Abstract
Background
In the present study we evaluated the functional success after macular hole surgery in correlation to visual quality of life and looked for predictive factors determining surgical success.
Methods
Fifty‐nine patients that underwent pars plana vitrectomy for idiopathic macular hole were included. Follow‐up visits were performed in regular intervals after surgery and included a clinical examination, optical coherence tomography (OCT) and measurement of visual acuity. To assess the visual quality of life patients filled out the National Eye Institute 25‐item Visual Function Questionnaire (VFQ‐25) before and three months and one year after surgery.
Results
Macular hole closure was achieved in 57 of 59 patients (97%). Mean visual acuity increased from 20/100 preoperatively to 20/34 one year after surgery (p = 0.02). Despite good visual acuity (20/27) in the fellow eye, visual quality of life (VFQ composite score) rose from 75.9 ± 14.4 (SD) to 81.5 ± 14.2 one year after surgery (p<0.001). Although there was no correlation between the increase in visual quality of life and visual acuity, the increase in VFQ‐25 could be well predicted: low visual acuity and significant impairment on VFQ‐25 testing preoperatively made patients most likely to benefit from macular hole surgery. A relatively high retinal thickness measurement at the hole border measured on OCT further increases the predictive value.
Conclusion
Macular hole surgery is associated with an increase in visual quality of life despite good visual acuity of the fellow eye. Preoperative visual acuity, VFQ‐25 value and partly OCT may help to predict the increase in patients' vision related quality of life after surgery.
The first report of successful closure of a macular hole was published 1991 by Kelly and Wendel.1 Since then, the surgical technique has been refined and peeling of the internal limiting membrane (ILM) has become a widely accepted surgical step to improve anatomic and functional success.2,3,4 The anatomical closure rate after two surgical interventions usually approaches 96% and a significant improvement of visual acuity is seen following surgery in most cases.5
Complications of macular hole surgery include cataract formation, retinal tears, retinal detachment and the reopening of macular holes.6,7 Despite good anatomical outcome an individual risk‐benefit analysis for each patient is important.
In general, visual acuity and morphological parameters like anatomical closure of the macular hole are used as major outcome parameters to assess the benefit of macular hole surgery. However, traditional clinical measures of vision may fail to give consideration to important aspects of visual function and patientś well being. Instruments to measure patientś vision‐targeted quality of life can close that gap as reported recently and may serve as additional tools to assess surgical success.8 The aim of this prospective study was to evaluate changes in patient́s visual quality of life following vitreoretinal surgery for idiopathic macular holes with a one year follow‐up. We further analysed, whether preoperative data can predict the patientś benefit from macular hole surgery, as determined by visual quality of life.
Patients and methods
A consecutive series of 59 eyes of 59 patients with idiopathic macular hole were included. Patientś informed consent was obtained at study entry and the study was approved by the local ethics committee. Exclusion criteria were high myopia >−8 D, traumatic macular holes, or any other macular disease limiting visual acuity. Patients were examined one day preoperatively and 3 and 12 months postoperatively. On each visit, clinical examinations included measurement of best‐corrected visual acuity (logarithmic steps on standardised Snellen projection), applanation tonometry, and slit lamp biomicroscopy using a 78 diopter lens. In all patients, optical coherence tomography (OCT) (OCT Model 2000, Carl Zeiss Meditec, Dublin, CA, USA) of the macula region was performed preoperatively. Six radial scans were applied by an experienced technician masked to all clinical and surgical data. Proper fixation was ensured by the operator and the macular center was scanned in cases of paracentral fixation. Two macular hole diameters were measured: The “base diameter” at the level of the retinal pigment epithelium and the “minimum diameter” of the hole. The hole form factor (HFF) was calculated according to Puliafito.9 Base diameter, minimum diameter, hole height and HFF were correlated to the outcome parameter visual quality of life.
The surgical technique consisted of standardised pars plana vitrectomy, removal of the ILM and intraocular gas tamponade (15% hexafluorethane gas mixture) as described previously.5 In the first days after surgery patients had to position face down.
