Abstract
Background
This study was performed to determine clinical features of dysthyroid optic neuropathy (DON) across Europe.
Methods
Forty seven patients with DON presented to seven European centres during one year. Local protocols for thyroid status, ophthalmic examination and further investigation were used. Each eye was classified as having definite, equivocal, or no DON.
Results
Graves' hyperthyroidism occurred in the majority; 20% had received radioiodine. Of 94 eyes, 55 had definite and 17 equivocal DON. Median Clinical Activity Score was 4/7 but 25% scored 3 or less, indicating severe inflammation was not essential. Best corrected visual acuity was 6/9 (Snellen) or worse in 75% of DON eyes. Colour vision was reduced in 33 eyes, of which all but one had DON. Half of the DON eyes had normal optic disc appearance. In DON eyes proptosis was > 21 mm (significant) in 66% and visual fields abnormal in 71%. Orbital imaging showed apical muscle crowding in 88% of DON patients. Optic nerve stretch and fat prolapse were infrequently reported.
Conclusion
Patients with DON may not have severe proptosis and orbital inflammation. Optic disc swelling, impaired colour vision and radiological evidence of apical optic nerve compression are the most useful clinical features in this series.
Dysthyroid optic neuropathy (DON) is impairment of optic nerve function due to Graves' orbitopathy (GO).1,2,3 This potentially blinding complication occurs in up to 5%2 of patients with GO. While often obvious at presentation, the diagnosis may be missed in patients without obvious proptosis. Orbitopathy occurs before, during or after the onset of hyperthyroidism and, less frequently, in euthyroid or hypothyroid patients.2
As controversy exists in assessment4,5,6 and management7,8,9 of DON there is a need to evaluate and improve diagnostic criteria for this syndrome. EUGOGO aims to standardise clinical assessment10 in order to perform multi‐centre trials. Each team comprises an ophthalmologist and an endocrinologist running a joint clinic for patients with GO. This survey aimed to identify clinical features of DON in specialist units managed by members of EUGOGO.
Patients and methods
Patients considered to have features suspicious of DON were included in the study. Each centre receives untreated or partially treated referrals who may require treatment urgently before all assessments are complete. New cases of DON were identified using centre‐specific clinical practice criteria and each eye was categorised as follows:
(i) Definite DON ‐ diagnosis was considered certain
(ii) Equivocal DON ‐ diagnosis was likely but not certain
(iii) No DON ‐ no evidence to support the diagnosis.
All patients with suspected or definite DON in at least one eye were recruited over a 12 month period; clinical information was entered onto a standard pro forma and ophthalmic co‐morbidity recorded.
Clinical assessment
A clinical activity score (CAS)1,11,12 was obtained by assessing two symptoms (orbital ache and gaze‐evoked pain) and five signs (conjunctival redness, eyelid erythema, eyelid oedema, chemosis and swelling of the plica or caruncle) for each eye. Patients were also asked if they had excessive watering, photophobia, grittiness, double vision and blurred vision.
Best corrected visual acuity (BCVA) was expressed as a Snellen fraction and colour vision was tested using Ishihara colour plates. The presence of a relative afferent pupillary defect (RAPD) was noted.
Ocular motility was assessed with regard to manifest strabismus and monocular ductions were measured in degrees of excursion from the primary position. The severity of diplopia was evaluated using a Gorman13 score: no diplopia (1), intermittent diplopia (2), inconstant (gaze‐evoked) diplopia (3) or constant diplopia in the primary position or on reading (4).
Keratopathy, superior limbic keratoconjunctivitis or corneal ulceration at slit lamp examination was documented. Observers had to choose whether the optic disc was normal, oedematous or pale and record any choroidal folds.
Proptosis and eyelid position were recorded. Proptosis measurements were performed with a wide variety of exophthalmometers including the Oculus, Keeler, Zeiss and the Inami.
Imaging modalities included computed tomography and magnetic resonance imaging (MRI) and were performed at different centres on different manufacturer's equipment with no predetermined protocol. Both axial and coronal computed tomography imaging were available using a thin slice thickness and the images were reviewed on both soft tissue and bone windows. The MRI protocol included T1W axial and coronal slices with short inversion time recovery or T2W Fat Saturation sequences. The observer reported whether increases in orbital tissue volume were mainly muscle or fat. If it was fat, the observer reported any optic nerve stretch; if it was muscle, apical muscle crowding was assessed.
