Abstract
Aim
To evaluate the recently introduced Travatan Dosing Aid (TDA) for its accuracy in recording and dispensing eyedrops.
Methods
The number of eyedrops dispensed with each lever depression and agreement of total number of drops dispensed with that recorded by the device was evaluated in a controlled setting.
Results
The TDA correctly recorded a drop being dispensed 100% of the time with full TDA lever depression for <3 s. Under these conditions, agreement between numbers of drops dispensed and recorded was 99%. However, failure to fully depress the lever or prolonged lever depression for >4 s resulted in unreliable TDA recording.
Conclusion
Eyedrops were reliably recorded by the TDA after each full lever depression. However, patients need to be instructed about optimal technique so that evaluation of compliance is not confounded by mechanical factors.
Patient compliance is critical in decision making concerning glaucoma treatment. Patients often present with evidence of progressing glaucoma despite reaching target intraocular pressure (IOP) or with increased IOP despite testaments to strict compliance. In these common scenarios, patients may use eyedrops only before visiting the office, or may feel uncomfortable discussing compliance issues.
Reports of non‐compliance with glaucoma drugs are as high as 59%.1 Gurwitz et al2 discovered that 23% of patients aged >65 years had not filled any drug prescriptions for glaucoma over a 12‐month period after the initiation of treatment. Studies have shown that patients markedly overestimate their level of compliance.3 In one study, self‐reported compliance with glaucoma treatment was 77% (200/260); however, only 52% knew the name and dosing frequency of their drugs.4 Decisions to alter a medical regimen or perform surgery must be made based partly on misleading intraocular measurements and inaccurate patient self‐reporting.
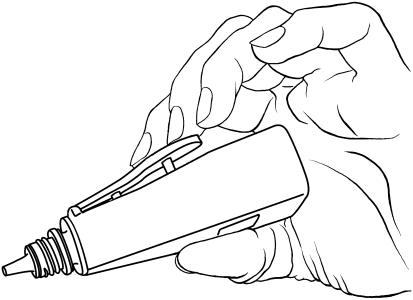
The recently introduced Travatan Dosing Aid (TDA; Alcon, Fort Worth, Texas, USA) is an eyedrop monitoring device developed to help healthcare providers discern between insufficient drug efficacy and non‐compliance. The TDA's external lever is depressed to squeeze the bottle (containing the drug) it holds and administers an eyedrop (fig 1). The device records the time, date and number of drops dispensed. As forgetfulness has been cited as the most common reason for non‐compliance, the TDA has an audible and visual alarm component.1,5
Figure 1 Features of the Travatan Dosing Aid (TDA). TDA lever depression dispenses a drop from the travoprost bottle.
In this study, we evaluated the ability of the TDA to accurately record eyedrop dispensing, and explored the mechanical variables that may influence its accuracy.
Methods
The TDA was loaded with a 2.5‐ml travoprost bottle. The TDA was held vertically, and the number of drops dispensed and recorded by the device was documented. The mechanical variables studied included the duration of TDA lever depression, the lever depression distance and the quantity of the drug remaining in the bottle.
Firstly, we tested the possibility of the device administering a drop without recording it. The TDA lever was fully depressed starting with a full bottle for 1–5 s.
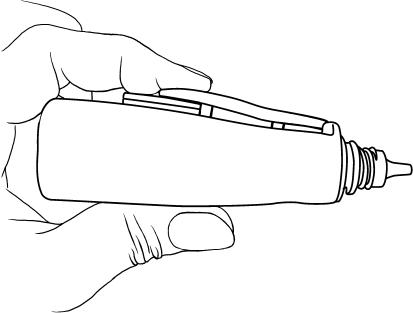
Next, we tested the lever depression distance required to record the administration of an eyedrop. The TDA was loaded with a full bottle and one drop was dispensed for the following lever depression distances: d mm (7 mm), d−1.75 mm (5.25 mm), and d−2(1.75) mm (3.75 mm), where d was full lever depression and 1.75 mm was the standardised width of a US quarter coin. The quarters were taped in place beneath the TDA lever (fig 2).
Figure 2 Testing the effect of the completeness of lever compression on Travatan Dosing Aid (TDA) accuracy. An increasing number of US quarter coins were taped beneath the TDA lever to vary the distance of lever compression.
Finally, we tested the effect of the quantity remaining in the bottle on the reliability of TDA drop administration. We quantified the total drops dispensed with complete lever depression of from the TDA loaded with a full bottle. When drops could no longer be dispensed, the bottle was removed from the TDA and manual pressure was exerted to remove any remaining drug. The total number of drops dispensed from each bottle was calculated and divided into thirds to compare the accuracy of drops administered with the quantity remaining in the bottle.
Results
Effect of duration of TDA lever depression on reliability
Full lever depression resulting in administration of one drop was recorded by the TDA with 100% accuracy if the duration of depression was <3 s, and with 99% accuracy with duration <5 s. Lever depression for ⩾5 s was not recorded by the TDA.
