Abstract
Aim
To evaluate the effects of unilateral compressive optic neuropathy on amplitude and latency of multifocal visual evoked potentials (mfVEPs).
Methods
Static automated perimetry and mfVEP recordings were obtained from six patients with presumed meningiomas affecting one optic nerve. Monocular and interocular amplitude and latency analyses were performed and compared with normal control subjects.
Results
The change in the mfVEP amplitude agreed with visual field findings with regard to topography and severity of deviation from normal. The delay in recordable responses from affected eyes ranged from 7.6 to 20.7 ms (interocular analysis) and 7.9 to 13.9 ms (monocular analysis).
Conclusions
Compressive optic neuropathy decreases the amplitude and increases the latency of the mfVEP. The changes in latency were similar to those seen in optic neuritis but larger than those in ischaemic optic neuropathy and glaucoma.
Proper clinical diagnosis and accurate means for follow‐up of patients with compressive optic neuropathy, especially for optic nerve meningiomas, have become more important than ever because of the improvements in treatment. Improved surgical and radiation approaches, which require therapeutic decisions that are based on the most‐reliable available clinical data, are now available. However, the current standard of care in this area is limited to subjective tests of vision. Objective measurements of visual function would be ideal, and multifocal visual evoked potential (mfVEP) recording may be able to suit this need. mfVEP testing is beginning to show promise in the management of patients with glaucoma,1,2,3 and interesting findings are becoming available from the study of patients with ischaemic optic neuropathy and optic neuritis.4,5 However, to date, little information is available regarding the effect of compressive lesions on the mfVEP. This study was designed to evaluate mfVEP findings in patients with compressive lesions. We were interested in the effects of compression on both the amplitude and latency of the mfVEP.
Patients
Six patients with unilateral compressive optic neuropathy were selected from the neuro‐ophthalmology practices of the New England Eye Center, Boston, Massachusetts, USA, and the Harkness Eye Institute, New York, New York, USA. Patients were included for analysis if they had strictly unilateral compressive lesions, diagnosed radiologically as meningiomas. None of the patients had received previous treatment. All patients had visual acuities in the affected eye of better than 20/100 allowing for stable fixation during both visual‐field and mfVEP testing. Table 1 summarises the relevant clinical information from the patients.
Table 1 Clinical information from the patients.
| Case | Age (years)/sex | Affected eye | VA | MD of HVF |
|---|---|---|---|---|
| 1 | 48/F | OS | 20/15 | –6.5 |
| 20/80 | ||||
| 2 | 63/F | OD | 20/60 | –9.4 |
| 20/25 | ||||
| 3 | 46/F | OS | 20/20 | –13.2 |
| 20/40 | ||||
| 4 | 49/F | OS | 20/20 | –14.1 |
| 20/50 | ||||
| 5 | 79/F | OS | 20/60 | –18.8 |
| 20/40 | ||||
| 6 | 47/M | OD | 20/60 | –23.6 |
| 20/20 |
F, female; HVF, Humphrey visual field; M, male, MD, mean deviation; OD, right eye;
OS, left eye; VA, visual acuity.
Methods
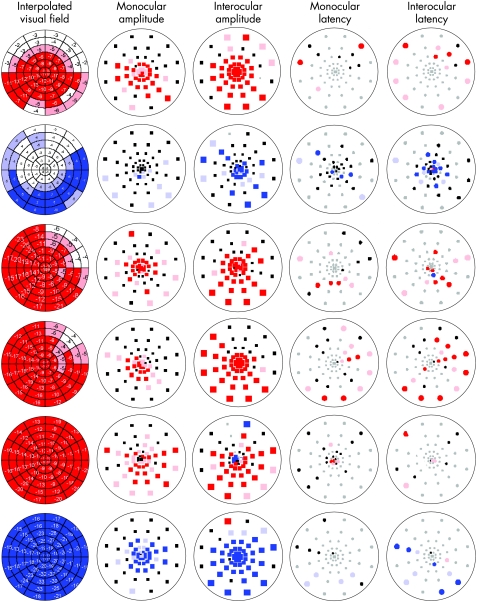
Static automated perimetry (SAP) was performed using Humphrey Visual Field Program 24–2 or 30–2 (Humphrey system, Dublin California, USA). To allow a comparison of the visual‐field sensitivity to the mfVEP responses, estimates of sensitivity for each sector of the multifocal stimulus were obtained from the visual field values (total deviation). These estimates were obtained by computer (MATLAB software; The Mathworks, Natick, Massachusetts, USA).3,5 The resulting interpolated visual‐field plots were colour coded by comparing the deviation in each segment to a group of 100 control patients. The saturated colour, red for left eye and blue for right eye, indicates a deviation from normal with a p value <0.01, and the desaturated colour indicates a deviation from normal with a p value <0.05 (fig 1).
