Summary
A large number of Gram-negative bacteria employ N-acyl homoserine lactones (AHLs) as signaling molecules in quorum sensing, which is a population density-dependent mechanism to coordinate gene expression. Antibody RS2-1G9 was elicited against a lactam mimetic of the N-acyl homoserine lactone and represents the only reported monoclonal antibody that recognizes the naturally-occuring N-acyl homoserine lactone with high affinity. Due to its high cross-reactivity, RS2-1G9 showed remarkable inhibition of quorum sensing signaling in Pseudomonas aeruginosa, a common opportunistic pathogen in humans. The crystal structure of Fab RS2-1G9 in complex with a lactam analog revealed complete encapsulation of the polar lactam moiety in the antibody combining site. This mode of recognition provides an elegant immunological solution for tight binding to an aliphatic, lipid-like ligand with a small head group lacking typical haptenic features, such as aromaticity or charge, which are often incorporated into hapten design to generate high-affinity antibodies. The ability of RS2-1G9 to discriminate between closely-related AHLs is conferred by six hydrogen bonds to the ligand. Conversely, cross-reactivity of RS2-1G9 towards the lactone is likely to originate from conservation of these hydrogen bonds as well as an additional hydrogen bond to the oxygen of the lactone ring. A short and narrow tunnel exiting at the protein surface harbors a portion of the acyl chain and would not allow for entry of the head group. The crystal structure of the antibody without its cognate lactam or lactone ligands revealed a considerably altered antibody combining site with a closed binding pocket, suggestive of an induced fit mechanism for ligand binding. Curiously, a completely buried ethylene glycol molecule mimics the lactam ring and, thus, serves as a surrogate ligand. The detailed structural delineation of this quorum-quenching antibody will now aid in further development of an antibody-based therapy against bacterial pathogens by interference with quorum sensing.
Keywords: Crystal Structure, hapten complex, quorum sensing, quorum quenching, N-acyl homoserine lactone
Introduction
Quorum sensing is a sophisticated system for coordinated regulation of gene expression via cell-to-cell communication in single-cell microorganisms. The term “quorum sensing” refers to the dependence of the signaling activity on the population density and provides bacteria with a means to act as a multicellular unit. Cooperativity in gene expression increases the effectiveness of processes, such as biofilm formation, sporulation, competence, conjugation, virulence factor expression, antibiotic production, swarming motility, or bioluminescence 1; 2. The bacterial population density is sensed by detection of the local concentration of soluble, small hormone-like signaling molecules, also known as autoinducers or quormones, which are constitutively produced at a low basal level. Many Gram-negative bacteria produce N-acyl homoserine lactones (AHLs), whereas Gram-positive species primarily employ peptides or peptide derivatives 2. The interspecies signaling molecule autoinducer-2 (AI-2), a furanose derivative, is utilized by both Gram-positive and Gram–negative bacteria. Two proteins are key components of an AHL-based quorum sensing circuit: an autoinducer receptor (R protein), which functions as a transcriptional activator, and an autoinducer synthase (I protein), whose gene expression, among others, is often activated by the autoinducer receptor in a positive feedback loop. As the autoinducer concentration rises as a function of increasing cell-population density, the detection of a minimal threshold stimulatory concentration of the autoinducer leads to alteration of gene expression and, consequently, to a physiological response.
Quorum sensing is crucial for virulence in many pathogenic bacteria that pose significant medical and agricultural threats. In the opportunistic bacterium Pseudomonas aeruginosa, quorum sensing signaling controls the expression of several hundred genes, constituting about 6% of the genome 3. One of these regulated processes is the production of biofilms that are associated with a variety of chronic infections 4. Biofilms consist of sessile bacterial colonies encased in polysaccharide matrices that have been shown to be resistant to antimicrobials and host immune cells; hence, treatment of infected cystic fibrosis patients and immune-compromised individuals has proven difficult. Moreover, AHLs themselves induce biochemical changes and exert cytotoxicity in mammalian cells, which further highlights their importance in pathogenicity of Gram-negative bacteria and the need for effective therapeutic countermeasures 5; 6. Thus, strategies to interfere with quorum sensing provide new avenues to combating bacterial diseases in humans, animals, and plants. In fact, several antagonistic approaches, such as heterologous overexpression of quorum-quenching lactonases or discovery of inhibitors against the I or R proteins using combinatorial chemistry, have recently been reported 7; 8; 9; 10. Interference with quorum sensing affords the great benefit of controlling infectious bacteria without interfering with growth, thus avoiding the type of selection pressure that frequently results in development and selection of resistant bacterial strains to antibiotics when traditional antibiotic treatments are used 11.
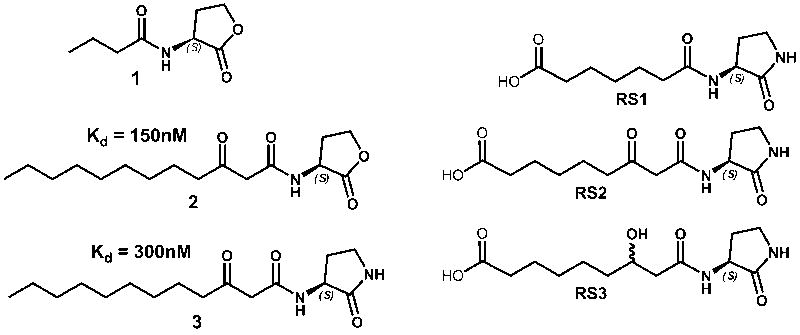
A completely different approach to quorum quenching has recently been described by harnessing the immune system to counteract the quorum sensing system of the opportunistic pathogen P. aeruginosa 12; 13. In one case, immunization of mice with an AHL-protein conjugate appears to prevent lethality in a P. aeruginosa infection model 13. However, AHLs are inherently instable at physiological pH and lead to breakdown products through formation of a ring-opened hydrolyzed form of the AHL, as well as the generation of a tetramic acid species 14. Thus, in another immunopharmacotherapeutic strategy, the lactone ring of the hapten was replaced with the more stable lactam moiety 12. Subsequent immunization of mice with three different lactam haptens, RS1, RS2, and RS3 (Figure 1), that closely resemble the two AHLs of P. aeruginosa, N-butanoyl-homoserine lactone (C4-AHL, 1), and N-(3-oxododecanoyl)-homoserine lactone (3-oxo-C12-AHL, 2), yielded numerous monoclonal antibodies (mAbs) 12. Affinity measurements using competition ELISA revealed six RS2-antibodies with submicromolar affinity, ranging from 150 to 800 nM, for the 3-oxo-C12-lactam 3, while none of the RS1- or RS3-antibodies possesses an affinity below 10 μM. Consistent with the well-documented specificity of antibodies for their antigens, five of the six RS2-antibodies recognize the original lactam immunogen significantly better than the corresponding lactone 2. For example, antibody RS2-1A4 approximately affords an impressive 1000-fold discrimination between lactone 2 and lactam 3, although these compounds only differ in a single functional group.
