Abstract
Background
In human albinism the plasticity of the visual system is challenged, as part of the temporal retina projects abnormally to the contralateral hemisphere.
Aim
To investigate whether the sensitivity of the abnormally projecting temporal retina is preserved.
Methods
Light spot detection sensitivities were assessed in the central 20° of the visual field in 15 patients with albinism and electrophysiologically determined extent of the retinal projection abnormality, and in 6 controls. The sensitivities were determined monocularly with static white‐on‐white perimetry using Octopus 101.
Results
In the patients with albinism, the sensitivity of the abnormally projecting part of the temporal retina was not selectively reduced. Apart from the vicinity of the papilla, there was no significant sensitivity difference between nasal and temporal retina in both patients with albinism and controls.
Conclusions
While in the present study the papilla‐induced scotoma was detected in those with albinism tested, there was no indication of a selective visual field defect induced by the projection abnormality. This contrasts with the selective visual field defects observed in some animal models of albinism, and indicates that, in humans, mechanisms of cortical self‐organisation make the abnormal representation available for visual perception.
In humans, the nasal hemiretina normally projects to the contralateral hemisphere, whereas the temporal hemiretina projects ipsilaterally. Consequently, the line of decussation that divides crossed from uncrossed fibres coincides with the vertical meridian through the fovea. This normal projection of optic nerve fibres is severely altered in albinism, in which the line of decussation is shifted into the temporal retina, such that a great number of fibres from the temporal retina cross the midline and project contralaterally.1,2,3,4,5,6,7 As a consequence of this projection abnormality, the primary visual cortex receives, in addition to the normal input from the contralateral hemifield, abnormal input from the ipsilateral hemifield. This abnormal visual field representation makes albinism a promising model to examine mechanisms of cortical self‐organisation in humans.
How and to what extent is the visual function of the abnormal cortical visual field representation preserved in albinism? In some albino animals, the abnormal projection of the temporal retina results in a visual field defect.8,9 This indicates that cortical self‐organisation does not always make the abnormal representation available for visual processing. A few studies have assessed visual fields in humans with albinism.10,11,12 They found that visual fields can be contracted,11,12 and that the sensitivity of the visual field centre can be reduced10,12; the extent of both features varies between subjects. In one of these studies, the sensitivities of the nasal and the temporal retina were compared with each other to assess the effects of the misrepresentation of the temporal retina in albinism on visual performance. A reduced contrast sensitivity of the temporal retina for grating detection was reported in a subset of the individuals tested.11 In this study, the part of the temporal retina that erroneously projects to the contralateral hemisphere was not identified, which hampers the interpretation of this study: as the extent of the projection abnormality associated with albinism varies greatly between subjects, it is important to determine whether the part of the retina under investigation is actually affected by the abnormality.13 To deal with this issue, we measured the retinal sensitivities along the horizontal meridian using static perimetry in subjects with albinism, in whom the horizontal extent of the projection abnormality had previously been quantified with visual evoked potentials.13 Thus, we were able to test whether potential sensitivity differences of the temporal and nasal retina are related to the extent by which the line of decussation is shifted into the temporal retina.
Methods
Subjects
In all, 15 subjects with albinism (aged 21–71 years; misrouting confirmed with visual evoked potentials (VEPs)13,14) and 6 subjects with visual acuity >1.0 (controls; age range 22–29 years) were investigated. All subjects gave their informed written consent before the study. The procedures followed the tenets of the Declaration of Helsinki,15 and were approved by the ethics committee of the University of Freiburg, Germany.
In the 15 subjects with albinism, monocular visual field perimetry was conducted for each eye. The subjects had previously taken part in an investigation of the extent of the projection abnormality:13 with visual evoked potentials the central ±27° of the horizontal meridian was sampled for the abnormality associated with albinism within a vertical aperture of ±12°. The VEPs indicated that the misprojection was limited to the central part of the retina. Its extent varied between subjects, the border between the crossed and uncrossed projection—that is, the line of decussation—was shifted by 2°–15° from the retinal midline into the temporal retina (median 8°). As spontaneous nystagmus was evident in most subjects with albinism, horizontal nystagmus amplitude was determined monocularly for each eye using the horizontal electro‐oculogram obtained during the VEP recordings of the previous study.13 Horizontal nystagmus amplitudes ranged from ⩽0.4° to 10.6° (median 3.5°).
