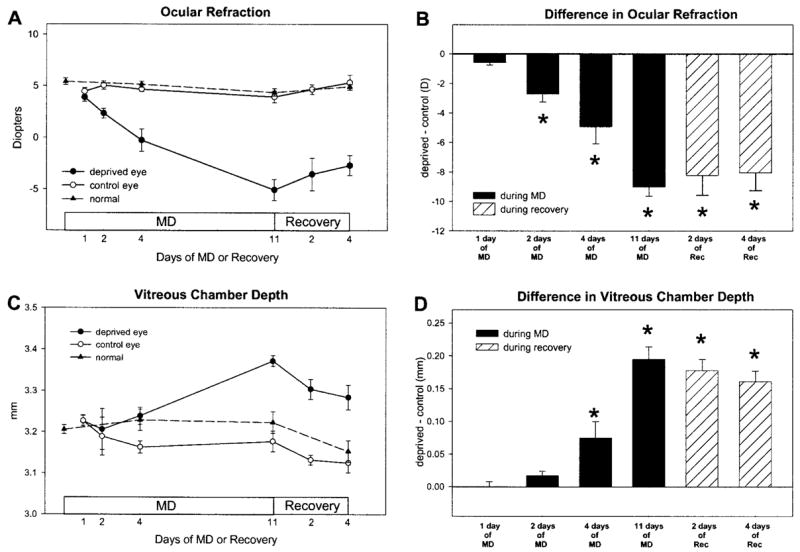
Ocular refraction and vitreous chamber depth during MD and recovery. Compared with their fellow control eyes, the deprived eyes became increasingly myopic (A) and their vitreous chambers became increasingly deep (C) as a function of days of MD. During recovery, the progression of myopia and the increase in vitreous chamber depth abruptly reversed. Data from normal animals (average of left and right eyes) are shown for comparison. (B, D) The difference between the deprived and control eyes, respectively (n = 5 in each group). Results are expressed as the mean ± SEM (*P < 0.05).
mRNA Levels
Figures 3A to 3E show the mRNA levels (average mRNA copy number) during MD and recovery. Figure 4A to 4E show the difference between the deprived and control eye or the recovering and control eye, derived from Figure 3. Figure 4F shows the difference in creep rate, derived from Figure 1. Similar to the refractive, axial, and creep rate changes, initial signs of change in some mRNA levels occurred after 1 or 2 days of MD, with statistically significant differences between the deprived and control eyes for several mRNAs noted after 4 days of treatment. MD produced two general types of effects on mRNA levels: differential changes in the deprived eye compared with the fellow control eye that were either transient or sustained and binocular changes that occurred in both the deprived and control eyes compared with age-matched normal eyes. In some cases, differential effects were superimposed on binocular effects. During recovery, the differential effects produced by MD were absent by 2 days of recovery and generally were reversed after 4 days. Most of the binocular effects had dissipated after 4 days of recovery.
