Abstract
Aim
To prospectively evaluate the efficacy of subtenon injection of triamcinolone acetonide (TA) before laser grid pattern photocoagulation (G‐PC) for the treatment of diffuse diabetic macular oedema (DDME).
Methods
42 eyes of 37 consecutive patients with DDME were studied. 1 week before G‐PC, 21 eyes received TA subtenon injection, and the other eyes served as control. The clinical course of visual acuity (VA) and foveal thickness (FT) was monitored for up to 24 weeks after G‐PC. Mean deviation (MD) of perimetry with 30‐2 program on Humphrey Perimeter (Zeiss‐Humphrey, Dublin, California, USA) was also measured. The average laser intensity was recorded.
Results
After TA injection, FT and VA were improved, and subsequent G‐PC maintained the improvement for up to 24 weeks without recurrence of diffuse diabetic macular oedema. In contrast, G‐PC without TA injection induced transient worsening of FT and VA, then both were gradually improved. At 24 weeks after G‐PC, MD in the TA‐injected eyes was better than those in control. The required laser intensity in TA‐injected eyes was less than that for control.
Conclusion
Subtenon injection of TA prior to G‐PC allows for treatment with a lower intensity of laser spots and also prevents the decrease in central visual field sensitivity, all of which have clinical advantages for G‐PC.
Macular oedema is the most common cause of vision loss in patients with diabetes.1,2 Reports from the Early Treatment Diabetic Retinopathy Study (ETDRS) indicate that focal grid laser photocoagulation for clinically significant macular oedema effectively reduces the risk of progressive visual loss in 50% of patients with diabetes.3,4,5 In the ETDRS protocol, discrete areas of leakage were treated with focal laser coagulation, and areas of non‐perfusion or diffuse leakage associated with macular oedema were treated with grid coagulation.
In a typical grid treatment, 100–200 µm grey‐white spots are placed around the fovea.6 This conventional photocoagulation protocol is effective for treating diffuse diabetic macular oedema (DDME); however, it causes visible laser scars that can enlarge postoperatively, leading to decreased vision.7,8 Even when carefully performed in conventional technique, it is difficult to minimise the laser power for grid photocoagulation against “oedematous” retina. Recently, posterior subtenon injection of triamcinolone acetonide (TA) was reported to effectively reduce macular oedema; however, the efficacy is likely to be temporary.9,10,11 We hypothesised that subtenon injection of TA prior to grid photocoagulation would reduce “oedematous” macula, allowing for grid photocoagulation to be performed with minimal laser power and better visual prognosis of visual acuity (VA), macular thickness and visual field sensitivity. The effectiveness of this combination therapy against DDME was evaluated in a prospective controlled study using an interventional case series design to investigate changes in foveal thickness (FT) and VA during the clinical course of DDME and the effectiveness of laser photocoagulation compared to grid treatment alone.
Methods
Patients
In this prospective interventional case series, 42 eyes of 37 consecutive patients with diabetes (18 men and 19 women) with DDME were studied. Patient age ranged from 58 to 80 years (mean (SD) 69.9 (6.2) years). All patients had type 2 diabetes. The duration of diabetes ranged from 6 to 20 years with a mean (SD) of 11.8 (3.8) years. Of the 37 patients, 23 (62%) had a history of hypertension controlled by oral systemic hypertensive drugs. Of the 37 patients, 8 had non‐high risk proliferative diabetic retinopathy (PDR) and the others had severe non‐proliferative diabetic retinopathy (NPDR). The haemoglobin A1c (HbA1c) and total cholesterol averaged 7.1 (1.1)% and 185.4 (12.6) mg/dl before starting the study. Five of 37 patients had bilateral and symmetrical DDME (patients no 33–37) with severe NPDR, and one eye received the TA injection and the other eye served as a control. In bilateral cases, TA‐injected eye was determined at random by throwing dice (if got odd number, chose right eye, and if even number, left eye). In all 32 patients had unilateral cases. In order to make possible equal conditions of macular oedema between TA‐injected and control eyes, consecutive eligible eyes were assigned into three groups according to initial FT of <300 μm, 300–500 μm and >500 μm. Then, in each group, TA‐injected grid pattern photocoagulation (G‐PC) and only G‐PC was performed at random. There is no statistically significant difference of age (p = 0.396), duration of diabetes mellitus (p = 0.497), HbA1c (p = 0.836) and total cholesterol level (p = 0.611) between TA‐injected and control eyes (table 1).