Although the TDA reliably recorded drop delivery for full lever depression of <5 s, not every depression resulted in drop delivery. At 1 and 2 s of full lever depression, each depression delivered a drop 70% and 71% of the time, respectively. This accuracy declined for each additional second of lever depression: 51%, 16% and 13% for 3, 4 and 5 s, respectively. The discordance between lever depression and drop delivery increased with increasing duration of lever depression owing to the delivery of multiple drops despite the device's recording of a single drop. The average number of drops dispensed with each full lever depression was 0.7, 0.7, 1.4, 1.6 and 1.9 for 1–5 s of depression, respectively.
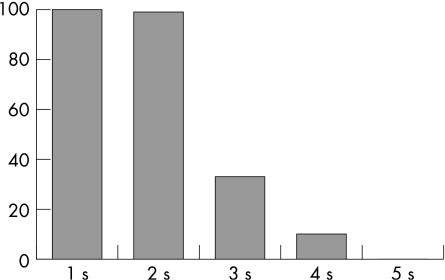
The delivery of multiple drops with increasing duration of lever depression also created a discrepancy between drops delivered and drops recorded by the TDA. Agreement between the quantities of drops delivered and drops recorded with full lever depression for <3 s was 99%. However, agreement between drops dispensed and recorded decreased to 33% and 10% for 3 and 4 s, respectively. Agreement was 0% between drops delivered and recorded at 5 s owing to lack of recording with this prolonged duration of lever depression (table 1, fig 3).
Table 1 Effect of duration of Travatan Dosing Aid (TDA) full lever depression on the accuracy of TDA recording and dispensing of drops.
| Duration of TDA full lever depression | |||||
|---|---|---|---|---|---|
| 1 s (%) | 2 s (%) | 3 s (%) | 4 s (%) | 5 s (%) | |
| Accuracy of TDA recording of the administration of a single‐drop with full lever depression | 100 | 100 | 93 | 100 | 0 |
| Accuracy of TDA single‐drop administration with full lever depression | 70 | 71 | 51 | 16 | 13 |
| Agreement between TDA drop administration and recording | 100 | 99 | 33 | 10 | 0 |
TDA, Travatan Dosing Aid.
Figure 3 Percentage agreement between Travatan Dosing Aid device administration and recording of a single eye drop with respect to duration of complete lever depression. Agreement was 100%, 99%, 33% and 10% for 1, 2, 3, and 4 s of lever depression, respectively. No drops were recorded at 5 s of lever depression.
Effect of distance of TDA lever depression on reliability
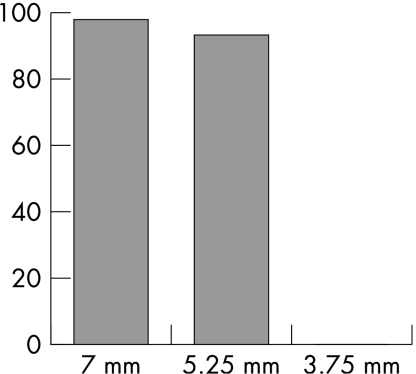
Irrespective of the duration of lever depression, full lever depression until a single eyedrop was delivered was recorded by the TDA 97% of the time. Exactly one drop was administered 98% of the time and recorded 95% of the time. At 5.25 mm of lever depression, agreement between drop delivery and recording was 93%; one drop was administered 94% of the time and recorded 88% of the time. No drops were recorded at 3.75 mm of lever depression, despite the TDA device delivering a drop 99% of the time (table 2, fig 4).
Table 2 Effect of the distance of Travatan Dosing Aid (TDA) lever depression on the accuracy of TDA recording and dispensing of eye drops.
| Distance of TDA lever depression | |||
|---|---|---|---|
| 7 mm (%) | 5.25 mm (%) | 3.75 mm (%) | |
| Accuracy of TDA recording of the administration of a single drop | 95 | 88 | 0 |
| Accuracy of TDA single‐drop administration | 98 | 94 | 99 |
| Agreement between TDA drop administration and recording | 97 | 93 | 0 |
TDA, Travatan Dosing Aid.
Figure 4 Percentage agreement between Travatan Dosing Aid device administration and recording of a single eye drop with respect to distance of lever depression. Agreement at full lever depression (7 mm) was 97%. Agreement was 93% at 5.25 mm. No drops were recorded at 3.75 mm of lever depression.
Effect of quantity of drug remaining in the bottle on TDA reliability
The travoprost bottles contained an average of 87 drops (range 82–107 drops, median 84 drops). Although the time needed to deliver exactly one drop was inversely proportional to the quantity remaining in the bottle, the TDA's ability to accurately dispense drops was unaffected by the quantity remaining in the bottle. For example, full lever depression for 1 s resulted in single‐drop administration 80% of the time when the bottle was more than two thirds full, but only 53% of the time when the bottle was less than one‐third full. Similarly, full lever depression for 2 s resulted in single‐drop administration 95% of the time when the bottle was more than two thirds full, but only 42% of the time when the bottle was less than one third full. When the lever was depressed for 3 s, multiple drops were dispensed, thereby altering the accuracy of single‐drop administration to 32% when the bottle was more than two thirds full, yet increasing to 84% of the time when the bottle was less than one third full, as multiple drops were less likely (table 3).