Specialised investigations included visual field examination (static automated perimetry or Goldmann) and visual evoked potential (VEP).
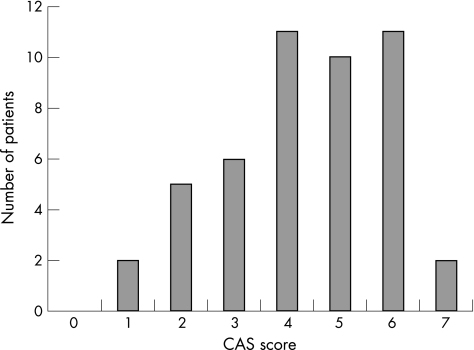
Figure 1 Clinical Activity Score in DON
Results
Forty seven patients (32 females, 15 males), mean age 56 years (range 31–81 years) were recruited. Graves' thyrotoxicosis was present before or at the time of diagnosis of GO in 44 patients. In addition to antithyroid drugs, treatment with radioiodine(14%) and thyroidectomy(18%) was noted. Three patients were taking thyroxine for primary hypothyroidism. Two thirds of the patients had FT3, FT4 and TSH in the normal range; hypo‐ and hyperthyroidism occurred equally among the remainder. Thyroid peroxidase antibodies and Thyrotrophin receptor antibodies (measured by binding inhibition assay) were positive in 27 of 28 and 30 of 32 patients tested respectively. A family history of thyroid disease was present in 10 patients. There were 24 cigarette smokers and 10 ex‐smokers in the group. Three patients had type II diabetes mellitus.
Twenty six patients received oral steroids prior to presentation and eight were taking them at the time of the study. Seven patients received orbital irradiation prior to study entry and four had undergone prior decompression surgery.
Ninety four eyes were assessed; 55 had definite DON while 17 were considered to have equivocal DON. Of these 72 eyes, 40 were right eyes and 32 were left. The patients comprised two groups:
(a) Unilateral DON
Twenty two patients had unilateral DON, six of whom had equivocal features. One of the equivocal cases only had unilateral Graves' orbitopathy. The 22 unaffected eyes of patients with unilateral DON formed a “no DON” group.
(b) Bilateral DON
DON was considered to be bilateral and symmetrical in 18 patients, two of whom had equivocal features. In addition DON was definite on one side and equivocal on the other in seven patients.
The clinical features and test results in each group are presented in table 1.
Table 1 The frequency of an abnormal finding is expressed as a fraction of the total number of eyes in the group that were tested.
| Eyes with definite DON | Eyes with equivocal DON | Eyes with no DON | Eyes reported | |
|---|---|---|---|---|
| Reduced colour vision | 23/30 (77%) | 9/16 (56%) | 1/15 (7%) | 61 |
| Visual acuity ⩽0.67 | 44/55 (80%) | 10/17 (59%) | 7/22 (32%) | 94 |
| Apical crowding | 40/42 (95%) | 9/14 (64%) | 6/14 (43%) | 70 |
| Optic disc swelling | 30/54 (56%) | 3/17 (18%) | 1/22 (5%) | 93 |
| Optic disc pallor | 2/54 (4%) | 2/17 (12%) | 0/22 (0%) | 93 |
| Visual field defects | 30/42 (71%) | 10/14 (71%) | 2/16 (13%) | 72 |
| Abnormal VEP latency | 8/11 (73%) | 6/7 (86%) | 0/4 (0%) | 22 |
| amplitude | 6/11 (55%) | 4/7 (57%) | 0/4 (0%) | 22 |
| Proptosis >21 mm | 33/53 (62%) | 13/16 (81%) | 12/19 (63%) | 88 |
| Optic nerve stretch | 5/15 (33%) | 0/5 (0%) | 3/7 (43%) | 27 |
| RAPD | 15/33 (45%) | 2/5 (40%) | N/A | 76 |
| Clinical Activity Score ⩾3 | 31/51 (61%) | 6/17 (86%) | 7/17 (41%) | 85 |
| Elevation < 30° | 36/51 (71%) | 13/17 (76%) | 11/21 (52%) | 89 |
| Depression < 30° | 9/50 (18%) | 2/17 (12%) | 0/19 (0%) | 86 |
| Abduction < 30° | 15/45 (33%) | 2/16 (12.5%) | 1/20 (5%) | 81 |
| Adduction < 30° | 7/47 (15%) | 0/17 (0%) | 3/20 (15%) | 84 |
Colour vision was tested in 30 eyes with definite DON and was reported to be reduced in 23.