Perimetry
Light spot detection thresholds were determined with automated static white on white perimetry (Octopus 101 Perimeter; Haag‐Streit, Koeniz, Switzerland) using a standard paradigm customised to include the position of the blind spot (background luminance: 4 asb; target size: Goldmann size III (0.4° diameter); target exposition: 100 ms). At 75 visual field positions, evenly distributed in the visual field (25°) with 5.5° spacing, detection thresholds were determined with the dynamic strategy16 incorporated in Octopus 101. Threshold sensitivity is given as S[dB] = 10×log(Lmax/Lstimulus), where Lmax equals 1000 asb. The subjects wore their prescribed refractive correction and looked straight ahead during testing. The reliability factor of the perimetric measurements—that is, the percentage of false positives and false negatives in the catch trials—never exceeded 17% (median 0% in controls and subjects with albinism). For a direct comparison with age‐matched controls, we used the reference data supplied with Octopus 101. In these reference data, a measure for the retinal sensitivity at the location of the actually blind papilla is provided by a linear interpolation of the sensitivities of the locations neighbouring the papilla. Thus, the papilla‐related sensitivity reduction is compensated for in the reference data. Consequently, the papilla‐induced scotoma can be assessed from the sensitivity reduction relative to the reference data. Further, it should be noted that the terminology nasal and temporal refers to the retina and not to the visual field; thus, we use the term “papilla” instead of “blind spot”.
Results
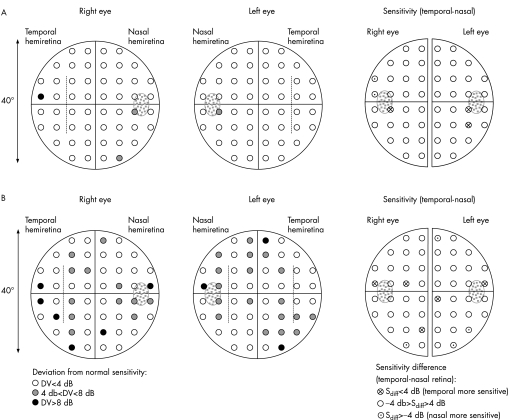
Figure 1 depicts the data of two patients with albinism as a projection of the visual field sensitivities onto the retina. In the left‐hand panels the deviations (DVs) of the visual field sensitivities from age‐matched reference data supplied with Octopus 101 are shown. In the right‐hand panel, sensitivity differences of the temporal and nasal retina are shown, which allows one to assess whether the sensitivity of the temporal retina is selectively reduced. In fig 1A, data are presented for a subject with a low nystagmus amplitude (<1.5°) and a visual acuity of 0.5, which is high in comparison to the average of our albinism group (visual acuity17: range 0.1–0.6; average 0.2). Only few visual field deficits (DV>4 dB) are evident—that is, in the vicinity of the papilla and some in the periphery. No selective defects correspond with the site of the pronounced projection abnormality of the temporal retina (extending 12° (left eye) and 9° (right eye) into the temporal retina; indicated by dotted lines in fig 1), as is also evident from the sensitivity differences in the right‐hand panel of fig 1A. In a subject with lower visual acuity—that is, 0.2, and horizontal nystagmus of >3.5° (fig 1B), scattered sensitivity reductions are evident, but again, no selective defects correspond to the misprojecting temporal retina (see the right‐hand panel with sensitivity differences). These features are assessed below in a quantitative analysis.
Figure 1 Retinal sensitivities in two patients with albinism. (A) Subject with a visual acuity of 0.5 and nystagmus amplitude <1.5°; (B) subject with a visual acuity of 0.2 and nystagmus amplitude >3.5°. In the left‐hand panels the deviation (DV) of the sensitivities from age‐matched reference data is given. An estimate of the horizontal extent of the abnormally projecting temporal retina is indicated by dotted lines. In the right‐hand panel, the sensitivity differences of temporal and nasal retina are given for each eye. Reduced sensitivities are evident, particularly in the nasal retina in the vicinity of the papilla (dotted in grey). No selective visual field defect of the abnormal temporal retina is indicated.