Preoperatively, 3 months and one year after surgery patients were asked to complete the interviewer administered form of the National Eye Institute Visual Function Questionnaire 25 (VFQ‐25) to assess vision‐targeted health‐related quality of life.10,11,12 The VFQ‐25 is a vision‐specific quality of life instrument derived from a multi‐condition focus group process. Each question (item) is assigned to one of 12 subscales (see table 1), and a composite score can be calculated. Scores range from 0 to 100 with higher scores indicating better functioning. The VFQ‐25 has been proven to be a reliable and valid instrument to measure vision‐targeted quality of life.12 Mean scores and standard deviations were calculated for the VFQ‐25 subscales as well as for the VFQ‐25 composite score. Visual acuity mean values were calculated after transforming the mean angle of resolution values to negative mean angle of resolution (–logMAR) values. Parametric methods included independent t‐test, ANOVA and linear regression in case of normally distributed variables; otherwise the corresponding non‐parametric equivalents were chosen. All tests were considered to be statistically significant for p<0.05.
Table 1 The National Eye Institute Visual Function Questionnaire 25 (VFQ‐25) consists of 25 items, each item is assigned to one of 12 subscales. The composite score as well as the subscales “general vision”, “near activities”, “social functioning”, “role difficulties” and “peripheral vision” increased significantly 1 year after surgery for macular hole (p values: all non‐parametrical Wilcoxon test). In the subscales “distance activities”, “social functioning”, “role difficulties” and “peripheral vision” the 3 months differed from the 12 months results.
| VFQ‐25 Questionnaire Scale | n | Mean score preoperative | Mean score 3 months postoperatively | p Value 3 months to preoperatively | Mean score one year postoperatively | p Value one year to preoperatively |
|---|---|---|---|---|---|---|
| General health | 58 | 57.8 (19.8) | 61.1 (18.4) | 0.840 | 63.5 (18.9) | 0.052 |
| General vision | 56 | 51.8 (23.1) | 62.6 (21.6) | 0.002 | 63.1 (19.9) | 0.001 |
| Ocular pain | 57 | 87.7 (19.6) | 88.0 (18.0) | 0.724 | 88.6 (16.3) | 0.858 |
| Near activities | 57 | 59.6 (23.1) | 71.3 (20.4) | <0.001 | 75.8 (19.9) | <0.001 |
| Distance activities | 59 | 85.2 (15.8) | 80.0 (20.1) | 0.019 | 85.2 (15.8) | 1.00 |
| Social functioning | 57 | 82.2 (20.0) | 84.7 (18.6) | 0.183 | 86.7 (18.4) | 0.008 |
| Mental health | 56 | 86.2 (19.3) | 88.5 (19.8) | 0.313 | 88.6 (20.7) | 0.132 |
| Role difficulties | 57 | 66.4 (25.1) | 71.1 (28.2) | 0.210 | 75.2 (24.8) | 0.006 |
| Dependency | 56 | 87.7 (19.4) | 88.7 (18.7) | 0.469 | 90.1 (19.3) | 0.140 |
| Driving | 38 | 70.7 (29.4) | 66.8 (30.5) | 0.205 | 66.9 (29.9) | 0.642 |
| Colour vision | 57 | 91.0 (9.8) | 92.1 (10.4) | 0.751 | 93.3 (10.1) | 0.762 |
| Peripheral vision | 57 | 83.3 (22.3) | 85.8 (20.5) | 0.246 | 89.8 (16.8) | 0.031 |
| Composite score | 59 | 75.9 (14.4) | 79.1 (15.4) | 0.031 | 81.5 (14.2) | <0.001 |
Results
Patient demographics
Fifty nine eyes (32 right eyes and 27 left eyes) of 59 consecutive patients were examined. Of these, 44 were females and 15 males; mean patients age was 67 years (range 50 to 78).