Figure 3.
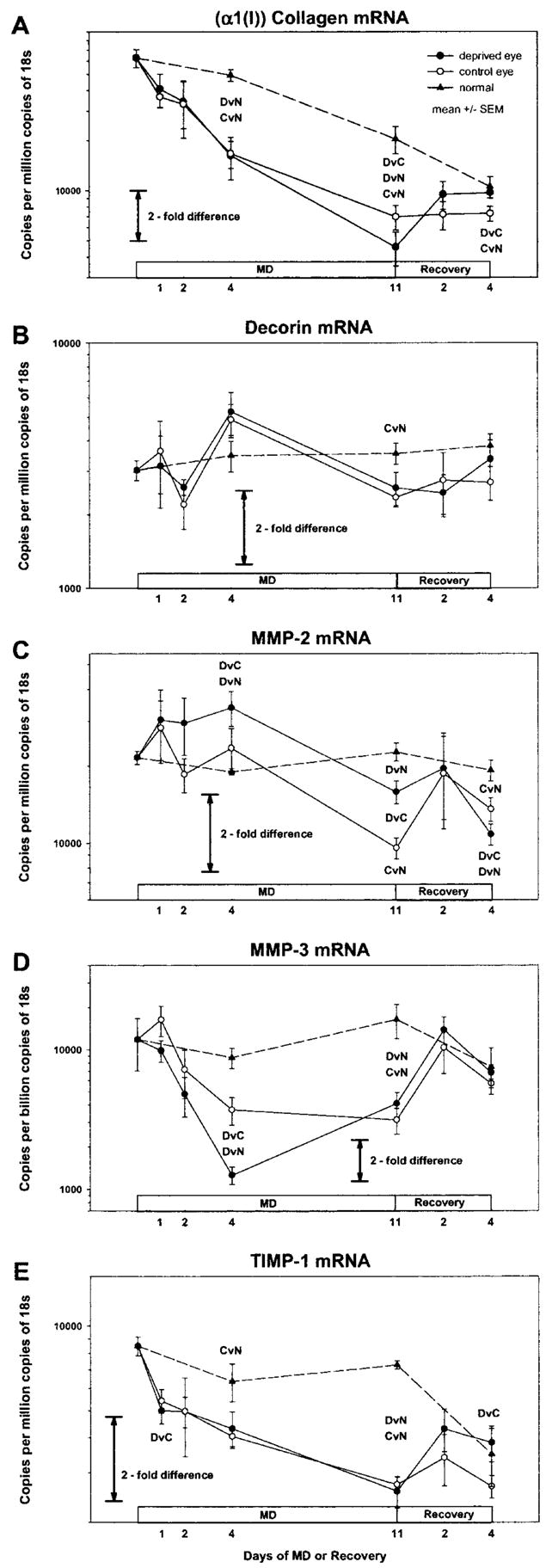
mRNA levels in the deprived and control eyes of animals in groups with different durations of MD or MD/recovery. Dashed lines: data from normal animals (left and right eye averaged). (A) Type I collagen (α1 chain), (B) decorin core protein, (C) MMP-2, (D) MMP-3, and (E) TIMP-1. All mRNA levels are expressed as copies per million copies of 18s, except for MMP-3 levels, which are copies per billion copies of 18s. Error bars are SEM. DvC, deprived eyes different from control eyes (paired-test, P < 0.05); DvN, deprived eyes different from normal eyes, (ANOVA, LSD, P < 0.05); CvN, control eyes different from normal eyes (ANOVA, LSD, P < 0.05). Deprived or control eye data could be compared statistically to normal eye data only at the four points at which age-matched normal data were available.
Figure 4.
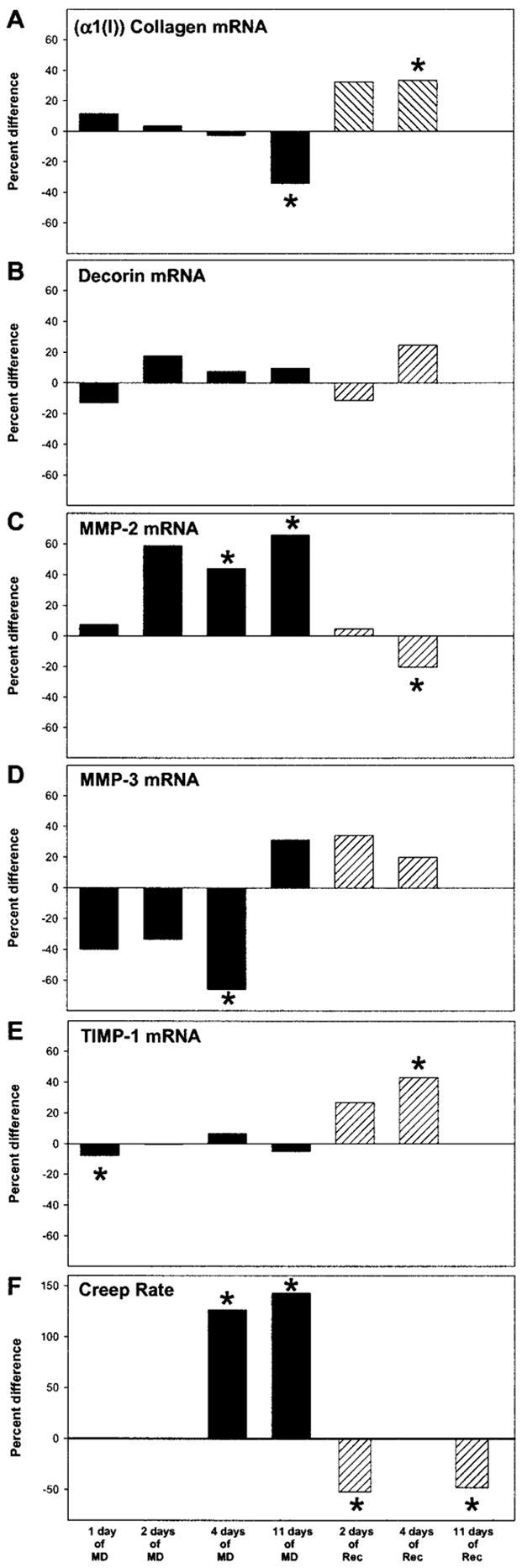
The difference in mRNA levels compared with the difference in creep rate. Shown is the difference in the average mRNA copy number for (A) Type I collagen (α1 chain), (B) decorin core protein, (C) MMP-2, (D) MMP-3, and (E) TIMP-1 in the deprived and control eyes from Figure 3. As computed, this difference has no associated error bars. *P < 0.05, as shown in Figure 3. (F) Difference in the average creep rate in the deprived and control eyes from Siegwart and Norton20 in which the animals received similar periods of MD and MD/recovery.