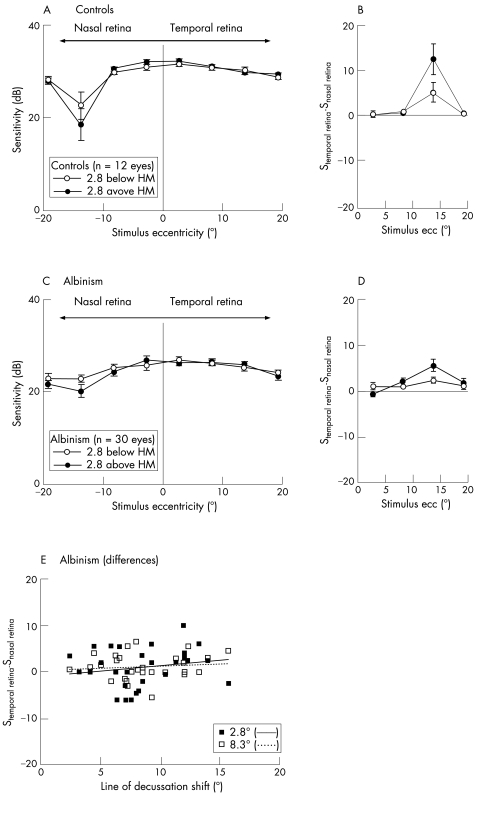
In fig 2A,C, the mean visual field sensitivities to light spots along the retinal horizontal meridian (ie, at +2.8° and −2.8° elevations) are given for 12 control eyes as a reference and for the group of subjects with albinism. As expected, the sensitivities are reduced for both groups in the vicinity of the papilla—that is, at the nasal retina at around 14° eccentricity, particularly above the horizontal retinal meridian. Although the papilla induces an absolute scotoma, the average sensitivity did not drop to or tend to 0, as the test stimulus did not coincide with the papilla in all subjects.
Figure 2 Mean sensitivities (±SEM) above and below (2.8°) the horizontal retinal meridian of 12 control eyes (A) and 30 eyes of 15 subjects with albinism (C). (B,D) Sensitivity differences of mirror‐symmetrical positions in the nasal and temporal retina. In both groups, a reduction in the sensitivity is evident in the nasal retina at around 14°, near the papilla. The sensitivity of the temporal retina is not specifically reduced in the patients with albinism. (E) Sensitivity differences versus shift of the line of decussation in patients with albinism for two stimulus eccentricities below the horizontal retinal meridian. No significant dependence of the sensitivity differences on the extent of misrouting is evident.
Visual field sensitivities are affected by a number of factors—for example, nystagmus, potential residual refractive errors, age and visual acuity.10,18 Caution should therefore be exercised during the direct comparison of the control group and the group of subjects with albinism. The problem can be dealt with by the use of an internal reference for each eye. We obtained a measure of a relative reduction in the retinal sensitivity by calculating the sensitivity difference between the temporal and nasal retina at locations that were mirror‐symmetrical across the vertical meridian. Figure 2B,D depicts the data for the resulting sensitivity differences along the horizontal meridian, where positive values indicate a relative reduction in the sensitivity of the nasal retina, and negative values a relative reduction in the sensitivity of the temporal retina. The sensitivity differences depended on eccentricity (p<0.001; analysis of variance), but post‐hoc tests showed that there were no significant differences between mirror‐symmetrical positions of the nasal and the temporal retina apart from the vicinity of the papilla (above the retinal horizontal meridian at 14° of eccentricity; p<0.01; Student–Newman–Keuls post hoc test), where the sensitivity of the nasal retina is, as a matter of course, reduced for both subjects with albinism and controls. This also applies if the four eyes with a particularly small misrouting (<5°) are excluded from the analysis. To further assess the influence of the size of the shift of the line of decussation on the sensitivity, the sensitivity differences at eccentricities of 2.8° and 8.3° are depicted in fig 2E as a function of the shift of the line of decussation, which had previously been determined electrophysiologically13 (see subsection Subjects under Methods). In a multiple regression analysis, no significant relationship was found, either for the sensitivity differences of the temporal retina below (fig 2E) or above the retinal horizontal meridian (data not shown). This also applies if only the subjects' leading eyes are included in the analysis.