Figure 1 Multifocal visual evoked potential (mfVEP) data. Interpolated visual fields: estimated visual loss in decibels in 60 mfVEP sectors. Monocular and interocular amplitude plots: black squares indicate that there was no significant difference between the amplitude of the affected eye and normal eyes (monocular) or between the two patient's eyes (interocular), the coloured squares (right eye, blue and left eye, red) indicate that there were significant differences between the affected eye and normal eyes (monocular) or between the patient's eyes (interocular) either at p<0.5 (desaturated colour ) or p<0.1 (saturated colour) level. Grey squares indicate a signal‐to‐noise ratio <1.7. Monocular and interocular latency plots: black ovals indicate that there were no significant differences between the latency of the affected eye and normal eyes (monocular) or between the two patient's eyes (interocular), the coloured squares (right eye, blue and left eye, red) indicate that there were significant differences between the latency of the affected eye and normal eyes (monocular) or between the patient's eyes (interocular) either at p<0.5 (desaturated colour ) or p<0.1 (saturated colour) level. Grey ovals indicate a logarithm signal‐to‐noise ratio <0.23.
MfVEP recordings were obtained using VERIS software (Electro‐Diagnostic Imaging, San Mateo, California, USA). The stimulus was a scaled dartboard with a diameter of 44.5°, contained 60 sectors, each with 16 checks alternating, 8 white and 8 black. The sectors were cortically scaled with eccentricity to stimulate approximately equal areas of the visual cortex.6 The dartboard pattern reversed according to a pseudorandom m‐sequence at a frame rate of 75 Hz.7 Three recording channels were connected to gold cup electrodes. For the midline channel, electrodes were placed 4 cm above the inion (active), at the inion (reference) and on the earlobe (ground). For the other two active channels, the same ground and reference electrodes were used but the active electrode was placed 1 cm up and 4 cm lateral to the inion on either side. By subtracting different combinations of pairs of channels, three additional “derived” channels were obtained resulting effectively in six channels representing the six possible pairs of the four electrodes. The channel providing the best recording for each sector was selected during the analysis as “best channel response”.3,8
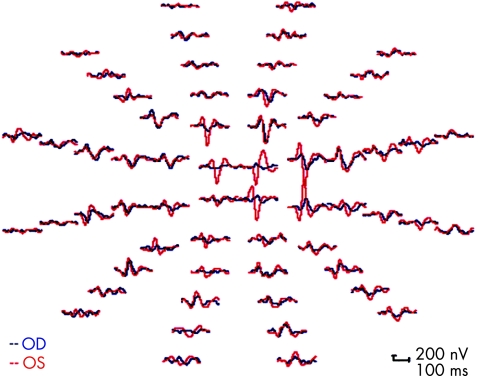
Each patient was tested twice in the same sitting or twice within 11 weeks, so that the results could be combined for analysis. The second‐order kernel best channel responses were extracted using VERIS 4.9.1 software (Electro‐Diagnostic Imaging). The analyses were done with programs written in MATLAB.5 Figure 2 shows sample records from one of the patients. All patients read and signed an informed consent approved by our institutional review board.
Figure 2 Multifocal visual evoked potential responses obtained from the left (OS, red) and right (OD, blue) eyes of case 2. The responses from both eyes in this patient were larger than those seen in the other cases.
Amplitudes of the responses were calculated by obtaining root mean square (RMS) of the amplitude for each mfVEP response over time intervals from 45 to 150 ms.3,4,8 Signal‐to‐noise ratios were calculated for each response by dividing the RMS of the signal window by the average of the 60 RMS values of the noise‐only window.8,9 Each of these values was compared to values for normal control subjects.10 The results are displayed as a monocular probability plot with coloured squares showing a difference between the patient and the control responses that was significant at p <0.01 (saturated colour) or at a p value <0.05 (desaturated colour). Black squares indicate no significant difference (fig 1). Interocular amplitude differences for each patient were also calculated by taking the logarithm of the interocular ratio at each location.11 Interocular probability plots were generated using a similar colour‐coding scheme with saturated red squares (left eye), saturated blue squares (right eye) depicting a difference with p <0.01 and desaturated colours, p <0.05.