Ocular co‐morbidity was present in 13 patients, two of whom had dual pathology. There were three cases of glaucoma, five of cataract, three of amblyopia; one corneal ulcer and a hypopyon which prevented visualisation of the optic disc; one case of macular drusen, one with a previous branch retinal vein occlusion and one patient with optic nerve head drusen.
The CAS score varied from 1 to 7 (median 4) (fig1). Although skewed towards higher values, 28% of patients had scores of 3 or less.
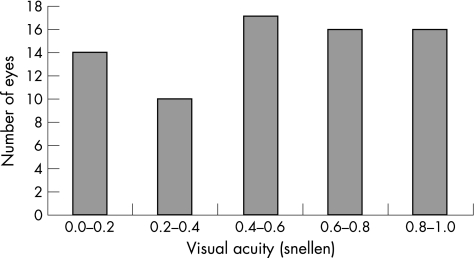
The median BCVA of eyes with DON at enrolment was 0.4 (range 0.0–1.0) (fig 2). In eyes with recorded Snellen fractions of 1.0 (6/6), five were considered to have DON and six were equivocal cases.
Figure 2 Best corrected visual acuity in DON
Colour vision assessment was available in 33 patients (61 eyes). In 33 eyes with abnormal colour vision, DON was considered to be present in all but one: definite in 23 and equivocal in nine. Conversely, colour vision was normal in 14 out of 15 eyes with no DON.
There were 15 patients with an RAPD and one patient with a bilateral afferent pupillary defect. In two cases with an RAPD, DON was bilateral. In 13 patients an RAPD was associated with definite DON on that side and either no or equivocal DON on the other side
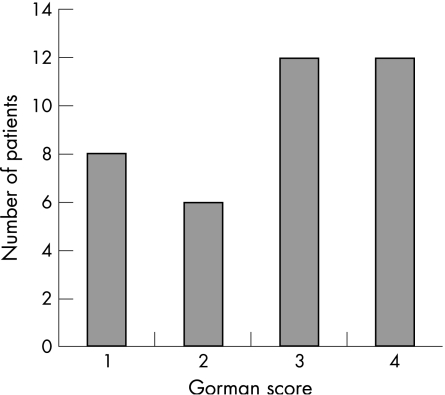
The Gorman diplopia scores were available in 38 patients (fig 3). A high proportion of patients had inconstant (gaze‐evoked) diplopia or constant diplopia in the primary position or on reading (score 3 or 4). The Gorman score distribution was very similar for unilateral compared with bilateral DON. Elevation was restricted to 30° or less in 49 out of 68 (72%) of eyes with DON, compared with the same degree of restriction in abduction in 17 out of 61 (28%), adduction in seven out of 64 (11%) and depression in 11 out of 67 (16%).
Figure 3 Gorman Diplopia Score
Keratopathy was found in 20 patients, 16 of whom had DON and corneal ulceration in three, all of whom had definite DON. Superior limbic keratoconjunctivitis was seen in seven eyes, six of which had DON. Thirty eyes had disc oedema and four had simultaneous disc pallor. Optic disc appearances were normal in 55% of the eyes with DON and 20 out of 21 (95%) of eyes with no DON. One equivocal case showed optic nerve head drusen and choroidal folds were noted in only one eye.
Mean proptosis was 22.1 mm on the right (range 13–30 mm, SD 4.2 mm) and 22.1 mm on the left (range 14–29 mm, SD 3.9 mm) with no significant gender difference. Proptosis measured 21 mm or less in 18 out of 49 eyes with definite DON.
Imaging at presentation was performed in 44 patients. Computed tomography was performed in 42 and was the only form of imaging in 34. Only two patients had MRI performed alone and six had an MRI together with computed tomography. One patient had orbital ultrasound and one had an octreoscan, both in conjunction with computed tomography. Bilateral muscle enlargement occurred in 37 patients and apical muscle crowding in 49 out of 56 orbits with DON, bilaterally in 28/44 patients. In contrast, optic nerve stretch was observed bilaterally in two patients and unilaterally in only one; these patients had symmetrical proptosis measurements of 23 mm and 25 mm respectively. Volume increase was predominantly fat in only four patients. Two patients had predominantly fat increase on one side and muscle increase on the other. Fat prolapse through the superior orbital fissure was assessed in 40 orbits. It was seen in only 10, all of which had DON.