Table 1 Baseline clinical characteristics of 37 patients with diffuse diabetic macular oedema.
| Patients no | Age | Sex | Duration of DM (years) | HbA1c (%) | HT (medication) | tChL (mg/dl) |
|---|---|---|---|---|---|---|
| Unilateral TA‐injected cases | ||||||
| 1 | 72 | M | 16 | 7.5 | − | 191 |
| 2 | 77 | F | 20 | 9.1 | + | 185 |
| 3 | 80 | F | 8 | 6.7 | + | 206 |
| 4 | 67 | M | 15 | 6.9 | − | 179 |
| 5 | 72 | M | 17 | 7.3 | + | 197 |
| 6 | 74 | F | 10 | 6.7 | + | 179 |
| 7 | 59 | M | 8 | 5.6 | − | 157 |
| 8 | 68 | F | 6 | 7.3 | + | 185 |
| 9 | 63 | F | 12 | 6.3 | − | 171 |
| 10 | 69 | M | 9 | 6.6 | + | 209 |
| 11 | 71 | F | 13 | 7.2 | − | 181 |
| 12 | 73 | F | 17 | 9.2 | + | 199 |
| 13 | 68 | M | 9 | 7.8 | + | 193 |
| 14 | 76 | M | 14 | 6.1 | + | 185 |
| 15 | 78 | M | 12 | 6.6 | + | 189 |
| 16 | 79 | F | 15 | 6.1 | + | 183 |
| Mean | 71.6 | (M) 8 | 12.56 | 7.06 | (−) 5 | 186.81 |
| SD | 5.8 | (F) 8 | 3.98 | 1.00 | (+) 11 | 12.89 |
| Unilateral control cases | ||||||
| 17 | 68 | F | 13 | 8.3 | + | 171 |
| 18 | 73 | F | 11 | 7.9 | + | 167 |
| 19 | 80 | M | 17 | 8.2 | + | 185 |
| 20 | 75 | M | 13 | 6.0 | + | 175 |
| 21 | 69 | M | 8 | 5.6 | − | 193 |
| 22 | 64 | F | 8 | 5.8 | + | 179 |
| 23 | 73 | F | 15 | 6.9 | + | 185 |
| 24 | 60 | M | 11 | 7.4 | − | 189 |
| 25 | 80 | F | 18 | 8.9 | + | 200 |
| 26 | 58 | M | 7 | 5.8 | − | 159 |
| 27 | 61 | F | 6 | 7.0 | − | 185 |
| 28 | 66 | F | 8 | 6.4 | − | 189 |
| 29 | 77 | M | 14 | 5.7 | + | 207 |
| 30 | 72 | M | 18 | 9.3 | + | 201 |
| 31 | 65 | F | 12 | 6.3 | − | 167 |
| 32 | 71 | F | 8 | 7.0 | + | 188 |
| Mean | 69.5 | (M) 7 | 11.69 | 7.03 | (−) 6 | 183.75 |
| SD | 6.8 | (F) 9 | 3.98 | 1.19 | (+) 10 | 13.42 |
| Bilateral cases | ||||||
| 33 | 70 | M | 12 | 6.8 | − | 177 |
| 34 | 62 | F | 10 | 8.6 | + | 189 |
| 35 | 68 | M | 8 | 7.2 | − | 199 |
| 36 | 64 | M | 9 | 8.4 | + | 191 |
| 37 | 65 | F | 11 | 7.5 | − | 175 |
| Mean | 65.8 | (M) 3 | 12.56 | 7.06 | (−) 3 | 186.20 |
| SD | 3.2 | (F) 2 | 3.98 | 1.00 | (+) 2 | 10.06 |
DM, diabetes mellitus; F, female; HbA1c, haemoglobin A1c; HT, hypertension; M, male; TA, triamcinolone acetonide; tChL, total cholesterol.
All the patients had pan‐retinal photocoagulation at least 12 months prior to entry into the study and DDME was shown within the past 1 year. All patients had a history of cataract surgery with no complications at least 6 months before. No concurrent retinal or optic nerve disorder other than diabetic retinopathy was present. The HbA1c had been controlled to be <9.5% for at least 6 months prior to the beginning of the study and for 6 months during the observational period.
After informing the patients of the purpose of this study and the possible outcomes, informed consent was obtained from all patients prior to the intervention. This study was also approved by the NTT East Japan Tohoku Hospital Clinical Research Ethics Committee. The procedures conformed to the tenets of the Declaration of Helsinki.
All patients received a comprehensive ocular examination before and after the treatment. DDME was defined as retinal thickening of two or more disc areas involving some portion of the foveal avascular zone.12 Direct and indirect ophthalmoscopy and slit‐lamp biomicroscopy of the posterior segment with a Volk Superfield contact lens (Volk, Mentor, Ohio, USA) were performed by at least two independent clinicians to establish the presence of DDME. Fundus photographs were taken at appropriate times. Intravenous fluorescein angiography (IVFA) was performed to disclose the grade of retinopathy (high‐risk NPDR or non‐high risk PDR), to establish the presence of DDME, and also to detect and assess diffuse leakage around the fovea. IVFA was also performed 24 weeks after the treatment.