Monocular and interocular latencies were measured as the temporal shift producing the best cross correlation value between the corresponding responses of the patient's eye and a template based on control eyes (monocular analysis)12 or between the corresponding responses from the two eyes (interocular analysis)13 using the cross‐correlation function in MATLAB. In particular, the best cross correlation was obtained by shifting the right eye's response in the window from 5 to 215 ms against the left eye's response from the same window. The shift necessary for the best cross correlation was taken as the interocular latency.13 The latency probability plots are colour coded in a manner similar to the amplitude plots with circles (saturated colour indicating increased latency with p <0.01, desaturated colour increased latency with p <0.05). Grey circles indicate a signal too small to be compared, the signal‐to‐noise ratio is <1.7.
Results
The mean deviation of the static automated visual fields ranged from –6.5 to –23.6 db (table 1). Table 1 displays the cases with respect to increasing mean deviation. Snellen visual acuities were decreased in all cases and ranged from 20/40 to 20/80.
In general, significant changes in monocular amplitude were seen in areas of corresponding visual‐field loss (fig 1). All patients had decreased visual acuity and both monocular and interocular mfVEP plots showed prominent loss centrally. In case 5, there was mild maculopathy reducing visual acuity to 20/60 in the right eye, contralateral to the compressed optic nerve. This was associated with significantly decreased amplitude on the interocular plot in the right eye, as well as the left eye, as indicated by blue and red squares.
Due to the reduced mfVEP amplitudes, latencies could not be measured from many areas where there was visual‐field loss (grey squares in the right‐most columns of fig 1). However, latency measurements could be calculated from areas of relatively preserved visual fields in all cases. These regions in the affected eyes with measurable responses tended to show an increase in latency (right‐most two columns in fig 1). For all six patients, 20–60% (monocular analysis) and 40–92% (interocular analysis) of measurable segments had significant delays. This compares with an average (median) of 9% (6%) and 6.7% (4%) from control eyes, for monocular and interocular analyses, respectively. The delay of the recordable responses from the affected eyes in this group of patients ranged from 7.9 to 13.9 ms (average 10.2 ms) for the monocular analysis and 7.6–20.7 ms (average 13.8 ms) for the interocular analysis (table 2).
Table 2 Average delay of responses for monocular and interocular analyses.
| Case | Average monocular latency (ms) | Average interocular latency (ms) |
|---|---|---|
| 1 | 9 | 17 |
| 2 | 7.9 | 7.6 |
| 3 | 13.9 | 20.7 |
| 4 | 10 | 11.1 |
| 5 | 11.9 | 12.3 |
| 6 | 8.9 | 14.2 |
Discussion
The amplitude changes on mfVEP in this series of patients corresponded topographically to defects seen on automated visual fields in most cases. Other authors have found similar topographical findings in patients with glaucoma, ischaemic optic neuropathy and optic neuritis.2,3,4,5,11,14,15,16 The interocular amplitude plots seem to correspond better with the interpolated visual fields than do the monocular plots, which is not surprising, given the sensitivity of the interocular analysis to monocular damage.3,16 The difference between the monocular and interocular amplitude plots was most apparent in case 2 in which the central field changes, although present on SAP and on the interocular plot, were relatively subtle on the monocular plot (fig 1). In this case, the mfVEP responses were extraordinarily large, including the affected eye (fig 2). Under these conditions, the monocular plot can under‐represent defects seen on SAP.16 Also, one should remember that there are inherent differences between subjective, psychophysical visual‐field data and physiological visual evoked potential data. In any case, when interpolated visual fields and mfVEP amplitudes were compared by quadrants, the least‐affected quadrants were the same except for case 3, where the mfVEP was less affected than the SAP in one quadrant.
We found mfVEP latency delay in all patients. Although the sampling of measurable latencies was limited by the number of locations with preserved responses, the majority of mfVEP signals, large enough to be measured, showed delays. These delays were close to those from patients with optic neuritis and much larger than those seen in glaucoma or ischaemic optic neuropathy. Recently, patients with glaucoma were found to have average monocular latency of 3.1 ms and average interocular latency of 1.3 ms.17 Also, patients with ischaemic optic neuropathy had median monocular latency of 2.2 ms and median interocular latency of 2.1 ms.18 However, for optic neuritis, median latencies were 11 ms for monocular and 11.5 ms for interocular analysis.18 In this series of compressive optic neuropathy, the average monocular latency was 10.2 ms and average interocular latency was 13.8 ms.