Visual fields were abnormal in 42 eyes, all assessed by static automated perimetry. Of 72 DON eyes, visual field was abnormal in 40 out of 56 tested. In eyes without DON visual field was abnormal in two patients, neither of whom had ocular co‐morbidity to explain their visual field loss.
VEPs were performed in 13 patients. In 18 eyes with DON the VEP was of abnormal amplitude in 10 and abnormal latency in 14. One patient with a normal VEP had bilateral DON, but had severe reduction in visual acuity to 0.05 bilaterally rendering the VEP testing inaccurate. Another patient with bilateral DON had a normal VEP test in one eye (visual acuity 0.4, abnormal colour vision and disc oedema).
Discussion
This series of DON cases was prospectively and simultaneously collected from European centres using a previously agreed pro forma. Despite this agreement the recorded details varied between units. This may reflect the complexity of dysthyroid optic neuropathy (DON) and the variability of presenting symptoms and signs of the condition. As an example of this complexity, while the median CAS score in this series was 4/7, it was 3 or less in more than 25% of patients with DON. Indeed, the diagnosis is not always clear. A previous series8 has reported only moderately intense soft tissue signs in DON patients. Twelve eyes with a CAS of 4 or less had a visual acuity of 0.5 or less.
Visual acuity was “normal” (Snellen fraction of 1.0, that is 6/6) in 12 out of 46 eyes with DON meaning that normal visual acuity did not preclude the diagnosis. However some of these patients previously could have recorded a visual acuity of 1.5 and a visual acuity of 1.0 therefore represented a deterioration of 2 lines on the Snellen chart.
In this patient cohort colour vision testing was not always recorded but results indicate that abnormal colour vision is an important positive finding. Ishihara colour plates are readily checked even in the “out of hours” clinical situation. However an alternative test, such as the Hardy‐Rand‐Rittler14 is probably a more reliable test of optic nerve function. Only one patient with definite bilateral DON had normal colour vision (and visual acuity of 1.0) in both eyes the diagnosis being based on the presence of bilateral disc oedema, abnormal visual fields (Humphrey 24–2) and computed tomography scan evidence of apical muscle crowding.
Restriction of elevation in 72% of eyes with DON was a striking abnormality which could relate to splinting of upgaze by an enlarged inferior rectus or medial rectus muscle; the remaining ductions were less affected. Symptomatic diplopia was common, but not a prerequisite for DON.
Optic disc appearance was normal in 55%, a trait which has previously been noted.15 However, the proportion of normal discs in eyes without DON was extremely high (95%), indicating that optic disc swelling, if present, is a very specific indicator of DON. The pro‐forma gave examiners the choice of normal, swollen or atrophic. The combination of atrophy and swelling due to chronic optic nerve compression could conceivably result in a normal appearance, possibly accounting for one or two of the “normal” appearing optic discs.
Males and females have different normal exophthalmometry values.15 We found slightly higher degrees of proptosis in males with DON, but these were not significant (mean 22.8 mm males, mean 21.8 mm females). Proptosis measurements alone did not correlate with the presence of DON and many patients with DON were not significantly proptosed. Twenty eyes with DON had exophthalmometry values of 20 mm or less compared with 13 eyes with values more than 25 mm. The absence of severe proptosis in many DON patients has been previously reported.16 Our series confirms this finding in European patients from a wide geographic area. Lack of proptosis could increase the orbital pressure, precipitating DON in some cases.
Computed tomography scanning demonstrated apical muscle crowding in 78 out of 94 eyes. In a previous study of computed tomography imaging in DON4 apical muscle crowding and intracranial fat prolapse were related to DON as independent variables. In the same paper, proptosis measurements taken from computed tomography did not correlate with the presence of DON. Computed tomography remains the preferred investigation in DON patients as it detects both apical muscle crowding and fat prolapse and assists surgical planning for decompression surgery. MRI, was not performed as regularly in this series. The rate of reporting the presence or absence of optic nerve stretch (27/94) and orbital fat prolapse through the supra‐orbital fissure (40/94) was low and this probably reflects uncertainty about identifying these features on computed tomography or MRI scans.