There were two instances in which an mRNA level was significantly different in the right and left eye of normal animals. In the 24N group, the average decorin mRNA level was 15.5% lower in the right eyes (P < 0.05) and the average α1(I) collagen mRNA level was 19.9% higher in the right eyes (P ≤ 0.05). There were no other instances of mRNA levels differing in the right and left eyes of the normal animals suggesting that, in general, mRNA levels are similar in both eyes of normally developing animals.
α1(I) Collagen
The level of α1(I) collagen (a representative structural protein) mRNA (Fig. 3A) decreased with age in the normal eyes (ANOVA, P < 0.05), in keeping with the leveling off of collagen (hydroxyproline) accumulation in the sclera in this same age range.18 During MD, α1(I) collagen mRNA levels showed an early binocular change, followed by a superimposed late-onset differential change that was present after 11 days of MD (Fig. 4A) when the level was 34% lower in the deprived eyes than in the control eyes (P < 0.01). Compared with normal eyes, (α1(I)) collagen mRNA levels decreased in both the deprived and control eyes during MD. After 4 days of MD, the level was 67% lower in the deprived eyes than in the 28-day normal eyes (P < 0.001) and was 66% lower than normal in the control eyes (P < 0.001). After 11 days of MD, the level was 78% lower in the deprived eyes than in the 35-day normal eyes (P < 0.001) and was 66% lower than normal in the control eyes (P < 0.01). After 2 days of recovery, the amount of α1(I) collagen mRNA in the deprived and control eyes was not significantly different. After 4 days of recovery, the level was 33% higher (P < 0.02) in the deprived eyes than in the control eyes, in which the level remained significantly lower than age-matched normal eyes.
Decorin
Decorin (a proteoglycan core protein) mRNA levels (Fig. 3B) were stable in normal eyes over the period examined (ANOVA, P > 0.05). The level of decorin mRNA was not significantly different between the deprived eyes and the control eyes at any point during either MD or recovery (Fig. 4B). With the exception of the control eyes in the 11-day MD group (−6% versus 35-day normal, P < 0.05), the levels in deprived and control eyes were not significantly different from those in the age-matched normal eyes at any time. Although there were no statistically significant differences between the deprived and control eyes, the decorin mRNA levels in both eyes of the deprived animals appeared to oscillate, compared with the very stable levels in normal eyes.
MMP-2
MMP-2 (gelatinase-A) mRNA levels (Fig. 3C) were stable over time in the normal eyes (ANOVA, P > 0.05). During MD, MMP-2 mRNA levels showed an early, differential increase in the deprived eyes compared with the control eyes and compared with age-matched normal eyes (Fig. 4C). After 4 days of MD, the level was 44% higher (P < 0.05) in the deprived eyes than in the control eyes, and 78% higher (P < 0.001) than in the 28-day normal eyes. After 11 days of MD, the level was 66% higher (P < 0.01) in the deprived eyes than in the control eyes. The sustained differential change was superimposed on a late-developing binocular decrease in MMP-2 mRNA levels. The MMP-2 mRNA levels in the deprived and control eyes were both significantly lower than in the 35-day normal eyes after 11 days of MD (deprived eyes, −30%, P < 0.01; control eyes, −58%, P < 0.001) suggesting a binocular decrease in MMP-2 mRNA levels starting sometime after 4 days of MD. After 2 days of recovery, MMP-2 mRNA levels were not significantly different in the deprived versus control eyes, and neither appeared different from normal. After 4 days of recovery (Fig. 4C), the mRNA level in the deprived eyes was 20% lower (P < 0.05) than in the control eyes, and both were significantly lower than in the age-matched normal eyes.
MMP-3
MMP-3 (stromelysin-1) mRNA levels (Fig. 3D) were stable over time in the normal eyes (ANOVA, P > 0.05). During MD, there was an early, transient differential change superimposed on a sustained binocular change. Initially, the mRNA level decreased rapidly in the deprived eyes compared with the control eyes and compared with normal eyes (Fig. 4D). After 4 days of MD, the MMP-3 mRNA level was 66% lower (P < 0.02) in the deprived eyes than in the control eyes and 86% lower (P < 0.05) than in the 28-day normal eyes. The level in the control eyes was not significantly different from the level in the age-matched normal eyes at 4 days of MD (P > 0.05). After 11 days of MD, the levels in deprived and control eyes were not different from each other, but both were significantly lower than in the 35-day normal eyes. After 2 and 4 days of recovery, MMP-3 mRNA levels were not significantly different in the deprived versus control eyes, and neither was significantly different from that in normal eyes.