Functional outcome
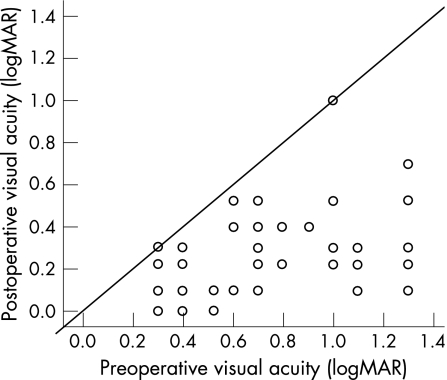
Median preoperative best‐corrected visual acuity of the operated eye was logMAR 0.68 (20/100; range 1.3 to 0.3). One year after surgery, median best‐corrected visual acuity increased to logMAR 0.23 (20/34; range 1.0 to 0, p<0.001, fig 1). An improvement of best‐corrected visual acuity was seen in 57 patients, in two patients the visual acuity remained unchanged. In no patient visual acuity had deteriorated one year after surgery and 46 cases (80%) achieved an at least 3 lines increase in visual acuity. The visual acuity of the fellow eye was logMAR 0.14 (20/28) before surgical intervention did not change significantly one year after surgery (logMAR 0.13 (20/27); p = 0.36). Most patients (57 of 59) presented with better visual acuity in the fellow eye than compared to the operated eye. On the one year follow up, 41 patients (69.5%) underwent cataract surgery in the meantime.
Figure 1 Development of the visual acuity for each patient. A highly significant (p<0.001) increase in visual acuity from median logMAR 0.68 (20/100) to 0.23 (20/34) one year after surgery was observed. Only two patients had no benefit, 80% achieved at least 3 lines increase and no patient worsened regarding visual acuity. The two diagonal lines delinate the interval between no change and a 3‐line increase in visual acuity.
Anatomic success in terms of closed macular hole was achieved in 57 eyes one year after surgery; in two patients the hole persisted (97% total closure rate). The preoperative base diameter (−0.39, p = 0.04) and hole height (r = −0.40, p = 0.03) measured by OCT were negatively correlated with anatomical hole closure.
Visual quality of life
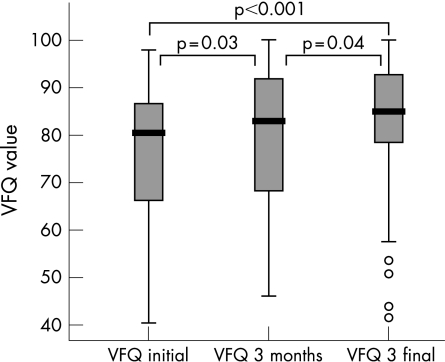
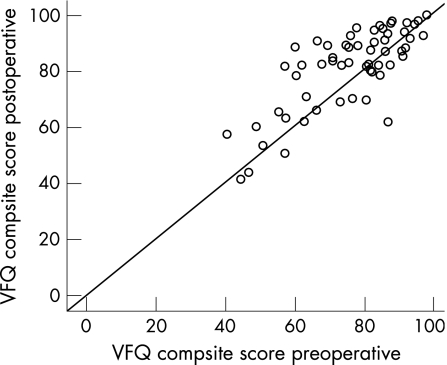
The preoperative VFQ‐25 composite score was 75.9 (SD 14.4). At the first follow up visit 3 months after surgery, the composite score increased to 79.1 (15.4; p = 0.03). One year after surgery, there was a further increase to 81.5 (14.2) (p = 0.04). The difference between preoperative VFQ‐25 composite score and VFQ‐25 composite score one year after surgery was highly significant (p<0.001; see fig 2). Figure 3 shows the development of visual quality of life for each patient. The majority of patients showed increased VFQ‐25 values postoperatively. In some patients lower VFQ‐25 values were found (n = 18), although none of these patients experienced a deterioration of visual acuity after surgery. When comparing those patients with a decreased VFQ‐25 composite score one year after surgery to the 41 patients with an increased VFQ‐25 composite score, two VFQ‐25 subscales were noted to differ: “near activities” (p = 0.002, all non‐parametrical Mann Whitney) and “role difficulties” (p = 0.001). There was no difference concerning clinical parameters such as lens status, age, visual acuity of the affected or fellow eye, or increase in visual acuity. Regarding the investigated preoperative parameters, the patients who did not benefit from surgery in terms of VFQ‐25 composite score values differed only in the VFQ‐25 subscale “ocular pain”, with remarkably low pain values before surgery (mean (SD) 94.1 (4.0) vs 85 (3.2) non‐benefiting group; p = 0.04). There was no change in visual function of the fellow eye; the function of the fellow eye was always good. The results of the VFQ‐25 subscales before surgery and 3 and 12 months postoperatively are listed in detail in table 1. Subscale analysis revealed a long term significant benefit from macular hole surgery in the following subscales: “social functioning”, “role difficulties”, “peripheral vision” and especially in “general vision” and “near activities”. Comparing the scores obtained 3 and 12 months postoperatively, a similar increase was seen for most subscales after surgery except for the subscales “distance activities”, “social functioning”, “role difficulties” and “peripheral vision”.