Experimental design
After the initial ocular and general examinations, grid photocoagulation was performed in all eyes. In the treated group, 1 week before starting the treatment (indicated as −1 week follow‐up), 0.5 ml of 20 mg TA was injected through the subtenon space reaching the posterior pole using a 21 gauge tri‐port subtenon cannula13 (Eagle Laboratories, Rancho Cucamonga, California, USA) by two ophthalmologists (TN and KY) who did not perform G‐PC. There was no injection in the control group. Subsequently, grid treatment was performed in both eyes (indicated as 0 week follow‐up). The best‐corrected VA with logarithm of the minimum angle of resolution (logMAR) chart (5m; NEITZ LVC‐10, Tokyo, Japan) and retinal thickness by optical coherence tomography (OCT; Zeiss‐Humphrey, Dublin, California, USA) were measured during the follow‐up examinations (before TA injection (1 week), before grid treatment (0 week) and at 2, 4, 8, 12 and 24 weeks follow‐up). A macular thickness map was made from six radial scans that intersected at the fovea using the OCT retinal‐mapping program (V 6.2). This program calculates mean thickness in nine regions; the 1000 μm central area and the four quadrants of the inner and outer ring. The diameters of the inner and outer rings were 1000–3000 and 3000–6000 μm, respectively.14,15 In this study, FT was defined as the value of a 1000 μm central area.
Perimetry comprises the assessment of visual field sensitivity of retinal locations eccentric from fovea. Grid laser photocoagulation to patients with DDME is reported to benefit reading vision owing to increased parafoveal vision and improved scanning ability.16 Other studies report the occurrence of paracentral scotoma following grid laser photocoagulation for patients with DDME.17,18 According to previous reports,19,20 visual field sensitivity measured by the mean deviation(MD) of static threshold perimetry with program 30‐2 on the Humphrey perimeter is impaired after panretinal or grid photocoagulation. In this study, to estimate the effect of grid photocoagulation on central visual field in patients with DDME, the MD of the 30‐2 static analysis was evaluated using a Humphrey field analyser at the initial examination and at the end of the observation period (24 weeks), its cost was covered by scientific grants in aid for clinical research by NTT East Japan Tohoku Hospital, Sendai, Miyagi, Japan.
Grid laser treatment was performed by the doctor (MS) who had no information about TA injection in all cases according to the guidelines of the ETDRS reports. The spots were 100 μm in size, non‐confluent, placed around the fovea and applied with the Volk Area Centralis contact lens (Volk, Mentor, Ohio, USA) and slit‐lamp system (SL‐130; Zeiss‐Humphrey systems, Carl Zeiss, Jena, Germany). No laser spots were placed within 500 μm of the centre of the fovea. Krypton red laser (920; Coherent, Palo Alto, California, USA) was used and the laser power that produced a grey appearance of the retina was recorded in each case. The total number of spots was about 100. Topical anesthesia was used in all cases, and all patients were treated as outpatients.
Statistical analyses
The significance of the differences between the pre‐treatment and post‐treatment data was assessed by the Mann–Whitney U test using a statistical program (SPSS, Chicago, Illinois, USA). The data are presented as mean (SD). p <0.05 was considered to be statistically significant.
Results
FT and VA at the initial examination
Before the administration of TA, mean FT was 504.8 (104.1) μm in the TA‐injected eyes, and 493.7 (110.7) μm in the control eyes, and there was no statistical significance between them (p = 0.687; table 2). Also, there was no significant difference between the initial VA (TA injected 0.48 (0.16), control 0.50 (0.21); p = 0.779). These results suggest that DDME in each group was symmetrical and adequate for a case–control study (table 3).
Table 2 Alteration of foveal thickness.
| TA injected | Control | ||||||
|---|---|---|---|---|---|---|---|
| Eye no | Initial (μm) | 1 week after TA (μm) | 24 weeks (μm) | Eye no | Initial (μm) | 1 week after TA (μm) | 24 weeks (μm) |
| 1 | 506 | 310 | 312 | 17 | 413 | – | 337 |
| 2 | 483 | 462 | 466 | 18 | 702 | – | 427 |
| 3 | 526 | 390 | 361 | 19 | 504 | – | 397 |
| 4 | 476 | 272 | 270 | 20 | 662 | – | 482 |
| 5 | 615 | 288 | 280 | 21 | 537 | – | 289 |
| 6 | 688 | 458 | 306 | 22 | 426 | – | 305 |
| 7 | 396 | 216 | 182 | 23 | 702 | – | 403 |
| 8 | 507 | 292 | 291 | 24 | 389 | – | 259 |
| 9 | 496 | 281 | 320 | 25 | 582 | – | 601 |
| 10 | 502 | 327 | 314 | 26 | 630 | – | 354 |
| 11 | 381 | 227 | 241 | 27 | 418 | – | 248 |
| 12 | 672 | 646 | 661 | 28 | 390 | – | 284 |
| 13 | 568 | 331 | 336 | 29 | 503 | – | 275 |
| 14 | 706 | 347 | 286 | 30 | 498 | – | 483 |
| 15 | 384 | 206 | 202 | 31 | 388 | – | 310 |
| 16 | 503 | 244 | 230 | 32 | 478 | – | 366 |
| 33 | 443 | 223 | 232 | 38 | 436 | – | 301 |
| 34 | 421 | 263 | 256 | 39 | 440 | – | 366 |
| 35 | 408 | 303 | 260 | 40 | 381 | – | 280 |
| 36 | 583 | 317 | 238 | 41 | 562 | – | 361 |
| 37 | 336 | 257 | 187 | 42 | 327 | – | 227 |
| Mean | 504.8 | 317.1 | 296.7 | 493.7 | – | 350.2 | |
| SD | 104.1 | 103 | 105.3 | 110.7 | – | 91.7 | |
| Max | 706 | 646 | 661 | 702 | – | 601 | |
| Min | 336 | 206 | 182 | 327 | – | 227 | |
Max, maximum; Min, minimum; TA, triamcinolone acetonide.