TIMP-1
Similar to α1(I) collagen mRNA levels, TIMP-1 (representative metalloproteinase inhibitor) mRNA levels (Fig. 3E) decreased with age in the normal eyes (ANOVA, P < 0.05). During MD, TIMP-1 mRNA levels showed a very early (after 1 day of MD), transient differential effect (Fig. 4E) and a sustained binocular effect. After 1 day of MD, mRNA levels were significantly lower in the deprived eyes than in the control eyes (P < 0.05), but differential effects were not found at any other time during MD. In addition, MD suppressed TIMP-1 mRNA levels in both the deprived and control eyes compared with that in normal eyes, so that the level in the control eyes was significantly lower than in age-matched normal eyes after 4 days of MD (ANOVA, P < 0.05). Levels in both the deprived and control eyes were lower than normal after 11 days of MD (ANOVA; T, P < 0.001; C, P < 0.001). During recovery, the amount of TIMP-1 mRNA increased in the deprived eyes compared with the control eyes and was 43% higher after 4 days of recovery (P < 0.02). The binocular effect appeared to dissipate, so that levels in neither group of eyes were significantly different from normal after 4 days of recovery.
Discussion
Significant differential (deprived eyes versus control eyes) changes in mRNA levels were found in four of the mRNAs examined, but not in the fifth (decorin core protein). The direction and time course of the differential mRNA changes are of interest because, if mRNA changes are the initial step in the tissue-remodeling process that produces the scleral and refractive changes, then the mRNA changes must precede, or at least accompany those changes. Changes in mRNA levels that occur after the scleral changes begin may be secondary responses not directly related to the initial signals that start the tissue-remodeling process. Binocular changes were also found in the levels of the four mRNAs that showed differential changes. The binocular changes are of interest, because they indicate significant effects on the control eye sclera, yet seem not to be reflected in any alteration in refractive state or creep rate in the control eyes compared with the normal eyes. The changes in mRNA levels during recovery are of interest, because many of the biochemical and creep rate changes that occurred during MD were reversed during recovery. Therefore, mRNA level changes involved in the scleral remodeling during MD might be expected to reverse during recovery.
Timing of mRNA Changes
Early Differential Changes during MD
The levels of three of the mRNAs studied showed significant differential changes that occurred as early as detectable increases in vitreous chamber depth (Fig. 2) and as early as creep rate changes (Fig. 4F) and biochemical changes that have been reported in other studies. The mRNA levels of MMP-2 increased, and the levels of MMP-3 and TIMP-1 decreased, in the deprived eyes compared with the control eyes, after 1 or 2 days of lens wear, whereas a difference in vitreous chamber depth was first detectable after between 2 and 4 days of MD.
The onset of these mRNA changes also appeared to match reasonably well with the onset of changes in scleral creep rate (Fig. 4F) from a previous study.20 It should be noted that Siegwart and Norton20 did not measure creep rate until after 4 days of MD. However, when axial elongation was induced with a –5-D lens, which produces a similar effect, an increase in creep rate was present after 2 days, but was not statistically significant until 4 days.20
In previous biochemical studies, it has been found that scleral glycosaminoglycan (GAG) content is decreased by the fourth day of minus-power lens treatment25 and that a decrease in sulfate incorporation into GAGs is detectable after 5 days of MD, with an elevation after 3 days of recovery from MD.17 A significant reduction in scleral dry weight after 5 days of MD has also been reported.17 Thus, biochemical changes in the sclera are present after 4 days, and possibly as early as after 2 days, of treatment.
Because most studies use a limited number of animals, which limits statistical power, it is difficult to determine exactly when a change begins. Within the limits of the temporal resolution of currently available data, the timing of the early, differential mRNA level changes found in MMP-2, MMP-3, and TIMP-1 in this study suggests that these changes in gene expression could be causally related to the physical changes.