Figure 1.
Structures of the two autoinducers (N-acyl homoserine lactones, AHLs) mediating quorum sensing in P. aeruginosa (1-2), of a lactam analog (3), and of haptens (RS1-3). The affinity constants of RS2-1G9 for 2 and 3 are listed.
However, cross-reactivity with the lactone is explicitly desired in this case, since the P. aeruginosa quorum sensing signaling molecules are indeed lactones, not lactams. Notably, one of the characterized antibodies, termed RS2-1G9, met this requirement and bound lactone 2 with an even higher affinity (Kd = 150 nM) than lactam 3 (300 nM). The use of an analog of the intended target in the immunization process to elicit antibodies against small molecules has been successfully used in the past for the generation of catalytic antibodies, in a so-called “bait-and-switch” strategy 15; 16. Consistent with high affinity recognition of AHL 2, subsequent reporter assays demonstrated that mAb RS2-1G9 effectively inhibits quorum sensing signaling in P. aeruginosa 12. At the same time, mAb RS2-1G9 was able to discriminate against the closely-related quorum sensing molecule C4-AHL 1, since this compound is bound with 1,000-fold lower affinity.
In order to gain insight into immune recognition of a quorum sensing molecule by an antibody and advance the development of antibody-based antimicrobial therapeutics that target quorum sensing, crystallographic studies of antibody RS2-1G9 were initiated. From a structural point of view, the generation of an antibody with nanomolar affinity against a lipid-like compound, such as 3, featuring a small head group that lacks typical haptenic features, such as aromaticity or charge, is quite remarkable 12; 17. Moreover, structure determination of a RS2-1G9-ligand complex has also provided a structural basis for its cross-reactivity with lactones and lactams and for its high specificity for the 3-oxododecanoyl substituent in AHLs.
Results and Disscussion
Quality of the RS2-1G9 lactam complex crystal structure
The crystal structure of the Fab fragment of antibody RS2-1G9 in complex with the AHL lactam analog 3 was determined by molecular replacement and refined to 3.18 Å resolution. The bound ligand has three additional methylene units in the acyl chain and lacks the terminal carboxyl group in comparison to the immunizing hapten RS2 (Figure 1). Despite the modest resolution, Rcryst and Rfree are better than average for this resolution range (Rcryst = 21.0% and Rfree = 26.5%, Table 1) 18. Only ThrL51 in the complementarity-determining region (CDR) L2 of both Fab molecules in the asymmetric unit are in the “disallowed” region of the Ramachandran plot, but both have well-defined electron density. ThrL51 is in a γ turn, as commonly observed in the canonical CDR L2 structure of other antibody structures19 and, hence, is not a true outlier despite the Procheck designation.
Table 1.
Data collection and refinement statistics of RS2-1G9 crystal structures
| AHL analog complex | ethylene glycol complex | |
|---|---|---|
| Space group | P43212 | P212121 |
| Unit cell dimensions (Å) | a=b=118.7, c=176.0 | a=56.3, b=72.0, c=116.1 |
| Resolution range (Å) | 50.0-3.18 (3.25-3.18) | 50.0-2.85 (2.92-2.85) |
| Unique reflections | 19,140 | 11,938 |
| Completeness (%) | 88.6 (95.0) | 99.6 (99.9) |
| Redundancy | 2.2 (2.1) | 3.6 (3.6) |
| Wilson B-value (Å2) | 79.9 | 83.5 |
| Rsym | 0.096 (0.508) | 0.062 (0.534) |
| <l/σ> | 14.2 (2.0) | 26.1 (2.7) |
| Rcryst/ Rfree | 0.210/ 0.265 | 0.200/ 0.263 |
| Fabs in asymmetric unit | 2 | 1 |
| Rmsd from ideal bond lengths (Å)/ angles (°) | 0.014/ 1.5 | 0.013/ 1.5 |
| Average B-values protein/ligand (Å2) | 75.3/ 59.0 | 60.3/ 58.8 |
| Ramachandran plot most favored/ additionally allowed/ generously allowed/ disallowed (%) | 83.2/15.1/1.4/0.3 | 84.9/14.6/0.3/0.3 |
* Highest resolution shell.
†
‡
§ Rfree is calculated as for Rcryst, but from 5% of the data that was not used for refinement.
¶ Root-mean-square deviation.
∥ ThrL51 (CDR L2) is the only residues in a disallowed region, but ThrL51 has well-defined electron density and is in a γ turn, as commonly observed in other antibody structures.
Overall, the electron density was of good quality and did not show any main-chain breaks at a contour level of 1σ throughout the entire structure. Even the notoriously poorly defined loop between SerH127 and GlnH135 of the heavy-chain constant domain CH1 was visible in the electron density map 20; 21, albeit exhibiting reduced electron density. Most importantly, clear electron density at the antibody combining sites in both Fabs became evident in σa-weighted 2Fo–Fc and Fo–Fc maps during refinement (Figure 2), which guided the incorporation of the lactam ligand into the structure. The terminal part of the acyl chain projects into solution and, accordingly, is less well-defined in the electron density map. The ligand refined well, as evidenced by a slightly lower average B-value calculated over all ligand atoms with respect to that of the protein (Table 1).
Figure 2.
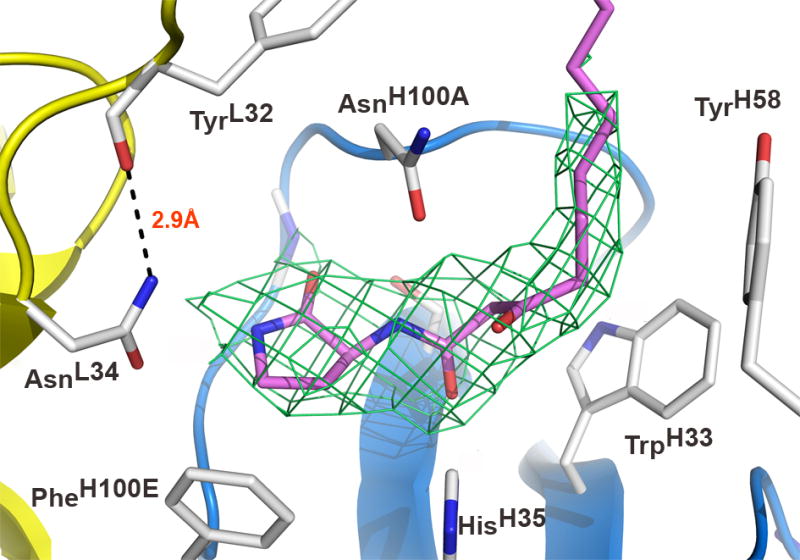
Antibody combining site of RS2-1G9 bound to an AHL lactam mimetic (pink). The light and heavy chains are colored in yellow and blue, respectively. The σa-weighted 2Fo-Fc electron density map around the ligand is contoured at 1.4σ. The microenvironment at the very bottom of the binding pocket tolerates high-affinity binding of both the lactam and the lactone, since AsnL34 does not form a hydrogen bond to the NH-group of the lactam. A potential hydrogen bond in the lactone complex model with the NH2-group of AsnL34 may account for its increased affinity with respect to the lactam. The displayed orientation of the terminal amide group of AsnL34 is preferred due to formation of a hydrogen bond with TyrL32. CDR L3 is omitted for clarity.