Figure 2 VFQ‐25 values increased significantly 3 months and 1 year after surgery.
Figure 3 VFQ‐25 composite scores preoperatively versus one year after surgery. A median increase of 5.6 points in VFQ‐25 scores was observed, although the increase was quite variable (SD 9.8 points, interquartile range13.8). Numerically most patients (N = 41) did profit significantly from macular hole surgery (p<0.001), but some patients (n = 18) had lower visual quality of life after surgery although no patient lost any line in visual acuity (see fig 5) and the fellow eye was stable.
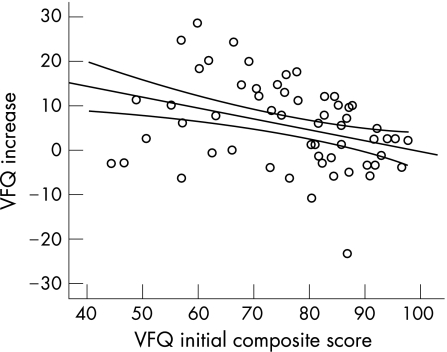
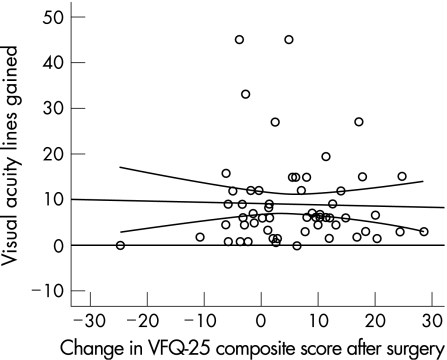
Patients with lower preoperative VFQ‐25 composite values showed a higher postoperative increase compared to those with higher preoperative scores (fig 4), disclosing a significant negative correlation of −0.28 (p = 0.04). There was no significant correlation between the increase of visual acuity and benefit in visual quality of life (fig 5).
Figure 4 There is a negative correlation (−0.28; p = 0.04) between the initial VFQ‐25 composite score and increase in visual quality of life after surgery.
Figure 5 There was no correlation between the increase of visual acuity and patients' visual quality of life values after surgery.
OCT data
Changes in VFQ‐25 composite score and subscales were correlated to preoperative OCT parameters. The only OCT parameter that differed between patients with increased or decreased VFQ‐25 values was the macular hole height with a higher mean value of 306 (SD 103) μm seen in patients with increased VFQ‐25 values in contrast to mean 238 (70) μm in patients with decreased VFQ‐25 values (p = 0.03).
Predictive model
A multiple stepwise regression model analysis was performed to predict the increase of the VFQ‐25 composite score following surgery. All preoperative VFQ 25 subscales, gender, visual acuity, age and all OCT parameters were included. The increase in VFQ‐25 composite score could be best predicted by the following preoperative parameters (R2 = 0.60): visual acuity (β = −0.84), VFQ‐25 composite score (β = −0.86) and OCT macular hole height (β = 0.24). In the regression analysis, the most significant subscales of VFQ‐25 were “distance activities” and “role difficulties”. A simple model including only these two preoperative factors yielded an adjusted R2 of 0.41 (moderate predictability). Remarkably the two major factors, visual acuity and the VFQ‐25 composite score are numerically equally important for predicting the increase in visual quality of life after surgery.