The reduced sensitivity in the vicinity of the representation of the papilla in subjects with albinism and in controls suggests that the data were not severely confounded by the nystagmus. To assess the effect of the nystagmus on the measured sensitivities in more detail, we compared the sensitivity differences of the two hemiretinae for two subgroups, one with a small and the other with a large horizontal nystagmus amplitude (<4° (n = 19) and >4° (n = 11), respectively), and did not find any significant difference (average across subjects, above and below the horizontal meridian, inner two eccentricities (standard error of mean) for nystagmus <4° v >4°: 1.05 (0.68) v 0.88 (0.423) dB; p = 0.8 (Student's t test)). Further, we tested the dependence between nystagmus amplitude and the sensitivity differences using multiple regression analysis, which allows for the separate assessment of each test location (4 locations 2.8° above and below the horizontal meridian at 2.8° and 8.3° from the visual field centre). No significant regression model fit the data (p>0.41), which indicates that there was no significant correlation between the nystagmus amplitudes and the sensitivity differences at the test locations.
Discussion
We used static perimetry to determine retinal sensitivities in subjects with albinism. No selective visual field defects corresponding to the abnormally projecting temporal retina were evident from our data. Furthermore, in both subjects with albinism and controls (present study, and Fahle and Schmid19), no reduction in the sensitivity of the central temporal retina relative to the central nasal retina was present. Our data therefore indicate that simple visual tasks, such as light spot detection in static perimetry, are not selectively impaired by the misrouting associated with albinism.
Normally, the primary visual cortex receives input from its contralateral visual hemifield, which is organised as a retinotopic map. In albinism, there is additional input from the ipsilateral visual field, and it is not a matter of course that this additional input can be used for visual perception. To make this abnormal input available for visual perception, it has to be accommodated in the visual cortex in addition to the normal retinotopic map of the contralateral visual field. From animal studies, evidence emerged for two principally different ways in which the additional input can be organised20:
(a) The additional input can be organised as a separate retinotopic map, which is located adjacent to the map of the normal input in the primary visual cortex. Thus, the entire input from the contralateral eye is organised as one contiguous retinotopic map, that comprises not only the contralateral but also part of the ipsilateral visual field. In this arrangement, the information from opposing visual hemifields is spatially separated on the visual cortex.
(b) The additional input can be organised as a retinotopic map, which is interleaved with the normal retinotopic map.21,22 In this arrangement of interleaved retinotopic maps, information from opposing hemifields is represented in close vicinity on the visual cortex, and mechanisms to avoid sensory conflicts are expected to be in place. Indeed, there is electrophysiological3 and behavioural evidence8,9 that in some animal models of albinism such conflicts are solved by a complete suppression of the abnormal input, which can result in a visual field defect.8,9
This stands in contrast with the situation in other models, particularly in non‐human and human primates.21,22,23 In these models, electrophysiological and functional magnetic resonance imaging studies have shown profound cortical activations due to the abnormal input from the ipsilateral temporal retina.13,23 The present study complements this picture, showing that in human albinism the abnormal representation is actually made available for visual perception.
We showed that in a light spot detection task the sensitivity of the temporal retina is not reduced in comparison with that of the nasal retina. An earlier study suggested that the contrast sensitivity of the temporal retina can be reduced in some subjects with albinism in a grating detection task.11 This implies that more complex tasks, which require an integration of information from neighbouring representations in the visual cortex, such as motion perception and pattern detection, might be affected by the abnormal cortical representation in albinism. Detailed studies are required to investigate whether tasks that require the spatial integration of information are selectively impaired by the representation abnormality associated with albinism.
Acknowledgements
We thank Guntram Kommerell for his comments, and the participants for their cooperation. This work was supported by the German Research Council (DFG HO‐2002/4‐1).