Overall Fab structure and architecture of the RS2-1G9 combining site
The structure of Fab RS2-1G9 strongly resembles other Fab molecules in its overall topology and features 22. Notably, the two copies of RS2-1G9 exhibit large elbow angles of 211° and 210°, respectively, consistent with RS2-1G9 possessing a λ light chain 23. The hapten analog 3 is bound in the center of the antibody combining site (Figure 3). As observed in other antibody complexes with small ligands 24, CDR H3 and L3 are primarily responsible for ligand recognition (43% and 19% of the total Fab surface area 25 contacting 3, respectively), H1 (16%), L1 (13%), and H2 (9%) make fewer contacts, whereas L2 does not contribute to ligand binding. Most striking, however, is the complete encapsulation of the lactam head group within the antibody combining site of RS2-1G9 (Figure 4), burying 97% of the surface area of the lactam ring and the first six carbon units of the C12 acyl chain. A narrow tunnel linking the lactam-binding cavity with bulk solvent harbors half of the acyl tail. Unlike many other anti-hapten antibodies that also provide deep, but rigid, hydrophobic pockets or slots 24; 26, mAb RS2-1G9 features a narrow constriction within the tunnel that envelopes the linkage between the acyl chain and the lactam. As a consequence of this architecture, facile diffusion of the ligand into and out of the binding site is not possible without major conformational changes. Among the few antibody-ligand structures that similarly clasp their ligands in deep, constricted cavities, are the catalytic antibodies 4C6 (95%), 1F7 (90%), or the Diels-Alder antibody 13G5 (99%) 27; 28; 29.
Figure 3.
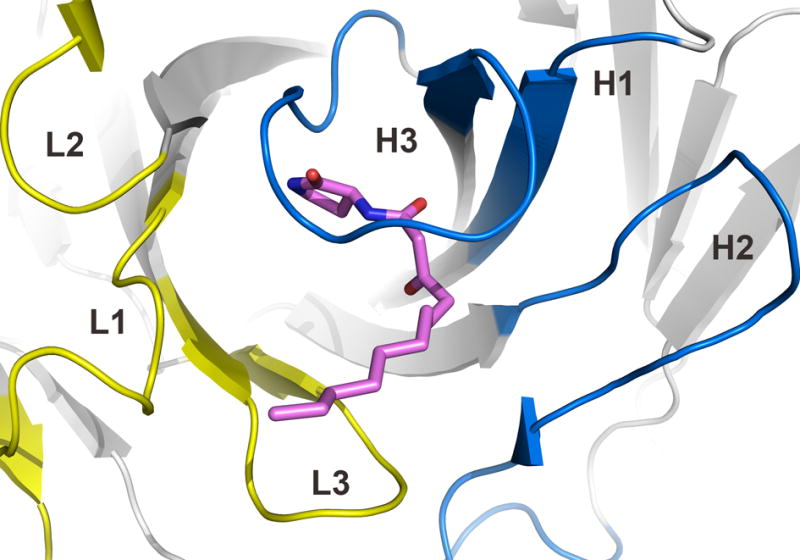
Architecture of the antibody combining site of RS2-1G9. The CDRs of light and heavy chains are highlighted in yellow and blue, respectively. The lactam 3 is shown in pink. The tip of the CDR H3 loop bends over the ligand and largely seals it from bulk solvent.
Figure 4.
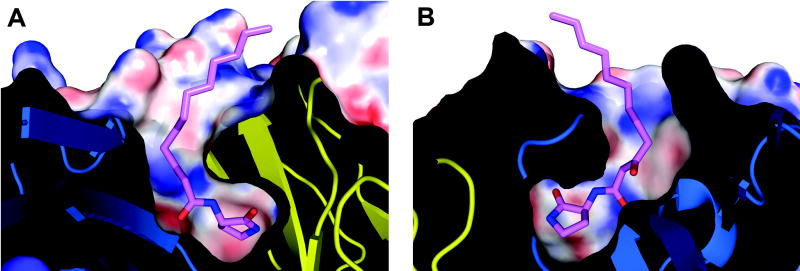
High electrostatic and shape complementarity of the hapten analog in the antibody-combining site. A slice through the center of the binding site is shown. (a) and (b) correspond to the front and back view. The electrostatic potential was calculated in APBS 64 and mapped onto the surface with the color code ranging from -30 kT/e (bright red) to +30 kT/e (dark blue).
In addition to complete burial of the ligand head group, RS2-1G9 also exhibits high shape complementarity (Sc parameter of 0.84 for the head group including the 3-oxo-group, 0.81 for the whole ligand) to the AHL lactam analog 3 30, thus ranking among antibody-hapten complexes which exhibit high Sc values, such as antibody 34E4 (Sc = 0.87) or 7A1 (Sc = 0.89) 31; 32. It is conceivable that the shape correlation in RS2-1G9 may even be higher, since no water molecules could be detected in the electron density due to the modest resolution. Three water molecules considerably contributed to the high Sc parameter in the antibody-hapten complex of 34E4 31.