Eyes 1–32 indicate unilateral cases, which correspond to patients 1–32.
Eyes 33–42 indicate bilateral cases, which correspond to patients 33–37.
Table 3 Alteration of logarithm of the minimum angle of resolution (logMAR) visual acuity.
| TA injected | Control | ||||||
|---|---|---|---|---|---|---|---|
| Eye no | Initial | 1 week after TA | 24 weeks | Eye no | Initial | 1 week after TA | 24 weeks |
| 1 | 0.6 | 0.3 | 0.2 | 17 | 0.5 | – | 0.2 |
| 2 | 0.8 | 0.8 | 1 | 18 | 0.8 | – | 0.4 |
| 3 | 0.7 | 0.5 | 0.4 | 19 | 0.4 | – | 0.3 |
| 4 | 0.4 | 0.1 | 0.1 | 20 | 0.3 | – | 0.2 |
| 5 | 0.5 | 0.2 | 0.2 | 21 | 0.7 | – | 0.3 |
| 6 | 0.3 | 0.3 | 0.1 | 22 | 0.2 | – | 0.1 |
| 7 | 0.3 | 0.2 | 0 | 23 | 0.9 | – | 0.4 |
| 8 | 0.5 | 0.3 | 0.3 | 24 | 0.4 | – | 0.2 |
| 9 | 0.3 | 0.3 | 0.3 | 25 | 0.3 | – | 0.6 |
| 10 | 0.6 | 0.2 | 0.3 | 26 | 0.5 | – | 0.5 |
| 11 | 0.7 | 0.6 | 0.4 | 27 | 0.6 | – | 0.3 |
| 12 | 0.4 | 0.4 | 0.8 | 28 | 0.4 | – | 0.3 |
| 13 | 0.5 | 0.4 | 0.3 | 29 | 0.7 | – | 0.3 |
| 14 | 0.5 | 0.1 | 0.2 | 30 | 0.3 | – | 0.6 |
| 15 | 0.3 | 0.2 | 0.2 | 31 | 0.6 | – | 0.4 |
| 16 | 0.4 | 0.1 | 0.2 | 32 | 0.6 | – | 0.3 |
| 33 | 0.3 | 0.1 | 0.15 | 38 | 0.3 | – | 0.2 |
| 34 | 0.5 | 0.3 | 0.4 | 39 | 0.7 | – | 0.5 |
| 35 | 0.5 | 0.2 | 0.2 | 40 | 0.4 | – | 0.3 |
| 36 | 0.7 | 0.3 | 0.5 | 41 | 0.8 | – | 0.5 |
| 37 | 0.3 | 0.15 | 0.1 | 42 | 0.2 | – | 0.15 |
| Mean | 0.48 | 0.29 | 0.30 | 0.50 | – | 0.34 | |
| SD | 0.16 | 0.18 | 0.23 | 0.21 | – | 0.14 | |
| Max | 0.8 | 0.8 | 1 | 0.9 | – | 0.6 | |
| Min | 0.3 | 0.1 | 0 | 0.2 | – | 0.1 | |
Max, maximum; Min, minimum; TA, triamcinolone acetonide.
Eyes 1–32 indicate unilateral cases, which correspond to patients 1–32.
Eyes 33–42 indicate bilateral cases, which correspond to patients 33–37.
Effects of subtenon injection of TA
One week after TA injection, FT was significantly reduced to 317.1 (103) μm in the TA‐injected eyes (p<0.001). VA in TA‐injected eyes (mean logMAR acuity) significantly improved from 0.48 (0.16; −1 week) to 0.29 (0.18; 0 week; p = 0.003). During the observational period, any complications related to TA injection, such as elevation of intraocular pressure, orbital hemorrhage or ptosis, had not been observed.