Abbreviations
VEP - visual evoked potential
Footnotes
Competing interests: None declared.
References
- 1.Lund R C. Uncrossed visual pathways of hooded and albino rats. Science 19651491505–1507. [DOI] [PubMed] [Google Scholar]
- 2.Creel D J. Visual system anomaly associated with albinism in the cat. Nature 1971231465–466. [DOI] [PubMed] [Google Scholar]
- 3.Kaas J H, Guillery R W. The transfer of abnormal visual field representations from the dorsal lateral geniculate nucleus to the visual cortex in Siamese cats. Brain Res 19735961–95. [DOI] [PubMed] [Google Scholar]
- 4.Guillery R W, Okoro A N, Witkop C J., Jr Abnormal visual pathways in the brain of a human albino. Brain Res 197596373–377. [DOI] [PubMed] [Google Scholar]
- 5.Hedera P, Lai S, Haacke E M.et al Abnormal connectivity of the visual pathways in human albinos demonstrated by susceptibility‐sensitized MRI. Neurology 1994441921–1926. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz B, Kasmann‐Kellner B, Schafer T.et al Monocular visual activation patterns in albinism as revealed by functional magnetic resonance imaging. Hum Brain Mapp 20042340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morland A B, Hoffmann M B, Neveu M.et al Abnormal visual projection in a human albino studied with functional magnetic resonance imaging and visual evoked potentials. J Neurol Neurosurg Psychiatry 200272523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elekessy E I, Campion J E, Henry G H. Differences between the visual fields of Siamese and common cats. Vision Res 1973132533–2543. [DOI] [PubMed] [Google Scholar]
- 9.Garipis N, Hoffmann K P. Visual field defects in albino ferrets (Mustela putorius furo). Vision Res 200343793–800. [DOI] [PubMed] [Google Scholar]
- 10.Abadi R V, Pascal E. Incremental light detection thresholds across the central visual field of human albinos. Invest Ophthalmol Vis Sci 1993341683–1690. [PubMed] [Google Scholar]
- 11.St John R, Timney B. Sensitivity deficits consistent with aberrant crossed visual pathways in human albinos. Invest Ophthalmol Vis Sci 198121873–877. [PubMed] [Google Scholar]
- 12.Edmunds R T. Vision of albinos. Arch Ophthalmol 194942755–767. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann M B, Lorenz B, Morland A B.et al Misrouting of the optic nerves in albinism: estimation of the extent with visual evoked potentials. Invest Ophthalmol Vis Sci 2005463892–3898. [DOI] [PubMed] [Google Scholar]
- 14.Apkarian P, Reits D, Spekreijse H.et al A decisive electrophysiological test for human albinism. Electroencephalogr Clin Neurophysiol 198355513–531. [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 20002843043–3045. [PubMed] [Google Scholar]
- 16.Weber J, Klimaschka T. Test time and efficiency of the dynamic strategy in glaucoma perimetry. Ger J Ophthalmol 1995425–31. [PubMed] [Google Scholar]
- 17.Bach M. The Freiburg Visual Acuity Test—automatic measurement of visual acuity. Optometry Vision Sci 19967349–53. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe G J, Alvarado J A, Juster R P. Age‐related changes of the normal visual field. Arch Ophthalmol 19861041021–1025. [DOI] [PubMed] [Google Scholar]
- 19.Fahle M, Schmid M. Naso‐temporal asymmetry of visual perception and of the visual cortex. Vision Res 198828293–300. [DOI] [PubMed] [Google Scholar]
- 20.Guillery R W. Neural abnormalities in albinos. Trends Neurosci 198618364–367. [Google Scholar]
- 21.Leventhal A G, Creel D J. Retinal projections and functional architecture of cortical areas 17 and 18 in the tyrosinase‐negative albino cat. J Neurosci 19855795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillery R W, Hickey T L, Kaas J H.et al Abnormal central visual pathways in the brain of an albino green monkey (Cercopithecus aethiops). J Comp Neurol 1984226165–183. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann M B, Tolhurst D J, Moore A T.et al Organization of the visual cortex in human albinism. J Neurosci 2003238921–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]