AHL lactam analog recognition in antibody RS2-1G9
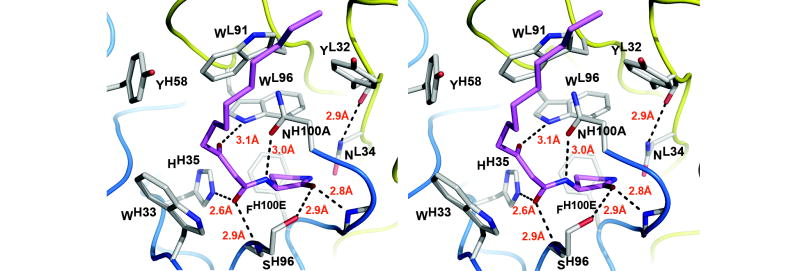
Ligand recognition in mAb RS2-1G9 is realized by a combination of 85 van der Waals’ contacts and six hydrogen bond interactions with the functional groups of the lactam analog (Figure 5) 33. Specifically, the aromatic side chains of TyrL32, TrpL91, TrpL96, TrpH33, TyrH58, and PheH100E snugly nestle around the ligand and provide 47% of the buried surface area of the Fab binding pocket. With 33Å2 each, TrpL91 and TrpL33 contribute most to this surface area. Interestingly, TrpL91 is the hallmark residue of CDR L3 in λ chains and its central role in ligand recognition observed here further underlines the common gene usage of λ chains in antibodies generated against various small molecules. For instance, the indole ring of TrpL91 is buttressed against the ligands in the complex structures of the anti-benzimidazolium antibody 34E4 31, the anti-nitrophenyl antibodies SPE7 34, N1G9 35, 88C6/12 20, the anti-carbohydrate antibody Se155-4 36, and the anti-indium(III)-benzyl-EDTA antibody CHA255 37, to cite just a few examples. Furthermore, in most of these antibodies, as well as in RS2-1G9 (Figure 5), the conserved TyrL32 and TrpL96 in the immediate vicinity of TrpL91 further complement its ligand interactions.
Figure 5.
Antibody combining site of RS2-1G9 (stereoview). Hydrogen bonds are shown as broken lines. Only Fab side chains that contact the lactam ligand (pink) are displayed. The hapten analog satisfies all its functional groups, except for the amide group of the lactam, which is crucial for the observed cross-reactivity of this antibody with an N-acyl homoserine lactone. A plethora of aromatic side chains surrounds the ligand. The residues between LeuH97 and AsnH100 of CDR H3 are omitted for clarity.
The engulfment of the ligand is structurally accomplished by a 90° bend of the tip of the CDR H3 loop over the ligand (Figure 3). Surprisingly, CDR H3 contributes more ligand contacts via main-chain (77) than via side-chain atoms (69), which is quite unusual for hapten recognition. The only other main-chain contacts are provided by SerL93 (6) in CDR L3. Of the CDR H3 residues, PheH100E (16) at the base of the binding pocket and AsnH100A (31) at the tip of CDR H3 play major roles in ligand binding. In addition, the side-chain carbonyl of the AsnH100A specifically interacts with the ligand by engaging in a hydrogen bond with the exocyclic amide (3.0 Å). The adjacent carbonyl group of the acyl tail undergoes simultaneous hydrogen bonding interactions with the backbone amide of SerH96 (2.9 Å) and Nε2 of HisH35 (2.6 Å). The 3-oxo group of the acyl chain hydrogen bonds with NHε1 of TrpL96 (3.1 Å). Lastly, the carbonyl oxygen of the lactam ring makes hydrogen bonds to the backbone amide of Gly100C (2.8 Å) and the hydroxyl of SerH96 (2.9 Å). Most of these hydrogen bonds display optimal geometry, as assessed by the program HBPLUS 38, thereby mediating strong specific interactions and providing convincing evidence for correct orientation of the ligand within the antibody-hapten complex despite the modest resolution of the data.
Structural basis for discrimination of RS2-1G9 against C4-AHL
The mAb RS2-1G9 shows high affinity for 3-oxo-C12-AHL (2), while the other quorum sensing molecule of P. aeruginosa, C4-AHL (1), is recognized with 1000-fold lower affinity 12. Based on the crystal structure, the missing 3-oxo group of AHL 1 would lead to the loss of a strong hydrogen bond interaction with NHε1 of TrpL96. Furthermore, the lack of eight methylene units in the acyl chain of C4-AHL would abrogate 11 van der Waals’ contacts that are observed in the 3-oxo-C12-lactam complex structure. Therefore, the combination of increased van der Waals’ contacts and the additional hydrogen bond appears to be responsible for the high specificity of RS2-1G9 for 3-oxo-C12-AHL vs. C4-AHL.
3-oxo-C12-AHL recognition: structural basis for cross-reactivity
Analysis of the RS2-1G9 ligand interactions reveals that the amide group of the lactam ring is the only functional group without satisfied hydrogen bonding potential and suggests that the chemical nature of this position is less critical for ligand recognition. The corresponding lactone featuring an oxygen atom at this location can easily be accommodated in place of the lactam without any detrimental effect on high-affinity binding, thus providing a structural basis for its cross-reactivity with lactone 2. Strikingly, the 3-oxo-C12-AHL 2 exhibits about twofold higher affinity for RS2-1G9 than the corresponding lactam 3 12. Detailed analysis of this part of the binding pocket can offer a plausible explanation for this binding behavior. The side-chain oxygen and nitrogen of AsnL34 are both in hydrogen-bonding distance to the lactam NH-group (2.9 Å and 3.3 Å, respectively). However, the lactam is unable to form a hydrogen bond with the AsnL34 carbonyl due to unfavorable geometry (Figures 2 and 5), as assessed by the program HBPLUS 38. By contrast, if the lactam NH group is substituted with O, the corresponding analysis of this lactone-complex model does, indeed, reveal possible formation of a hydrogen bond between the lactone oxygen and the NH2–group of AsnL34, albeit with suboptimal geometry. As this interpretation critically depends on the proper orientation of the AsnL34 carboxamide, the final RS2-1G9 structure was submitted to an all-atom contact analysis performed by the program MolProbity 39 with the terminal carboxamide group of AsnL34 in one of the two possible orientations and flipped by 180° in the other calculation. The analysis provided clear evidence for the rotamer displayed in Figure 2, which is favored by formation of a hydrogen bond of its NH2-group with the backbone carbonyl of TyrH32 (2.9 Å)(Figures 2 and 5). In summary, the microenvironment at the very bottom of the binding pocket tolerates high-affinity binding of both the lactam and the lactone without much discrimination; slightly improved hydrogen-bonding potential in the antibody-lactone complex may account for its increased affinity with respect to the lactam.