Discussion
A number of studies were performed to assess the quality of life in patients with vitreoretinal diseases, mainly age related macular degeneration.13,14 Patients with vitreoretinal diseases have a reduced visual quality of life that is often better correlated with self‐reported visual impairment than with the visual acuity in the better eye.15 Some studies estimated quality of life results for patients undergoing vitreoretinal surgery, e.g. for the removal of subfoveal choroidal neovascularisations in age‐related macular degeneration, macular translocation or vitreoretinal surgery for epiretinal membranes.16,17,18 There are only a few reports regarding visual quality of life after macular hole surgery.19,20,21,22 Tranos21 could show a beneficial effect on patientś subjective perception of visual function after surgery in a case‐series with a follow‐up time of 4 months. However, it should be kept in mind that the full functional rehabilitation after vitreomacular surgery may take up to one year. Therefore we investigated the VFQ‐25 values at two different time points at 3 and 12 months after surgery. A significant differences at least in some VFQ‐25 subscales when comparing 3 and 12 month results could be shown.
For ophthalmic surgeons, the knowledge of factors predicting the potential outcome of macular hole surgery are of special interest when discussing the risk and benefit of a surgical intervention with the patient. There are several factors one might take into consideration such as duration of symptoms, hole size, visual acuity. However, less attention has been paid to the individual visual quality of life.
The concept of visual quality of life evaluation has been reported to add useful information on the potential benefit of macular surgery: In a previous study, we examined the improvement of patientś quality of life after vitreoretinal surgery for epiretinal membranes16 and could demonstrate, that patients with preoperatively low life quality values and low visual acuity profited significantly from surgery. The increase in visual quality of life was better predictable than the increase of visual acuity. The preoperative visual quality of life and preoperative visual acuity had a surprisingly high predictive value for the patients benefit.
In this study, similar results were obtained with the current series of 59 patients undergoing macular hole surgery. Macular hole surgery lead to a significant increase in visual quality of life noted at the 3 and 12 months follow up visits. There was no correlation between the increase in visual quality of life and improvement of visual acuity. In many studies that estimated quality of life outcomes after ophthalmological interventions only week or absent correlation between increase in quality of life and improvement of visual acuity was observed, eg for cataract surgery.23 This highlights that visual acuity is only one aspect of visual functioning, which explains why despite increased visual acuity in several cases VFQ‐25 values decrease in our series. However, the benefit of macular hole surgery as determined by an increase in VFQ‐25 value could be well predicted by low preoperative visual acuity and low preoperative VFQ‐25 testing. The VFQ‐25 subscales “distance activities” and “role difficulties” appeared to be most relevant. A relatively high retinal thickness measurement at the hole border in OCT scans further increased the predictive value.
Conclusions
Measurement of visual quality of life is of increasing importance to assess the clinical outcome of an ophthalmological intervention. Macular hole surgery leads to a significant increase of patients visual quality of life despite good visual function of the fellow eye. There is no correlation between gain in visual acuity and visual quality of life. Low preoperative VFQ‐25 values (especially subscales “distance activities” and “role difficulties”), low visual acuity and high retinal thickness at the hole border on OCT are predictive parameters for the patient's perceived benefit. These findings are important when deciding on vitreoretinal surgery for macular holes as they may help to predict the individual postoperative success. For clinical practice this means that not only visual acuity should be impaired by the macular hole, also the patient should report a significant degree of subjective impairment to benefit most from surgery.
Abbreviations
HFF - hole form factor
ILM - internal limiting membrane
OCT - optical coherence tomography
VFQ‐25 - Visual Function Questionnaire
Footnotes
Competing interests: None.