Alterations of FT after grid photocoagulation
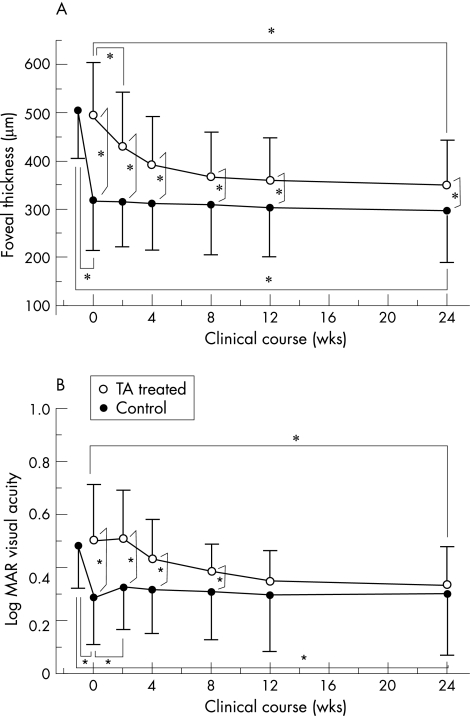
The dynamic change in FT after grid photocoagulation in TA‐injected eyes was different from that in control eyes. Two weeks after the grid treatment, FT in the TA‐injected eyes remained unchanged from 317.1 (103) μm to 315.9 (93.5) μm (p = 0.476). In contrast, FT in control eyes decrease from 493.7 (110.7) μm to 435.8 (118.4) μm (p = 0.001). FT in the TA‐injected eyes did not change during the clinical course up to 24 weeks, whereas that in the control eyes gradually decreased and saturated after 8 weeks (fig 1A). At all time points after 0 week, foveal thickness in the TA‐injected eyes was significantly lower than that in control eyes. Even at 24 weeks, there is significant difference in FT between TA‐injected and control eyes (p = 0.023). At 24 weeks, FT was significantly decreased from the initial thickness in both TA injected and control eyes (TA‐injected: p<0.0001, control: p<0.0001). There was no statistically significant change in TA‐injected eyes between at 0 weeks and 24 weeks (p = 0.065). Paradoxically significant increase in FT at 24 weeks (>105% of FT14 at 0 week) after G‐PC was observed in one of the 37 TA‐injected eye, but not observed in control eyes.
Figure 1 A. Comparison of the clinical course of foveal thickness (FT) between triamcinolone acetonide (TA)‐injected (filled circle) and control (open circle) eyes. Each point and vertical bar indicates mean FT (SD) of the mean. B. Comparison of the clinical course of logarithm of the minimum angle of resolution (logMAR) visual acuity (VA) between the TA‐injected (open circle) and control eyes (filled circle). Each point and vertical bar indicates mean logMAR visual acuity (standard deviation) of the mean. Asterisk (*) indicates statistically significant difference between the TA‐injected and control eyes at each time point (*p<0.05).
Alterations of logMAR VA after grid photocoagulation
Two weeks after the grid treatment, logMAR VA in the TA‐injected eyes significantly worsened from 0.29 (0.18) to 0.33 (0.16) (p = 0.041); in contrast, in control eyes remained unchanged from (0.50 (0.21) to 0.51 (0.18; p = 0.875)). After that, logMAR visual acuity in the TA‐injected eyes was unchanged during the clinical course up to 24 weeks, whereas that in the control eyes gradually improved (fig 1B) and after 12 weeks, there were no statistically significant differences in logMAR VA between TA‐injected and control eyes. At 24 weeks, in both TA‐injected and control eyes, logMAR VA reached to 0.30 (0.23) (TA‐injected) and 0.34 (0.14) (control) (p = 0.1866), which was significantly improved from the initial acuity (−1 week; TA‐injected: p = 0.0034, control: p = 0.0036). There was no statistically significant change in TA‐injected eyes between at 0 weeks and 24 weeks (p = 0.7983).
Alteration of MD of 30‐2 static analysis by Humphrey perimetry
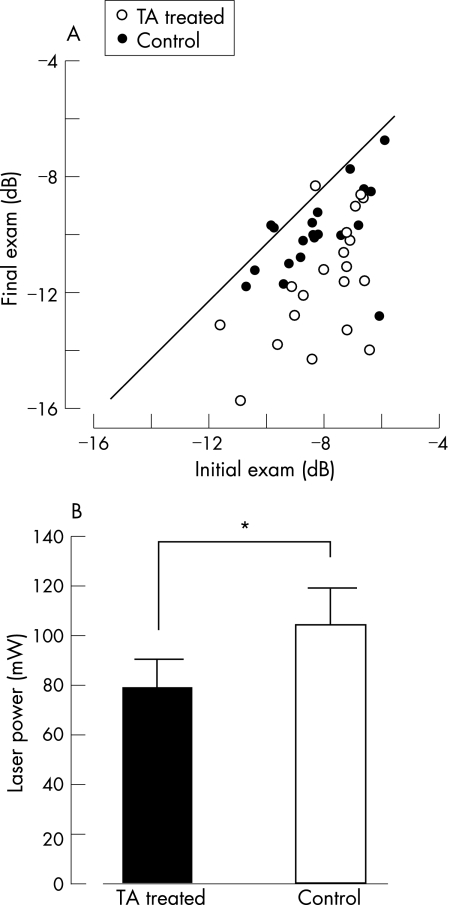
At the initial examination, there was no statistical difference in the MD in the Humphrey 30‐2 static analysis between TA‐treated eyes (−8.06 (1.58) dB) and control eyes (−7.91 (1.40); p = 0.6149); thus establishing that DDME was symmetrical. Twenty‐four weeks after the grid treatment, two of the five TA‐treated eyes showed regression of MD. All control eyes had decreased MD (fig 2A). Compared with the initial examination, MD in both TA‐treated and control eyes significantly decreased to −9.24 (1.97) (TA treated, p = 0.018), and to −11.1 (2.17) (control, p = 0.001) at 24 weeks. Interestingly, at 24 weeks, MD in TA treated eyes was significantly better than that in control (p = 0.013).