CDR H3 has uniquely evolved for high-affinity recognition of its immunogen
The successful selection of only a small number of RS2-antibodies with affinities in the submicromolar range from 68 mAbs illustrates the difficulties in generating high-affinity antibodies against small lipophilic structures 12. The crystal structure of one representative of these, RS2-1G9, in complex with its hapten has revealed that the attributes of high shape complementarity, deep burial, hydrogen bonding to all but one functional group of the ligand, and numerous van der Waals’ contacts can explain the high affinity of RS2-1G9 towards specific N-acyl homoserine lactones and lactam analogs. In particular, hapten recognition is primarily achieved by simultaneously optimizing shape complementarity and hydrogen bonding of CDR H3. In fact, this hypervariable loop has been found to dominate immune recognition in most antibody-hapten complexes and to be the most flexible and diverse CDR loop 24; 40; 41. Although the conformation of CDR H3 is somewhat correlated with length, the backbone conformation of CDR H3 is unique to RS2-1G9 and does not superimpose with those of other antibodies containing the same CDR H3 length , such as the anti-digoxin antibody 40-50, the anti-phospholipase Cδ1 antibody L5MK16 42, or the anti-neuraminidase antibody NC10 43. With five insertions after residue AsnH100, antibody RS2-1G9 has a relatively long CDR H3 loop for mouse antibodies. On average, the length of CDR H3 in murine anti-hapten antibodies is only 8.5 residues, while RS2-1G9 consists of 13 amino acids 40. Again, the crystal structure has illuminated the structural basis for the origin of the relatively long CDR H3 loop, as it forms a flap at its tip in order to make maximal interactions with the lactam ligand.
Crystal structure of RS2-1G9 in complex with ethylene glycol
The constriction of the antibody binding pocket harboring part of the acyl chain is too narrow to allow facile entrance or exit of the ligand head group. As a consequence, structural rearrangements of the antibody combining site must occur upon ligand binding and release. In order to experimentally address this issue, we crystallized RS2-1G9 in absence of its cognate lactam or lactone ligands. Notably, these crystals were indexed in a different space group than those of the lactam complex (Table 1), possibly indicating structural changes. However, the antibody in the crystal was not in its truly unliganded form, as ethylene glycol, which most likely originated from the cyroprotection buffer containing 25% ethylene glycol, occupied the region of the binding pocket that harbors the lactam moiety in the complex structure (Figure 6). This Fab structure was determined to 2.85 Å resolution and refined to an Rcryst of 20.0% and an Rfree of 26.3% (Table 1). Although the resolution of the ethylene glycol-bound RS2-1G9 structure is better than that of the lactam complex (3.18 Å), the framework regions of the variable part, as defined by Kabat and Wu 44, as well as the constant domains are essentially identical to its lactam complex with an rmsd of 0.6 Å for the Cα atoms, which validates the conclusions from the lower resolution structure of the RS2-1G9 lactam complex.
Figure 6.
Ethylene glycol acts as a surrogate ligand in the “unliganded” antibody RS2-1G9 (lactam complex in grey, ethylene glycol complex in green, stereoview). This solvent molecule (yellow) substitutes for the lactam ring of the bound hapten derivative (pink) and fills the bottom of the ligand cavity. Strikingly, the bound ethylene glycol adopts an unfavorable, nearly eclipsed conformation that faithfully mimics part of the lactam ring. Ethylene glycol was added for cryoprotection of the crystals prior to data collection at cryogenic temperatures. This view also illustrates how CDRs H3 and L3 rearrange to close the N-acyl harboring tunnel of the binding site.
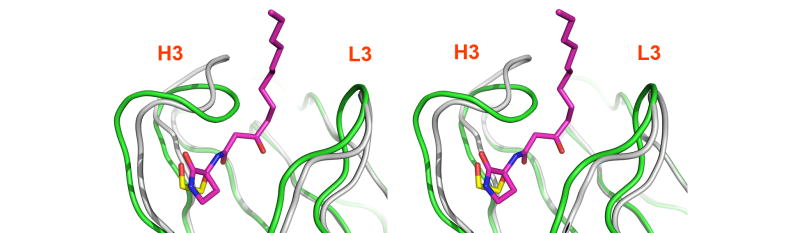
Antibody RS2-1G9 binds its cognate ligands via an induced fit mechanism
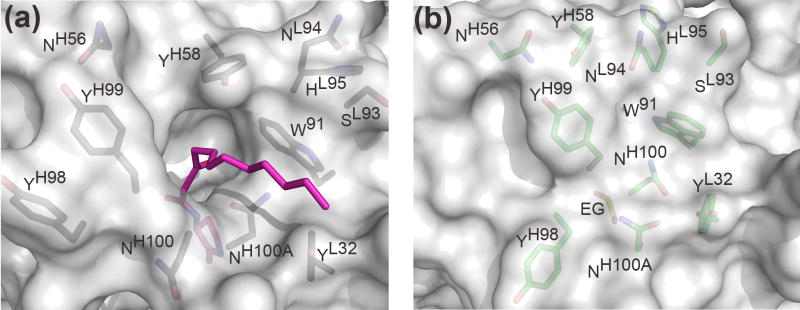
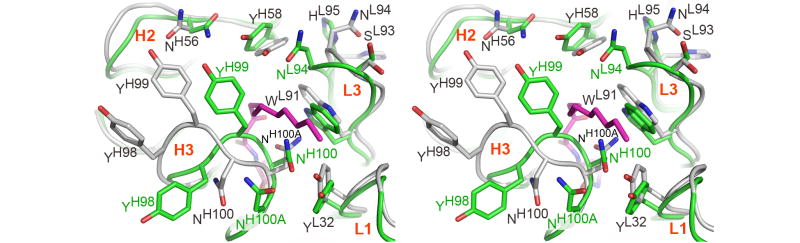
In contrast to the framework regions, the architecture of the RS2-1G9 combining site has significantly changed in absence of its bound lactam derivative. Importantly, the main chain, as well as side-chains, of the CDR loops have clear and distinctly different electron densities in both complexes. While the complementarity determining region of the lactam complex structure features a deep binding cavity, this region has transformed into a relatively flat surface in the ethylene glycol-bound form (Figure 7). The structural changes in CDRs H3 and L3 are primarily responsible for this remodeling. Instead of rigid-body movements of the H3 and L3 tips, individual residues undergo disparate structural changes that collectively lead to closure of the tunnel harboring the N-acyl chain in the hapten derivative complex (Figures 6 and 8). In quantitative terms, H3 undergoes the largest rearrangement (rmsd of 1.6Å for its Cα atoms), followed by L3 (0.9Å) (Figure 8). By contrast L1, L2, H2, and H1 do not undergo substantial movements: 0.7Å, 0.6Å, 0.5Å, and 0.3Å, respectively. The structural repositioning of the H3 loop is accompanied by even more pronounced reorientations of its side-chains (Figure 8). AsnH100A is displaced from the center to the edge of the antibody combining site in the ethylene glycol-bound structure. Conversely, AsnH100 and TyrH99 move towards the center and seal off the binding pocket via their side chains. By packing against the indole ring of TrpL91 and the phenol ring of TyrL32, AsnH100 partly covers their hydrophobic surfaces. Likewise, TyrH99 largely restricts solvent accessibility to TrpH33 and TyrH58. Finally, AsnL94 of L3 wedges between TyrH58 and TrpL91 and completes the formation of the flat molecular surface of the RS2-1G9 combining site in the “unliganded” state. On average, the side-chains atoms of TyrH98, TyrH99, AsnH100, AsnH100A, and AsnL94 (labeled green in Figure 8) have an rmsd of 5.6 Å in the two structures, highlighting the large structural plasticity in the antigen binding site of RS2-1G9.