Compressive optic neuropathy may have a more generalised effect on optic nerve axons as opposed to the more selective and segmental effect that occurs with glaucoma or ischaemic optic neuropathy. The mfVEP latency delays in the patients with meningioma in this study are comparable to those in patients affected by optic neuritis.5,18 This finding is not surprising, because, previous studies using conventional visual evoked potential showed latencies which were prolonged in patients with compressive lesions, although less than in patients with demyelinating lesions.19,20 It is known that compressive lesions may cause demyelination as well as distortion of the nodes of Ranvier, both of which may result in delay in conduction similar to that seen from inflammatory damage to the optic nerve.21 Although latency information may be useful in the differential diagnosis of different types of optic neuropathy, it is more likely that monitoring mfVEP amplitudes may offer a way to follow the clinical course of patients with compressive optic neuropathy in an objective manner.
Abbreviations
mfVEP - multifocal visual evoked potential
RMS - root mean square
SAP - static automated perimetry
Footnotes
Funding: This study was financially supported by the Swiss National Science Foundation Grant # PBBEB 104452.
Competing interests: None declared.
References
- 1.Goldberg I, Graham S L, Klistorner I. Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol 200213329–39. [DOI] [PubMed] [Google Scholar]
- 2.Hood D C, Thienprasiddhi P, Greenstein V G.et al Detecting early to mild glaucomatous damage: a comparison of the multifocal VEP and automated perimetry. Invest Ophthalmol Vis Sci 200445492–498. [DOI] [PubMed] [Google Scholar]
- 3.Hood D C, Greenstein V C. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 200322201–251. [DOI] [PubMed] [Google Scholar]
- 4.Hood D C, Zhang X, Greenstein V C.et al An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci 2000411580–1587. [PubMed] [Google Scholar]
- 5.Hood D C, Odel J G, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci 2000414032–4038. [PubMed] [Google Scholar]
- 6.Baeseler H A, Sutter E E, Klein S A.et al The topography of visual evoked response properties across the visual field. Electroencephalogr Clin Neurophysiol 19949065–81. [DOI] [PubMed] [Google Scholar]
- 7.Sutter E E. The fast m‐transform: a fast computation of cross correlations with binary m‐sequences. Soc Ind Appl Math 199126686–694. [Google Scholar]
- 8.Hood D C, Zhang X, Hong J E.et al Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol 2002104303–339. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Hood D C, Chen C S.et al A signal‐to‐noise analysis of multifocal VEP responses: an objective definition for poor records. Doc Ophthalmol 2002104287–302. [DOI] [PubMed] [Google Scholar]
- 10.Fortune B, Zhang X, Hood D C.et al Normative ranges and specificity of the multifocal VEP: questions of age, gender and race. Doc Ophthalmol 200410987–100. [DOI] [PubMed] [Google Scholar]
- 11.Hood D C, Greenstein V C, Odel J G.et al Visual field defects and multifocal visual evoked potentials. Evidence of a linear relationship. Arch Ophthalmol 20021201672–1681. [DOI] [PubMed] [Google Scholar]
- 12.Hood D C, Ohri N, Yang E B.et al Determining abnormal latencies of multifocal visual evoked potentials: a monocular analysis. Doc Ophthalmol 2004109189–199. [DOI] [PubMed] [Google Scholar]
- 13.Hood D C, Zhang Z, Rodarte C.et al Determining abnormal interocular latencies of multifocal visual evoked potentials. Doc Ophthalmol 2004109177–187. [DOI] [PubMed] [Google Scholar]
- 14.Klistorner A I, Graham S L, Grigg J R.et al Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 199839937–950. [PubMed] [Google Scholar]
- 15.Klistorner A, Graham S L. Objective perimetry in glaucoma. Ophthalmology 20001072283–2299. [DOI] [PubMed] [Google Scholar]
- 16.Hood D C, Zhang X, Winn B J. Detecting glaucomatous damage with the mfVEP: how can a monocular test work? J Glaucoma 2003123–15. [DOI] [PubMed] [Google Scholar]
- 17.Rodarte C, Yang E B, Grippo T.et al Evaluation of multifocal visual evoked potential latency in glaucoma. ARVO 2005, Poster 3753
- 18.Odel J G, Rodarte C, Yang E B.et al A quantitative measure of multifocal visual evoked potential latencies in ischemic neuropathy and optic neuritis. ARVO 2005, Poster 642.
- 19.Halliday A M, Halliday E, Kriss A.et al The pattern‐evoked potential in compression of the anterior visual pathways. Brain 197699357–374. [DOI] [PubMed] [Google Scholar]
- 20.Kupersmith M J, Siegel I M, Carr R E.et al Visual evoked potentials in chiasmal gliomas in four adults. Arch Neurol 198138362–365. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa J, Fowler T J, Gilliatt W. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat 1972113433–455. [PMC free article] [PubMed] [Google Scholar]