References
- 1.Kelly N E, Wendel R T. Vitreous surgery for macular holes. Results from a pilot study. Arch Ophthalmol 1991109654–659. [DOI] [PubMed] [Google Scholar]
- 2.Liesenhoff O, Messmer E M, Pulur A.et al Treatment of full thickness idiopathic macular holes. Ophthalmologe 199693655–659. [DOI] [PubMed] [Google Scholar]
- 3.Haritoglou C, Gass C A, Schaumberger M.et al Macular changes after peeling of the internal limiting membranes in macular hole surgery. Am J Ophthalmol 2001132363–368. [DOI] [PubMed] [Google Scholar]
- 4.Brooks H L., Jr Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107,19391948. [DOI] [PubMed]
- 5.Haritoglou C, Gass C, Schaumberger M.et al Long‐term follow‐up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol 2002134661–666. [DOI] [PubMed] [Google Scholar]
- 6.Park S S, Marcus D M, Duker J S.et al Posterior segement complications after vitrectomy for macular hole. Ophthalmology 1995102775–781. [DOI] [PubMed] [Google Scholar]
- 7.Banker A S, Freeman W R, Kim J W.et al Vision‐threatening complications of surgery for full‐thickness macular holes. Vitrectomy for Macular Hole Study Group. Ophthalmology 19971041442–1452. [DOI] [PubMed] [Google Scholar]
- 8.Hirneiss C, Neubauer A S, Welge‐Lüßen U.et al Bestimmung der Lebensqualität des Patienten in der Augenheilkunde. Ophthalmologe 20031001091–1097. [DOI] [PubMed] [Google Scholar]
- 9.Puliafito C A, Hee M R, Lin C P.et al Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995102217–229. [DOI] [PubMed] [Google Scholar]
- 10.Mangione C M, Lee P P, Berry S H.et al “Development of a questionnaire for assessment of visual function: Association for Research in Vision and Ophthalmology”. Invest Ophthalmol Vis Sci 1995361962 [Google Scholar]
- 11.Mangione C M, Berry S H, Spritzer K. “Identifying the content area for the 51‐item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. ” Arch Ophthalmol 1998116227–233. [DOI] [PubMed] [Google Scholar]
- 12.Mangione C M, Lee P P, Guitierrez P R, Spritzer K.et al National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25‐item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 20011191050–1058. [DOI] [PubMed] [Google Scholar]
- 13.Dong L M, Childs A L, Mangione C M.et al Submacular Surgery Trials (SST) Research Group. Health‐ and vision‐related quality of life among patients with choroidal neovascularisation secondary to age‐related macular degeneration at enrolment in randomised trials of submacular surgery: SST report no, 4. Am J Ophthalmol 200413891–108. [DOI] [PubMed] [Google Scholar]
- 14.Berdeaux G H, Nordmann J P, Colin E.et al Vision‐related quality of life in patients suffering from age‐related macular degeneration. Am J Ophthalmol 2005139271–279. [DOI] [PubMed] [Google Scholar]
- 15.Linder M, Chang T S, Scott I U.et al “Validity of the Visual Function Index (VF‐14) in Patients with Retinal Disease”. Arch Ophthalmol 19991171611–1616. [DOI] [PubMed] [Google Scholar]
- 16.Hirneiss C, Rombold F, Kampik A.et al Visual quality of life after vitreoretinal surgery for epiretinal membranes. Ophthalmologe 2006103109–113. [DOI] [PubMed] [Google Scholar]
- 17.Miskala P H, Bass E B, Bressler N M.et al Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularisation in age‐related macular degeneration: quality‐of‐life findings, SST report no. 12. Ophthalmology 20041111981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahill M T, Stinnett S S, Banks A D.et al Quality of life after macular translocation with 360 degrees peripheral retinectomy for age‐related macular degeneration. Ophthalmology 2005112144–151. [DOI] [PubMed] [Google Scholar]
- 19.Pearce I A, Branley M, Groenewald C.et al Visual function after macular hole surgery. Eye 199812651–658. [DOI] [PubMed] [Google Scholar]
- 20.Ellis J D, Malik T Y, Taubert M A.et al Surgery for full‐thickness macular holes with short‐duration prone posturing: results of a pilot study. Eye 200014307–312. [DOI] [PubMed] [Google Scholar]
- 21.Tranos P G, Ghazi‐Nouri S M, Rubin G S.et al Visual function and subjective perception of visual ability after macular hole surgery. Am J Ophthalmol. 2004 Dec 138995–1002. [DOI] [PubMed] [Google Scholar]
- 22.Tranos P G, Peter N M, Nath R.et al Macular hole surgery without prone positioning. Eye. 2006 (Epub ahead of print). [DOI] [PubMed]
- 23.Cassard S D, Patrick D L, Damiano A M.et al Reproducibility and responsiveness of the VF‐14: an index of functional impairment in patients with cataracts. Arch Ophthalmol 19951131508–1513. [DOI] [PubMed] [Google Scholar]