Figure 2 (A). Correlation between the initial mean deviation of Humphrey 30‐2 static perimetry and that at 24 weeks after the grid pattern photocoagulation. Open circles indicate triamcinolone acetonide (TA)‐injected eyes and filled circles indicate control eyes. The solid line indicates no change after the treatment. (B). The mean laser power that produced a grey appearance of the retina in TA‐injected (black bar) and control (white bar) eyes. Each longitudinal bar indicates the standard deviation of the mean. Asterisk (*) indicates statistically significant difference between the TA‐injected and control eyes (*p<0.05).
Alteration of IVFA findings
At the initial examination, diffuse leakage around the fovea was observed by IVFA in all cases, and eight patients with unilateral eligible eye (patient no 3, 6, 9, 12, 14, 17, 25 and 30) had non‐high‐risk PDR. Twenty‐four weeks after the treatment, diffuse leakage was not worsened in all cases and the grade of retinopathy had not been changed.
Laser power required for grid treatment
To compare the effect of grid treatment alone against DDME, the averaged laser power required for complete grid treatment was recorded in each case. For comparison, spot size was fixed to 100 μm in diameter. Mean laser power was significantly lower in TA‐treated eyes (78.8 (11.6) mW) compared with control eyes (104 (15.3) mW; p<0.001; fig 2B).
Discussion
Grid laser photocoagulation is beneficial for reducing vision loss in patients with DDME.4,17,18,21 Despite its effectiveness, some problems remain because laser photocoagulation irreversibly damages the focal retina, which leads to a decreased visual field.17,18,20,22 However, with conventional techniques, it is difficult to make enough (grey‐colour) laser burns under these conditions against “oedematous” retina before grid treatment. In this study, to reduce intrinsic damage from visible endpoint laser photocoagulation, treatment with posterior subtenon TA injection before grid treatment against DDME has been proposed. The main findings in this study are that grid photocoagulation with pre‐treatment of TA: (1) reduces the FT and improves VA faster than grid treatment alone in patients with DDME, and these effects were stable during the clinical course of up to 24 weeks; (2) requires less laser power to complete grid treatment and, therefore, (3) can minimise the grid photocoagulation‐induced visual field defect.
Several recent studies report regression of DDME with a single posterior subtenon injection of TA, leading to the critical question of why grid treatment is necessary after TA‐induced reduction of macular oedema. As previously reported from many institutes, TA injection often leads to the recurrence of macular oedema within 12–24 weeks.9,10,13 In contrast, there are no reports about grid treatment‐induced recurrence of macular oedema. In this pilot study, grid treatment was performed in both eyes, and within 24 weeks, there was no recurrence of DDME in any of the cases. Recent study revealed that TA‐assisted G‐PC for DDME reduces the recurrence of macular oedema compared to TA injection alone,23 and also supports our findings.
The mechanism of grid photocoagulation might be to produce an opening of new pathways in the retinal pigment epithelium barrier for fluid transportation between the retina and choriocapillaries and/or a decrease in the photoreceptor population, thereby reducing oxygen demand leading to reduced blood flow.24,25,26,27,28 These effects are thought to lead to the regression of macular oedema. Thermal destruction by retinal photocoagulation induces focal inflammation in the target retinal tissue29; therefore, some cytokines related to tissue inflammation, such as interleukin‐6 and interleukin‐8, are induced by laser photocoagulation,30 which leads to macular oedema. Thus, grid treatment is thought to have two opposite effects against DDME. In this study, grid photocoagulation alone induced temporary worsening, and subsequent regression of the macular oedema. In contrast, subtenon injection of TA might suppress the photocoagulation‐induced inflammation, thus this combination therapy leads to a better prognosis for at least 24 weeks.
Laser absorption and thermal injury are focused on the retinal pigment epithelium31 located on the most outer part of the retina, and the laser energy reached this area via retinal tissue. Therefore, the thicker the retina, the more laser power required for retinal photocoagulation. After the laser treatment, the atrophic scar enlarges with time to produce a zone of injury that is >200–300% larger than the original spot size.8,32 Patients who undergo laser photocoagulation often complain of paracentral, grid‐like scotomas in the treated eye, especially when the treatment is near the fovea.18,33 Postoperative retinal atrophy induced by laser burns is related to the laser power. Therefore, Sinclair et al34 recommended threshold‐level treatment (low power, short duration), when grid treatment is performed. As shown in this study, grid treatment FT in DDME can be reduced by subtenon TA injection, and treatment with low intensity and a minimal number of laser spots is possible, which also prevents the decrease in sensitivity of the central visual field, all of which have many clinical advantages for grid treatment.