Late Differential Changes during MD
Our previous study27 found differential changes in mRNA levels for collagen after 11 days of MD and a reversed pattern after 4 days of recovery. In the present study, the time course information from groups with shorter periods of MD suggests that the differential changes in collagen mRNA levels occurred after physical and biochemical changes had begun in the sclera. These late differential changes in collagen mRNA levels could have been a secondary response to physical changes within the sclera38 as the tissue remodeling proceeded, rather than a direct response to the initial signal. Previous studies have shown that a change in mechanical stress can produce changes in the expression of a variety of genes, including those of collagen and MMPs,39,40 and the tissue-remodeling process in the sclera probably alters the local stresses within the sclera. It is interesting to note, however, that most studies report that an increase in tension induces an increase in collagen expression, the opposite of what was found after 11 days of MD. This suggests that the decrease in collagen mRNA is not due to an increase in tension in the deprived eye sclera. The dissipation of the difference in MMP-3 mRNA level from 4 to 11 days of MD (Fig. 3D) could also be a secondary response to a change in scleral stress induced by the tissue remodeling.
Binocular Changes during MD
In addition to differential changes, some mRNA levels were significantly altered in the control eyes, compared with normal eyes, at various points during MD (Fig. 3). In all cases, the changes in the control eye mRNA levels were in the same direction, compared with levels in normal eyes, as the changes in the deprived eyes. Two points are of interest regarding the mRNA level changes in the control eyes: One is how they occur. The other is why, given that the mRNA changes were in the same direction as the changes in the deprived eye, they did not appear to the affect creep rate or other physical characteristics of the sclera in a similar fashion.
Previous studies in tree shrew and other species have shown that MD or monocular treatment with a minus-power lens can produce alterations in the open control eye.20,26,41 It is currently not known how monocular treatment produces an effect in the open control eye. Possible mechanisms include systemic effects such as altered choroidal blood flow, CNS feedback to both eyes, and modified visual input to the control eyes resulting from modified visual behavior. In the present study, the 24-, 28-, and 35-day normal animals all underwent a surgical procedure to install a goggle pedestal at 21 days of VE, as did the deprived animals, which eliminates the possibility of a systemic effect from the surgical procedure. In addition, there was an open goggle frame, rather than a plano lens, in front of the control eyes, and the effect in the control eyes therefore cannot be a lens effect, such as an increase in temperature. In a recent study,42 the investigators found that decreased illuminance in one eye produces a decrease in choroidal blood flow in both eyes. A similar binocular reduction was found in the chick after MD.43 How a change in blood flow, or other binocular effects might alter mRNA levels in the control eye is not known. However, these effects on mRNA levels in the control eyes emphasize the importance of also obtaining measurements in completely normal eyes. It clearly could be misleading to draw conclusions about the effects of monocular treatment based solely on relative differences between the treated and control eyes.
It is not clear why the changes in mRNA levels in the control eyes, which moved generally in the same direction as the changes in the deprived eyes, did not appear to cause similar physical changes. The vitreous chamber appeared to become slightly smaller in the control eyes during MD, and the creep rate in the previous study20 was slightly lower (not statistically significant), but these changes were in the opposite direction from the changes in the deprived eyes. This could indicate that there are gene products that were not examined in this study that have expression changes only in the deprived eye. It could also suggest that the differences between the treated and control eyes are functionally more important than the absolute levels.
Differential Changes during Recovery
During recovery, there is an abrupt reversal in creep rate (Fig. 1), so that the deprived eyes switch from having a much higher creep rate than the control eyes to having a creep rate that is significantly lower than in control eyes.20 A relative change in sulfate incorporation, from lower in the deprived eye after 5 days of MD to higher in the recovering eye after 3 days of recovery, has also been reported in tree shrews.17 A similar reversal occurred in several of the mRNAs that showed significant differential changes during MD. The level of mRNA for type I collagen, which was significantly lower in the treated eyes after 11 days of MD, was not different after 2 days of recovery. After 4 days of recovery, the levels were higher in the recovering eyes. The effect of recovery on MMP-2 mRNA levels was similar, but in the opposite direction. The levels were higher in the deprived eyes after 11 days of MD, were not different from the control eyes after 2 days of recovery, and were significantly lower after 4 days of recovery. mRNA levels of TIMP-1 were transiently lower in the deprived eye after 1 day of MD and then were not significantly different for the rest of the MD period. During recovery, TIMP-1 levels in the recovering eyes were at approximately normal levels after 2 days and were significantly higher in the recovering eye after 4 days. Although MMP-3 mRNA levels became higher in the recovering eye after 2 and 4 days of recovery, the differences were not statistically significant.
Binocular Changes during Recovery
In general, the binocular effects produced by MD receded rapidly during recovery. MMP-3 mRNA levels, which were significantly lower than normal in both eyes after 11 days of MD rapidly returned to normal levels during recovery. Collagen mRNA levels in both eyes moved back toward normal levels during recovery, but the level in the control eye remained significantly below normal. MMP-2 mRNA levels in both eyes transiently moved back to normal levels after 2 days of recovery, but then decreased back below normal after 4 days.