Figure 7.
Comparison of the molecular surface representation of (a) the lactam complex and (b) the ethylene glycol complex of antibody RS2-1G9 reveals profound differences in the architecture of the antibody combining site. The lactam and the buried ethylene glycol (EG) are colored in pink and yellow, respectively.
Figure 8.
Induced fit in RS2-1G9 (lactam complex in grey, ethylene glycol complex in green, stereoview). Upon ligand binding, the main-chain and side-chain atoms of CDR H3 undergo the largest rearrangements, while L3, L1, and H2 move to a minor extent. L2 and H1 are essentially not in contact to the hapten derivative and, hence, are not displayed. In particular, the tip of H3 completely reorganizes upon ligand binding to accommodate the lactam in the binding pocket (induced fit). For clarity, TyrH98, TyrH99, AsnH100, and AsnH100A are labeled in both structures.
Ethylene glycol serves as a surrogate ligand
Although the antibody combining site reveals considerable structural rearrangements at the tips of the CDR loops, the buried chamber that is occupied by the lactam moiety in the RS2-1G9-3 complex (Figure 4) is not subject to structural changes, but harbors ethylene glycol (Figure 6). Strikingly, ethylene glycol adopts an energetically unfavorable eclipsed conformation, thus somewhat mimicking the five-membered lactam ring (Figure 6). Precedence for antigen binding pockets that are occupied by molecules of the crystallization buffer in absence of their cognate haptens has been documented previously 27; 32. Moreover, closure of the ligand binding pocket in RS2-1G9 by antibody-derived residues that substitute for high-affinity ligands has also been observed in other anti-hapten antibodies 45; 46; 47; 48; 49. In the anti-progesterone antibody DB3, for instance, a tryptophan side chain partially occupies the steroid binding cavity in the free antibody and acts as an antibody-derived surrogate ligand to “solvate” an otherwise hydrophobic site in the absence of antigen 45; 46. Thus, partial closure of the highly-hydrophobic pockets in DB3 and RS2-1G9 appears to be thermodynamically driven, since the structural alteration reduces the percentage of non-polar residues exposed to the aqueous environment in the unliganded state.
Structure-based protein engineering of the quorum-quenching antibody RS2-1G9
The crystal structures of the quorum-quenching antibody RS2-1G9 provide an excellent starting point for protein engineering in order to increase the therapeutic potential of this antibody in its interference with quorum sensing of the human pathogen P. aeruginosa. In particular, site-directed mutagenesis is a powerful tool to fine-tune and to improve interactions of the antibody with AHL. Based on their vicinity to the AHL lactam analog, the following mutations are likely to influence ligand-antibody interactions in RS2-1G9: HisH35→Gln, TyrH58→Phe/Trp, AsnH100A→His/Gln, PheH100E→His/Trp, and AsnL34→Asp/Glu/His/Lys/Gln. Since these mutations are predominantly conservative, improved packing or hydrogen bonding to 3-oxo-C12-AHL may result from these substitutions. Moreover, the mutation of AsnL34 might introduce lactonase activity into the antibody due its juxtaposition to the ring carbonyl carbon, which is hydrolytically attacked in naturally-occurring quorum-quenching enzymes 50; 51. The introduction of catalytic activity would greatly enhance the potency of antibody RS2-1G9, since a single antibody molecule would degrade many AHLs, thus requiring significantly lower titers of antibody for effective treatment in vivo 52; 53. Importantly, mutagenesis may alternatively result in a covalent binding event between the RS2-1G9 mutants and 3-oxo-C12-AHL rather than catalysis, as it is conceivable that a lactone bond breaking event might take place without the subsequent replacement with a water molecule and release of the hydrolyzed 3-oxo-C12-AHL. Previously, such covalent catalysis has been achieved in catalytic aldolase antibodies that feature enzyme-like reaction rates and broad substrate specificity 54; 55. Finally, biochemical characterization of the mutations will complement our crystallographic characterization of ligand recognition by RS2-1G9 and corroborate key residues in the interaction between the antibody and AHL.
Conclusions
The crystal structure of antibody RS2-1G9 in complex with an AHL analog clearly illustrates how the immune system has evolved to potently bind and discriminate a low molecular weight lipophilic compound with a small head group, such as the AHL lactam analog 3, with nanomolar affinity. The head group is deeply buried within the protein and specifically recognized by hydrogen bonds to the functional groups. The tunnel towards the protein surface as well as the potential dynamics of the CDR H3 loop enable mAb RS2-1G9 to reversibly bind to its ligands. Evidence for substantial structural plasticity in the RS2-1G9 combining site was gleaned from the crystal structure of the free Fab in the absence of any cognate ligands. Although the antibody already achieves great shape and electrostatic complementarity towards the AHL mimic, further optimization of antibody-ligand affinity or introduction of lactonase activity by site-directed mutagenesis may represent promising routes to enhanced therapeutic potential of the quorum-quenching antibody RS2-1G9. Improved hapten design and the synthesis of new haptens specifically designed to emulate AHLs of bacteria other than P. aeruginosa may yield additional more potent antibodies with the desired properties of quorum quenching.