Besides this study, many modified methods of grid treatment to reduce retinal damage have been presented—for example, temporal grid treatment,35 subvisible clinical endpoint photocoagulation34,36 and minimal intensity diode laser photocoagulation.37 Although subtenon injection of TA is easy and safe procedure because it is performed in outpatient clinic and has lower risk of infection than intravitreous injection, possibilities of complications, including elevation of intraocular pressure, orbital haemorrhage and ptosis are not eliminated. Atraumatic low‐duty‐cycle diode micropulse photocoagulation is a better method for reducing complication of G‐PC, however, it needs new laser equipment.36,38 Therefore, TA assisted G‐PC, as modified conventional G‐PC technique, can be an easy and effective method.
This pilot study is not large enough to reach definitive conclusions. Interestingly, even in five cases of symmetrical and bilateral DDME (case 1–5) in this study, it was reached to the same conclusion. Our protocol was designed to detect differences between injected and control eyes with respect to anatomical outcome via OCT findings and functional outcome via VA and MD assessments. The results suggest that posterior subtenon injection of TA before grid treatment is useful for avoiding retinal damage. A larger number of cases and longer observation period are necessary to confirm our hypothesis.
Acknowledgements
The authors thank Dr Norio Sugimoto in Theranostic Instruments Research Laboratories for technical support of statistical analyses and useful comments. This study was supported by 2004–2006 scientific grants in aid for clinical research by NTT East Japan Tohoku Hospital
Abbreviations
DDME - diffuse diabetic macular oedema
ETDRS - early treatment diabetic retinopathy study
FT - foveal thickness
G‐PC - grid pattern photocoagulation
HbA1c - haemoglobin A1c
IVFA - intravenous fluorescein angiography
logMAR - logarithm of the minimum angle of resolution
MD - mean deviation
NPDR - non‐proliferative diabetic retinopathy
OCT - optical coherence tomography
PDR - proliferative diabetic retinopathy
TA - triamcinolone acetonide
VA - visual acuity
Footnotes
Competing interests: None.
References
- 1.Moss S E, Klein R, Klein B E K. The incidence of visual loss in a diabetic population. Ophthalmology 1988951340–1348. [DOI] [PubMed] [Google Scholar]
- 2.MacMeel J W, Trempe C L, Franks E B. Diabetic maculopathy. Trans Am Acad Ophthalmol Otolaryngol 197783476–487. [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group Focal photocoagulation treatment of diabetic macular edema. Report number 19. Arch Ophthalmol 19951131144–1155. [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema. Report #1. Arch Ophthalmol 19851031796–1806. [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group Treatmant. Techniques and clinical guidelines for photocoagulation of diabetic macular edema. Report #2. Ophthalmology 198794761–774. [DOI] [PubMed] [Google Scholar]
- 6.Bandello F, Lanzetta P, Menchini U. When and how to do a grid laser for diabetic macular edema. Doc Ophthalmol 199997415–419. [DOI] [PubMed] [Google Scholar]
- 7.Morgan C M, Schatz H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology 19899696–103. [DOI] [PubMed] [Google Scholar]
- 8.Schatz H, Madeira D, McDonald H R.et al Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 19911091549–1551. [DOI] [PubMed] [Google Scholar]
- 9.Bakri S J, Kaiser P K. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol 2005139290–294. [DOI] [PubMed] [Google Scholar]
- 10.Verma L K, Vivek M B, Kumar A.et al A prospective controlled trial to evaluate the adjunctive role of posterior subtenon triamcinolone in the treatment of diffuse diabetic macular edema. J Ocul Pharmacol Ther 200420277–284. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Takeda K, Morita K.et al Vitreous concentration of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol 20041381046–1048. [DOI] [PubMed] [Google Scholar]
- 12.Lee C M, Olk J, Akduman L. Combined modified grid and panretinal photocoagulation for diffuse diabetic macular edema and proliferative diabetic retinopathy. Ophthalmic Surg Lasers 200031292–300. [PubMed] [Google Scholar]
- 13.Shimura M, Yasuda K, Shiono T. Posterior subtenon injection of triamcinolone acetonide prevents pan‐retinal photocoagulation‐induced visual dysfunction in patients with severe diabetic retinopathy and good vision. Ophthalmology 2006113381–387. [DOI] [PubMed] [Google Scholar]