Relation of mRNA Levels to Specific Creep Rates
A pattern that might be expected to occur if an mRNA level is causally related to the scleral remodeling would be for the mRNA level to change in unison with the changes in creep rate and/or other scleral changes throughout MD and recovery. This type of pattern did not occur among the mRNAs examined in the present study. In general, there appeared to be a stronger association between initial changes in mRNA levels and initial changes in creep rate than between sustained mRNA levels and sustained creep rates. The closest to a continuous correlation was the mRNA level for MMP-2, except that there was a binocular decrease in mRNA levels by 11 days of MD that was not paralleled by creep rate or other scleral changes. Two factors should be considered with regard to there being no perfect correlation between any mRNA level and, for instance, creep rate. One is that there may be other mRNA levels, not examined in this study, that change in unison with creep rate. Another is that it is not necessarily the case that mRNA changes that initiate scleral changes must remain altered. For example, a transient change in the MMP-2 mRNA level may contribute to tissue remodeling that changes the creep rate, which then remains constant until another transient change in an mRNA level, such as an increase in TIMP-1 during recovery contributes to a decrease in the creep rate. Further, an elevated mRNA level and excessive production of a degradative enzyme such as MMP-2 may produce excessive tissue degradation if it were maintained too long.
Possible Functional Significance of Changes in mRNA Level
The changes in mRNA levels found in this study should be considered in light of known changes in protein levels during MD or minus-power lens wear, and with general principles of ECM remodeling from other tissue systems. A change in the level of a protein during tissue remodeling can be achieved through different combinations of synthesis and degradation and a key to understanding the remodeling process is to determine the relative contribution of the two distinct processes. Comparing changes in mRNA level with changes in protein level can help determine whether synthesis of the proteins is altered, and further, whether it can be controlled at the transcriptional level or at a later stage of gene expression.
Previous studies have found reduced amounts of type I collagen in the deprived-eye sclera compared with the control-eye sclera after 21 days of MD.14,18 This suggests that a reduction in the synthesis of type I collagen, the primary structural component of the sclera, could play an important role in scleral remodeling during MD. It is not known how quickly collagen protein levels change or whether there is a difference between the deprived and control eyes after several days of treatment when anatomic changes are first apparent. Therefore, it is not clear whether the early binocular and late differential mRNA changes are consistent with protein changes. The absence of an early difference in collagen mRNA level, and possibly protein level, between the deprived and control eyes does not necessarily mean that a change in collagen expression cannot play a role in the increase in creep rate in the deprived-eye sclera that is apparent by 4 days of MD.20 The rapid decrease in α1(I) collagen mRNA (compared with normal eyes) that occurs in both the deprived and control eyes, could be essential to the biomechanical changes that occur in the deprived-eye sclera without being sufficient to cause any changes in the control-eye sclera. An analogy is the effect of systemic lathyritic treatment in tree shrews, which undoubtedly affects collagen cross-linking in both the deprived and control eyes, but only enhances the effect of MD in the deprived eyes, without affecting the control eyes.44 The absence of an early difference in collagen mRNA between the deprived and control eyes suggests that a change in collagen expression alone is not sufficient to produce the early differential biomechanical effects between the deprived and control eyes and that another component, such as proteoglycans, may be involved. The substantial increase in the α1(I) collagen mRNA level in the deprived eyes after 2 days of recovery suggests that an increase in expression of type I collagen plays a role in the rapid decrease in creep rate that occurs during recovery.
That decorin mRNA levels did not change was an unexpected result. Proteoglycans, which consist of a core protein with attached GAG chains, are known to influence the mechanical properties of biological tissue.45 Decorin, a small proteoglycan with a single GAG chain, is the most abundant proteoglycan in the sclera46 making it a potentially important component in the scleral creep rate changes that occur during MD and recovery. Several previous studies have shown that MD and recovery alter the levels of sulfated and unsulfated GAGs in the sclera.12–14,24 The amount of 35S sulfate that is incorporated into sulfated GAGs is reduced in the deprived eyes compared with the control eyes, suggesting that MD and recovery may alter GAG or proteoglycan core protein synthesis. In the present study, MD and recovery did not alter decorin core protein mRNA levels in the deprived eyes compared with the control eyes. This suggests that the synthesis of the most abundant proteoglycan (core protein) in the sclera was not altered. This finding is not necessarily inconsistent with previous findings, because other mechanisms, including a change in the rate of proteoglycan degradation and modification of GAG change, length, and extent of sulfation could produce the observed changes in GAG levels and 35S sulfate incorporation. Data on GAG chain length and extent of sulfation and separate measures of proteoglycan core protein and GAG chain synthesis and degradation are needed to learn conclusively what mechanism is responsible for the observed changes in GAGs. In addition, other less abundant scleral proteoglycans, such as biglycan, lumican, and aggrecan should be examined.