Table 3 Effect of the quantity of drug remaining in the bottle on Travatan Dosing Aid reliability.
| Quantity remaining in the travoprost bottle | Duration of TDA full lever depression | |||
|---|---|---|---|---|
| 1 s (%) | 2 s (%) | 3 s (%) | No restriction (%) | |
| Full to 2/3 full | 80 | 95 | 32 | 98 |
| 2/3 full to 1/3 full | 76 | 76 | 32 | 100 |
| 1/3 full to empty | 53 | 42 | 84 | 93 |
TDA, Travatan Dosing Aid.
If exactly one eye drop was administered with full lever depression without restriction on duration of depression, agreement between drop administration and recording remained accurate at 98%, 100% and 93% for a bottle full to two thirds full, two thirds to one third full and one third full to empty, respectively (table 3). After the experiment, an average of one drop (range 0–4 drops, median one drop) remained in the bottle. Thus, on average, the TDA delivered 99% of the drug.
Discussion
Assessment of patient adherence is an elusive variable in glaucoma treatment. Both self‐reports and provider judgements of compliance have been shown to be inaccurate.3,4 A monitoring device capable of accurately reporting compliance would be invaluable in evaluating a patient's response to treatment.
There has been few monitoring devices to assist in assessment of patient compliance. Kass et al3,6,7 described a device that recorded the date and time of each eyedrop administered when the cap of the bottle containing the drug was removed and the bottle was inverted. A study investigating the TDA prototype reported 100% accuracy with the device's date stamping and noted that the device failed to record at least one drop in seven of the ten participants.8 Earlier models of adherence aids, such as the medication alarm device, Prescript TimeCap, have shown enhancement of patient compliance.9 Products such as Pfizer's XAL‐EASE dealt with commonly reported difficulties with self‐administration of drops: misdirection and difficulty squeezing the bottle.10 The TDA combines an eyedrop monitoring device with an adherence aid that alerts patients and provides ergonomic deliverance.
On the basis of the current results, our recommendations for optimal TDA accuracy include full lever depression for 2 s with an increase to 3 s once the travoprost bottle is less than one third full. In reality, most patients depress the lever until one drop is delivered. In this case, the TDA accurately recorded 97% of the time.
Our data suggested that the TDA did not compromise on reliability of drop administration at the expense of ease of use. The lever device at full depression delivered 99% of the drug with >90% agreement between drop administration and recording, regardless of the quantity remaining in the bottle.
The TDA administered drops but did not record administration under the following two conditions: lever depression of >4 s and 3.75 mm of lever depression. Therefore, failure to fully depress the TDA lever or prolongation of lever depression could potentially underestimate patient compliance. Prior studies with the TDA prototype have suggested that the device may underestimate compliance.8 To avoid compliance misrepresentation, the patient must be educated regarding proper use of the Dosing Aid. If used correctly, the TDA has the potential to positively effect the treatment of glaucoma.
Acknowledgements
We thank Alcon for generously providing samples of the Travatan Dosing Aids.
Abbreviations
TDA - Travatan Dosing Aid
Footnotes
Competing interests: RJN and MYK have received speaking fees from Alcon.
References
- 1.Patel P C, Spaeth G L. Compliance in patients prescribed eyedrops for glaucoma. Ophthamol Surg 199526233–236. [PubMed] [Google Scholar]
- 2.Gurwitz J H, Glynn R J, Monane M.et al Treatment for glaucoma: adherence by the elderly. Am J Public Health 199383711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass M A, Gordon M, Meltzer D W. Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy? Am J Ophthalmol 1986101524–530. [DOI] [PubMed] [Google Scholar]
- 4.Deokule S, Sadiq S, Shah S. Chronic open angle glaucoma: patient awareness of the nature of the disease, topical medication, compliance and the prevalence of systemic symptoms. Ophthal Physiol Opt 2004249–15. [DOI] [PubMed] [Google Scholar]
- 5.Taylor S A, Galbraith S M, Mills R P. Causes of non‐compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther 200218401–409. [DOI] [PubMed] [Google Scholar]
- 6.Kass M A, Gordon M, Meltzer D W. A miniature compliance monitor for eyedrop medication. Arch Ophthalmol 19841021550–1554. [DOI] [PubMed] [Google Scholar]
- 7.Kass M A, Gordon M, Meltzer D W.et al Compliance with topical pilocarpine treatment. Am J Ophthalmol 1986101515–523. [DOI] [PubMed] [Google Scholar]
- 8.Boden C, Sit A, Weinreb R N. Accuracy of an electronic monitoring and reminder device for use with travoprost eye drops. J Glaucoma 20061530–34. [DOI] [PubMed] [Google Scholar]
- 9.Laster S F, Martin J L, Fleming J B. The effect of a medication alarm device on patient compliance with topical pilocarpine. J Am Optom Assoc 199667654–658. [PubMed] [Google Scholar]
- 10.Winfield A J, Jessiman D, Williams A.et al A study of the causes of non‐compliance by patients prescribed eyedrops. Br J Ophthalmol 199074477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]