- 14.Massin P, Vicaut E, Haouchine B.et al Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol 20011191135–1142. [DOI] [PubMed] [Google Scholar]
- 15.Rivellese M, George A, Sulkes D.et al Optical coherence tomography after laser photocoagulation for clinically significant macular edema. Ophthalmic Surg Lasers 200031192–197. [PubMed] [Google Scholar]
- 16.McNaught E I, Foulds W S, Allan D. Grid photocoagulation improves reading ability in diffuse diabetic macular oedema. Eye 19882288–296. [DOI] [PubMed] [Google Scholar]
- 17.Olk R J. Modified grid argon laser photocoagulation for diffuse diabetic edema. Ophthalmology 198693938–950. [DOI] [PubMed] [Google Scholar]
- 18.Olk R J. Argon green (514 nm) versus krypton red (647 nm) modified grid laser photocoagulation for diffuse diabetic macular edema. Ophthalmology 1990971101–1113. [DOI] [PubMed] [Google Scholar]
- 19.Henricsson M, Heiji A. The effect of panretinal photocoagulation on visual acuity, visual field and on subjective visual impairment in preproliferative and early proliferative diabetic retinopathy. Acta Ophthalmol 199472570–575. [DOI] [PubMed] [Google Scholar]
- 20.Striph G G, Hart W M J, Olk R J. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology 1988951673–1679. [DOI] [PubMed] [Google Scholar]
- 21.Whitelock R A E, Kearns M, Blach R K.et al The diabetic maculopathies. Trans Ophthalmol Soc UK 197999314–318. [PubMed] [Google Scholar]
- 22.Hudson C, Flanagan J G, Turner G S.et al Influence of laser photocoagulation for clinically significant diabetic macular edema (DMO) on short‐wavelength and conventional automated perimetry. Diabetologia 1998411283–1292. [DOI] [PubMed] [Google Scholar]
- 23.Kang S W, Sa H S, Cho H Y.et al Macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema. Acta Ophthalmol 2006124653–658. [DOI] [PubMed] [Google Scholar]
- 24.Peyman G A, Spitznas M, Straatsma B R. Peroxidase diffusion in the normal and photocoagulated retina. Invest Ophthalmol Vis Sci 197110181–189. [PubMed] [Google Scholar]
- 25.Peyman G A, Spitznas M, Straatsma B R. Chorioretinal diffusion of peroxidase before and after photocoagulation. Invest Ophthalmol Vis Sci 197110489–495. [PubMed] [Google Scholar]
- 26.Peyman G A, Bok D. Peroxidase diffusion in the normal and laser‐coagulated primate retina. Invest Ophthalmol Vis Sci 19721135–45. [PubMed] [Google Scholar]
- 27.Wallow I H. Repair of the pigment epithelial barrier following photocoagulation. Arch Ophthalmol 1984102126–135. [DOI] [PubMed] [Google Scholar]
- 28.Bresnick G H. Diabetic maculopathy, a critical review highlighting diffuse macular edema. Ophthalmology 1983901301–1317. [DOI] [PubMed] [Google Scholar]
- 29.Nonaka A, Kiryu J, Tsujikawa A.et al Inflammatory response after scatter laser photocoagulation in nonphotocoagulated retina. Invest Ophthalmol Vis Sci 2002431204–1209. [PubMed] [Google Scholar]
- 30.Er H, Doganay S, Turkoz Y.et al The levels of cytokine and nitric oxide in rabbit vitreous humor after retinal laser photocoagulation. Ophthalmic Surg Lasers 200031479–483. [PubMed] [Google Scholar]
- 31.Gabel V P, Birngruber R, Hillenkamp F.Visible and near infrared light absorption in pigment epithelium and choroid. Kyoto, Japan: Excerpta Medica, 1997658–662.
- 32.Mainster M A, White T J, Tips J H.et al Retinal‐temperature increases produced by intense light sources. J Opt Soc Am 197060264–270. [DOI] [PubMed] [Google Scholar]
- 33.McDonald H R, Schatz H. Grid photocoagulation for diabetic macular edema. Retina 1985565–71. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair S, Alaniz R, Presti P. Laser treatment of diabetic macular edema: comparison of ETDRS\level treatment with threshold level treatment by using high contrast discriminant center visual field testing. Semin Ophthalmol 199914214–222. [DOI] [PubMed] [Google Scholar]
- 35.Shimura M, Yasuda K, Nakazawa T.et al Effective treatment of temporal grid pattern photocoagulation in patients with diffuse diabetic macular edema. Ophthalmic Surg Lasers Imaging 200435270–280. [PubMed] [Google Scholar]
- 36.Luttrull J K, Musch D C, Mainster M A. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular edema. Br J Ophthalmol 20058974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olk R J, Akduman L. Minimal intensity diode laser (810 nanometer) photocoagulation (MIP) for diffuse diabetic macular edema (DDME). Semin Ophthalmol 20011625–30. [DOI] [PubMed] [Google Scholar]
- 38.Luttrull J K, Spink C J. Serial optical coherence tomography of subthreshold diode micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging 200637370–377. [DOI] [PubMed] [Google Scholar]