The changes in MMP-2 mRNA levels generally matched the known changes in MMP-2 protein during MD and recovery.26 MMP-2 activity can be controlled at a number of levels, including transcription, activation, and inhibition by TIMPs. Although a change in mRNA stability cannot be ruled out, these data suggest that control at the transcriptional level is important in this system. In addition, the early onset of the MMP-2 mRNA level changes suggests that a change in MMP-2 transcription may be one of the initial responses of the scleral fibroblasts to signals from the retina.
The decrease in MMP-3 mRNA during MD was also an unexpected finding. Other studies23 suggest that one role of MMP-3 in tissue remodeling is to degrade proteoglycans by cleaving the core protein. Therefore, based on reports that MD produces a loss of GAGs in the deprived-eye sclera compared with the control eye sclera,14 we hypothesized that MMP-3 might degrade proteoglycans during MD and therefore that there might be an increase MMP-3 mRNA in the deprived-eye sclera during MD. Instead, the data suggest that MMP-3 mRNA levels decrease during MD and increase during recovery. Given this unexpected result, it is interesting to speculate the role that MMP-3 may play. It has been reported that MMP-3–null mice display delayed wound healing47 and that MMP-3–null dermal fibroblasts in culture contract collagen gels significantly less than wild-type fibroblasts.48 These data suggest that MMP-3 plays a role in scleral remodeling other than degrading proteoglycan core proteins.
Current evidence suggests that the primary role of the TIMPs is to inhibit MMPs, and TIMP-1 is thought to be the primary inhibitor of MMP-3. In many but not all systems that have been studied, TIMP levels tend to decrease under conditions of increased tissue degradation and to increase under conditions of decreased degradation.39,49 The changes in TIMP-1 mRNA levels in the present study were in general agreement with these findings in that TIMP-1 levels decreased during MD and increased during recovery. However, the changes in TIMP-1 mRNA levels were in the same direction as the changes in MMP-3 mRNA levels, which does not necessarily fit if the primary role of TIMP-1 is to inhibit MMP-3, and it should be kept in mind that inhibiting MMPs may be but one role that TIMPs can play.50
In conclusion, this study provides quantitative data on the changes in mRNA levels that occur during MD and recovery in a sample of important ECM proteins. Within the limits of resolution that these data provide, some of the mRNA changes appeared to occur at least as early as the physical changes, making it plausible that a change in gene expression initiates the scleral tissue remodeling. It is likely that a number of other genes play a role in the observed tissue remodeling. Together, the opposite responses of specific mRNA levels to MD and recovery, and the temporal correspondence between mRNA, biochemical, and biomechanical changes suggest that the visually guided emmetropization mechanism modulates scleral gene expression to control axial elongation rate and the refractive state of the eyes.
Acknowledgments
The authors thank Joel Robertson for excellent technical assistance.
Supported by National Eye Institute Grants RO1 EY05922 and Core Grant P30 EY03909.
Footnotes
Commercial relationships policy: N.