Materials and Methods
Fab preparation, crystallization, and structure determination of Fab RS2-1G9 in complex with an AHL lactam analog and with ethylene glycol
The Fab fragment was produced from murine mAb RS2-1G9 (IgG1, λ) by pepsin digest for 4 hours using standard protocols 56. Crystallization experiments were performed by the sitting drop vapor diffusion method at 22.5°C. The Fab, concentrated to 15mg/ml in 0.1M sodium acetate pH 5.5, was crystallized after several weeks in presence of 4-fold molar excess of lactam 3 from 1.0M Na/K tartrate, 0.2M NaCl, and 0.1M imidazole buffer pH 8.0. The ethylene-glycol bound RS2-1G9 (“unliganded”) was crystallized from 10% PEG 8000, and 0.1 M imidazole pH 6.5. For data collection, the crystals were flash cooled to 100K using 25% glycerol (lactam complex) and 25% ethylene glycol (ethylene glycol complex) as a cryoprotectant, respectively. Lactam-complexed Fab and “unliganded” Fab data were collected at synchrotron beamlines APS 23-ID-D and SSRL 11-1, respectively, and processed and scaled with HKL2000 57 (Table 1). The structure of the RS2-1G9-3 complex was determined by molecular replacement using the program Phaser 58 and the coordinates of scFv B1-8 (PDB ID code 1A6V) for the variable domain and Se155-4 (1MFB) for the constant domain. The structure of RS2-1G9 in complex with ethylene glycol was determined by molecular replacement using the same program and the RS2-1G9 coordinates of the lactam complex. The models were refined by alternating cycles of model building with the program O 59 and refinement with Refmac5 60. Given the modest resolution of 3.18 Å and 2.85 Å, tight non-crystallographic symmetry restraints were applied. The final statistics are shown in Table 1. The quality of the structures was analyzed using the programs MolProbity 39, WHAT IF 61, and PROCHECK 62. All figures were prepared with PyMol 63. The coordinates and the structure factors are deposited at the PDB under accession code 2NTF (RS2-1G9-3 complex) and 2OP4 (RS2-1G9 ethylene glycol complex), respectively.
Acknowledgments
We are grateful to S. Ferguson for help with Fab preparation, the APS staff at beamline 23-ID-D and the SSRL staff at beamline 11-1 for their assistance, R. Stanfield, X. Dai, J. Stevens, and D. Shore for help with data collection, R. Sartorio, S. Lee, and B. Clapham for synthesis of compound 3, and the TSRI Antibody Production Core Facility. This research was supported by NIH grants GM38273 (IAW) and AI055781 (KDJ), a Skaggs predoctoral fellowship and a Jairo H. Arévalo fellowship from the TSRI graduate program (EWD), and The Skaggs Institute for Chemical Biology. This is publication 18564-MB from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 2.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6:56–60. doi: 10.1016/s1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 5.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, Pan Q, Fearns C, Knaus UG, Meijler MM, Janda KD, Ulevitch RJ. N-(3-oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J Biol Chem. 2006;281:28822–28830. doi: 10.1074/jbc.M606613200. [DOI] [PubMed] [Google Scholar]
- 6.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geske GD, Wezeman RJ, Siegel AP, Blackwell HE. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J Am Chem Soc. 2005;127:12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]
- 8.Smith KM, Bu Y, Suga H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem Biol. 2003;10:81–89. doi: 10.1016/s1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 9.Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 10.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. Embo J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006;296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann GF, Sartorio R, Lee SH, Mee JM, Altobell LJ, 3rd, Kujawa DP, Jeffries E, Clapham B, Meijler MM, Janda KD. Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. J Am Chem Soc. 2006;128:2802–2803. doi: 10.1021/ja0578698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyairi S, Tateda K, Fuse ET, Ueda C, Saito H, Takabatake T, Ishii Y, Horikawa M, Ishiguro M, Standiford TJ, Yamaguchi K. Immunization with 3-oxododecanoyl-L-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol. 2006;55:1381–1387. doi: 10.1099/jmm.0.46658-0. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci USA. 2005;102:309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janda KD, Weinhouse MI, Schloeder DM, Lerner RA, Benkovic SJ. Bait and switch strategy for obtaining catalytic antibodies with acyl-transfer capabilities. J Am Chem Soc. 1990;112:1274–1275. [Google Scholar]
- 16.Xu Y, Yamamoto N, Janda KD. Catalytic antibodies: hapten design strategies and screening methods. Bioorg Med Chem. 2004;12:5247–5268. doi: 10.1016/j.bmc.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 17.Boullerne A, Petry KG, Geffard M. Circulating antibodies directed against conjugated fatty acids in sera of patients with multiple sclerosis. J Neuroimmunol. 1996;65:75–81. doi: 10.1016/0165-5728(96)00010-0. [DOI] [PubMed] [Google Scholar]
- 18.Kleywegt GJ, Brunger AT. Checking your imagination: applications of the free R value. Structure. 1996;4:897–904. doi: 10.1016/s0969-2126(96)00097-4. [DOI] [PubMed] [Google Scholar]
- 19.Al-Lazikani B, Lesk AM, Chothia C. Standard conformations for the canonical structures of immunoglobulins. J Mol Biol. 1997;273:927–948. doi: 10.1006/jmbi.1997.1354. [DOI] [PubMed] [Google Scholar]
- 20.Yuhasz SC, Parry C, Strand M, Amzel LM. Structural analysis of affinity maturation: the three-dimensional structures of complexes of an anti-nitrophenol antibody. Mol Immunol. 1995;32:1143–1155. doi: 10.1016/0161-5890(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 21.Sheriff S, Jeffrey PD, Bajorath J. Comparison of CH1 domains in different classes of murine antibodies. J Mol Biol. 1996;263:385–389. doi: 10.1006/jmbi.1996.0582. [DOI] [PubMed] [Google Scholar]
- 22.Davies DR, Chacko S. Antibody structure. Acc Chem Res. 1993;26:421–427. [Google Scholar]
- 23.Stanfield RL, Zemla A, Wilson IA, Rupp B. Antibody elbow angles are influenced by their light chain class. J Mol Biol. 2006;357:1566–1574. doi: 10.1016/j.jmb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IA, Stanfield RL. Antibody-antigen interactions. Curr Opin Struct Biol. 1993;3:113–118. doi: 10.1016/0959-440x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 25.Connolly ML. Analytical molecular surface calculation. J Appl Cryst. 1983;16:548–558. [Google Scholar]
- 26.MacCallum RM, Martin AC, Thornton JM. Antibody-antigen interactions: contact analysis and binding site topography. J Mol Biol. 1996;262:732–745. doi: 10.1006/jmbi.1996.0548. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Heine A, Monnat F, Houk KN, Janda KD, Wilson IA. Structural basis for antibody catalysis of a cationic cyclization reaction. J Mol Biol. 2003;329:69–83. doi: 10.1016/s0022-2836(03)00406-6. [DOI] [PubMed] [Google Scholar]