References
- 1.Norton TT. Animal models of myopia: learning how vision controls the size of the eye. Inst Lab Anim Res J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 2.Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthal Physiol Opt. 1997;17:279–290. [PubMed] [Google Scholar]
- 3.Lauber JK, McGinnis J, Boyd J. Influence of mitotics, diamox and vision occluders on light- induced buphthalmos in domestic fowl. Proc Soc Exp Biol Med. 1965;120:572–575. doi: 10.3181/00379727-120-30593. [DOI] [PubMed] [Google Scholar]
- 4.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 5.Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68:364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol. 1992;170:565–574. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- 7.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 8.Tigges M, Tigges J, Fernandes A, Eggers HM, Gammon JA. Postnatal axial eye elongation in normal and visually deprived rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:1035–1046. [PubMed] [Google Scholar]
- 9.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys [see comments] Mature Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 10.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 12.Christensen AM, Wallman J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 1991;32:2143–2150. [PubMed] [Google Scholar]
- 13.Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- 14.Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 15.Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41:2050–2058. [PubMed] [Google Scholar]
- 16.Gentle A, McBrien NA. Modulation of scleral DNA synthesis in development of and recovery from induced axial myopia in the tree shrew. Exp Eye Res. 1999;68:155–163. doi: 10.1006/exer.1998.0587. [DOI] [PubMed] [Google Scholar]
- 17.McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000;41:3713–3719. [PubMed] [Google Scholar]
- 18.Norton TT, Miller EJ. Collagen and protein levels in sclera during normal development, induced myopia, and recovery in tree shrews [ARVO Abstract] Invest Ophthalmol Vis Sci. 1995;36(4):S760. Abstract nr 3517. [Google Scholar]
- 19.Walls G. The vertebrate eye and its adaptive radiations. Bloomfield Hills, MI: The Cranbrook Press; 1942. [Google Scholar]
- 20.Siegwart JT, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 21.Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000;41:2028–2034. [PubMed] [Google Scholar]
- 22.Hay ED. Extracellular matrix, cell skeletons, and embryonic development. Am J Med Genet. 1989;34:14–29. doi: 10.1002/ajmg.1320340107. [DOI] [PubMed] [Google Scholar]
- 23.Birkedal-Hansen H, Moore WGI, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 24.Norton TT, Rada JA, Clark EC. Proteoglycans in tree shrew sclera [ARVO Abstract] Invest Ophthalmol Vis Sci. 1998;39(4):S505. Abstract nr 2312. [Google Scholar]
- 25.German A, Baker J, Norton TT. Changes in hyaluronan, chondroitin sulfate and dermatan sulfate in sclera of tree shrews with induced myopia [ARVO Abstract] Invest Ophthalmol Vis Sci. 1999;40(4):S453. Abstract nr 2387. [Google Scholar]
- 26.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1380–1395. [PubMed] [Google Scholar]
- 27.Siegwart JT, Norton TT. Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery. Invest Ophthalmol Vis Sci. 2001;42:1153–1159. [PubMed] [Google Scholar]
- 28.Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab Animal Sci. 1994;44:292–294. [PubMed] [Google Scholar]
- 29.Norton TT, Siegwart JT, German A, Robertson J, Wu W. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia [ARVO Abstract] Invest Ophthalmol Vis Sci. 2000;41(4):S563. Abstract nr 2990. [Google Scholar]
- 30.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 31.McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Doc Ophthalmol. 1981;28:187–192. [Google Scholar]
- 32.Andison ME, Siegwart JT, DeCarlo AA, Leonard CL, Norton TT. Identification of gelatinase-A, Stromelysin-1 and decorin mRNA in tree shrew sclera [ARVO Abstract] Invest Ophthalmol Vis Sci. 1999;40(4):S453. Abstract nr 2388. [Google Scholar]
- 33.Siegwart JT, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 34.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 35.Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258. [PubMed] [Google Scholar]
- 37.Hung LF, Wallman J, Smith EL., III Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41:1259–1269. [PubMed] [Google Scholar]
- 38.Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol. 1996;74:737–744. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- 39.Kessler D, Dethlefsen S, Haase I, et al. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem. 2001;276:36575–36585. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- 40.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 41.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- 42.Fuchsjager-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vision Res. 2001;41:2919–2924. doi: 10.1016/s0042-6989(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 43.Shih Y-F, Fitzgerald MEC, Norton TT, Gamlin PDR, Hodos W, Reiner A. Reduction in choroidal blood flow occurs in chicks wearing goggles that induce eye growth toward myopia. Curr Eye Res. 1993;12:219–227. doi: 10.3109/02713689308999467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res. 1994;59:475–486. doi: 10.1006/exer.1994.1133. [DOI] [PubMed] [Google Scholar]
- 45.Cribb AM, Scott JE. Tendon response to tensile stress: an ultra-structural investigation of collagen: proteoglycan interactions in stressed tendon. J Anat. 1995;187:423–428. [PMC free article] [PubMed] [Google Scholar]
- 46.Rada JA, Achen VR, Perry CA, Fox PW. Proteoglycans in the human sclera: evidence for the presence of aggrecan. Invest Ophthalmol Vis Sci. 1997;38:1740–1751. [PubMed] [Google Scholar]
- 47.Bullard KM, Lund L, Mudgett JS, et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bullard KM, Mudgett J, Scheuenstuhl H, Hunt TK, Banda MJ. Stromelysin-1-deficient fibroblasts display impaired contraction in vitro. J Surg Res. 1999;84:31–34. doi: 10.1006/jsre.1999.5599. [DOI] [PubMed] [Google Scholar]
- 49.Fassina G, Ferrari N, Brigati C, et al. Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metastasis. 2000;18:111–120. doi: 10.1023/a:1006797522521. [DOI] [PubMed] [Google Scholar]
- 50.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]