- 28.Haynes MR, Stura EA, Hilvert D, Wilson IA. Routes to catalysis: structure of a catalytic antibody and comparison with its natural counterpart. Science. 1994;263:646–652. doi: 10.1126/science.8303271. [DOI] [PubMed] [Google Scholar]
- 29.Heine A, Stura EA, Yli-Kauhaluoma JT, Gao C, Deng Q, Beno BR, Houk KN, Janda KD, Wilson IA. An antibody exo Diels-Alderase inhibitor complex at 1. 95 angstrom resolution. Science. 1998;279:1934–1940. doi: 10.1126/science.279.5358.1934. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 31.Debler EW, Ito S, Seebeck FP, Heine A, Hilvert D, Wilson IA. Structural origins of efficient proton abstraction from carbon by a catalytic antibody. Proc Natl Acad Sci USA. 2005;102:4984–4989. doi: 10.1073/pnas.0409207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu X, Dickerson TJ, Rogers CJ, Kaufmann GF, Mee JM, McKenzie KM, Janda KD, Wilson IA. Complete reaction cycle of a cocaine catalytic antibody at atomic resolution. Structure. 2006;14:205–216. doi: 10.1016/j.str.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Sheriff S, Hendrickson WA, Smith JL. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987;197:273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- 34.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 35.Mizutani R, Miura K, Nakayama T, Shimada I, Arata Y, Satow Y. Three-dimensional structures of the Fab fragment of murine N1G9 antibody from the primary immune response and of its complex with (4-hydroxy-3-nitrophenyl)acetate. J Mol Biol. 1995;254:208–222. doi: 10.1006/jmbi.1995.0612. [DOI] [PubMed] [Google Scholar]
- 36.Cygler M, Rose DR, Bundle DR. Recognition of a cell-surface oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science. 1991;253:442–445. doi: 10.1126/science.1713710. [DOI] [PubMed] [Google Scholar]
- 37.Love RA, Villafranca JE, Aust RM, Nakamura KK, Jue RA, Major JGJ, Radhakrishnan R, Butler WF. How the anti-(metal chelate) antibody CHA255 is specific for the metal ion of its antigen: X-ray structures for two Fab’/hapten complexes with different metals in the chelate. Biochemistry. 1993;32:10950–10959. doi: 10.1021/bi00092a004. [DOI] [PubMed] [Google Scholar]
- 38.McDonald IK, Thornton JM. Satisfying hydrogen bonding potential in proteins. J Mol Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- 39.Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 40.Collis AV, Brouwer AP, Martin AC. Analysis of the antigen combining site: correlations between length and sequence composition of the hypervariable loops and the nature of the antigen. J Mol Biol. 2003;325:337–354. doi: 10.1016/s0022-2836(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 41.Morea V, Tramontano A, Rustici M, Chothia C, Lesk AM. Conformations of the third hypervariable region in the VH domain of immunoglobulins. J Mol Biol. 1998;275:269–294. doi: 10.1006/jmbi.1997.1442. [DOI] [PubMed] [Google Scholar]
- 42.Perisic O, Webb PA, Holliger P, Winter G, Williams RL. Crystal structure of a diabody, a bivalent antibody fragment. Structure. 1994;2:1217–1226. doi: 10.1016/s0969-2126(94)00123-5. [DOI] [PubMed] [Google Scholar]
- 43.Malby RL, Tulip WR, Harley VR, McKimm-Breschkin JL, Laver WG, Webster RG, Colman PM. The structure of a complex between the NC 10 antibody and influenza virus neuraminidase and comparison with the overlapping binding site of the NC41 antibody. Structure. 1994;2:733–746. doi: 10.1016/s0969-2126(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 44.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. 5. National Institutes of Health; Bethesda: 1991. [Google Scholar]
- 45.Arévalo JH, Hassig CA, Stura EA, Sims MJ, Taussig MJ, Wilson IA. Structural analysis of antibody specificity. Detailed comparison of five Fab’-steroid complexes. J Mol Biol. 1994;241:663–690. doi: 10.1006/jmbi.1994.1543. [DOI] [PubMed] [Google Scholar]
- 46.Arévalo JH, Stura EA, Taussig MJ, Wilson IA. Three-dimensional structure of an anti-steroid Fab’ and progesterone-Fab’ complex. J Mol Biol. 1993;231:103–118. doi: 10.1006/jmbi.1993.1260. [DOI] [PubMed] [Google Scholar]
- 47.Gruber K, Zhou B, Houk KN, Lerner RA, Shevlin CG, Wilson IA. Structural basis for antibody catalysis of a disfavored ring closure reaction. Biochemistry. 1999;38:7062–7074. doi: 10.1021/bi990210s. [DOI] [PubMed] [Google Scholar]
- 48.Charbonnier J-B, Carpenter E, Gigant B, Golinelli-Pimpaneau B, Eshhar Z, Green BS, Knossow M. Crystal structure of the complex of a catalytic antibody Fab fragment with a transition state analog: structural similarities in esterase-like catalytic antibodies. Proc Natl Acad Sci USA. 1995;92:11721–11725. doi: 10.1073/pnas.92.25.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golinelli-Pimpaneau B, Gigant B, Bizebard T, Navaza J, Saludjian P, Zemel R, Tawfik DS, Eshhar Z, Green BS, Knossow M. Crystal structure of a catalytic antibody Fab with esterase-like activity. Structure. 1994;2:175–183. doi: 10.1016/s0969-2126(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Lepore BW, Petsko GA, Thomas PW, Stone EM, Fast W, Ringe D. Three-dimensional structure of the quorum-quenching N-acyl homoserine lactone hydrolase from Bacillus thuringiensis. Proc Natl Acad Sci USA. 2005;102:11882–11887. doi: 10.1073/pnas.0505255102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim MH, Choi WC, Kang HO, Lee JS, Kang BS, Kim KJ, Derewenda ZS, Oh TK, Lee CH, Lee JK. The molecular structure and catalytic mechanism of a quorum-quenching N-acyl-L-homoserine lactone hydrolase. Proc Natl Acad Sci USA. 2005;102:17606–17611. doi: 10.1073/pnas.0504996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsushita M, Hoffman TZ, Ashley JA, Zhou B, Wirsching P, Janda KD. Cocaine catalytic antibodies: the primary importance of linker effects. Bioorg Med Chem Lett. 2001;11:87–90. doi: 10.1016/s0960-894x(00)00659-4. [DOI] [PubMed] [Google Scholar]
- 53.Deng SX, de Prada P, Landry DW. Anticocaine catalytic antibodies. J Immunol Methods. 2002;269:299–310. doi: 10.1016/s0022-1759(02)00237-5. [DOI] [PubMed] [Google Scholar]
- 54.Barbas CF, 3rd, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjornestedt R, List B, Anderson J, Stura EA, Wilson IA, Lerner RA. Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 55.Wagner J, Lerner RA, Barbas CF., 3rd Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 56.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. [Google Scholar]
- 57.Otwinowski Z, Minor W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 58.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr D. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 59.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 60.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 61.Vriend G. WHAT IF: a molecular modeling and drug design program. J Mol Graph. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- 62.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 63.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; San Carlos, CA, USA: 2002. [Google Scholar]
- 64.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]