Static perimetry, the commonest form of visual field analysis, was abnormal in at least 71% of eyes with DON when it was tested. As there is a learning curve for this test, and an abnormal result must be taken in the context of other findings.
Visual evoked potentials (VEPs) have previously been reported to be useful in both diagnosis and monitoring of DON.17 In this study VEPs were performed in less then a third of patients, reflecting variation in facilities rather that the efficacy of the test. Results were abnormal in 8 out of 11 definite cases of DON. However, all the “no DON” eyes which were tested had normal VEPs, suggesting high specificity. Some cases of DON can be refractory to treatment and can present or recur despite oral steroids, radiotherapy or decompression. Previous treatment may have affected the CAS score but it did not prevent DON.
Conclusion
This study sought to identify clinical features leading to a diagnosis of DON in EUGOGO centres across Europe. Optic disc swelling and impaired colour vision and radiological evidence of apical optic nerve compression were frequently present when a diagnosis of DON was made. Fat prolapse through the superior orbital fissure and an abnormal VEP were also linked to a diagnosis of DON but were reported in less than a quarter of the series. Neuro‐radiological expertise in this field is required to diagnose fat prolapse and an accurate VEP may not be accessible in all units.
We suggest that impairment of colour vision and optic disc swelling, together with radiological evidence of apical optic nerve compression, are clinical features which are frequently used to make the diagnosis of DON across Europe. These features, together with more specific tests for DON, could be evaluated to identify diagnostic criteria in future studies.
Acknowledgements
We thank Dr Margaret Hourihan, Consultant Neuroradiologist, University Hospital of Wales for imaging advice. This paper is dedicated to Dr Mark Prummel, a founder member of EUGOGO, who died in 2005.
Abbreviations
BCVA - best corrected visual acuity
CAS - clinical activity score
DON - dysthyroid optic neuropathy
EUGOGO - European Group on Graves' orbitopathy
GO - Graves' orbitopathy
MRI - magnetic resonance imaging
RAPD - relative afferent pupillary defect
VEP - visual evoked potential
References
- 1.Mourits M P, Koorneef L, Wiersinga W M.et al Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. British Journal of Ophthalmology 198973639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartalena L, Wiersinga W M, Pinchera A. Graves' ophthalmopathy: state of the art and perspectives. J Endocrinol Invest 200427295–301. [DOI] [PubMed] [Google Scholar]
- 3.Boulos P R, Hardy I. Thyroid‐associated orbitopathy: a clinicopathologic and therapeutic review. Current Opinion in Ophthalmology 200415389–400. [DOI] [PubMed] [Google Scholar]
- 4.Giaconi J A, Kazim M, Rho T.et al CT scan evidence of dysthyroid optic neuropathy. Ophthalmic Plastic and Reconstructive Surgery 200218177–182. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosio G, Ferrara G, Vitale R.et al Visual evoked potentials inpatients with Graves' ophthalmopathy complicated by ocular hypertension and suspect glaucoma or dysthyroid optic neuropathy. Doc Ophthalmol 2003 Mar 10699–104. [DOI] [PubMed] [Google Scholar]
- 6.Neigel J M, Rootman J, Belkin R I.et al Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology 1988951515–1521. [DOI] [PubMed] [Google Scholar]
- 7.Klingele T G, Hart W M, Burde R M. Management of dysthyroid optic neuropathy. Ophthalmologica 197774327–335. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuka K, Nakamura Y. Results of transmedial – canthal ethmoidal decompression for severe dysthyroid optic neuropathy. Jpn J Ophthalmol. 1999 Sep–Oct 43426–432. [DOI] [PubMed] [Google Scholar]
- 9.Soares‐Welch C V, Fatourechi V, Bartley G B.et al Optic neuropathy of Graves' disease: results of transantral orbital decompression and long term follow up in 215 patients. Am J Ophthalmol. 2003 Sep 136433–441. [DOI] [PubMed] [Google Scholar]
- 10.Prummel M F, Bakker A, Wiersinga W M.et al Multicenter study on characteristics and treatment strategies of patients with Graves' orbitopathy: The first EUGOGO experience. Eur J Endocrinol. 2003;148;491–495. [DOI] [PubMed]
- 11.Mourits M P, Prummel M F, Wiersinga W M.et al Clinical activity score as a guide to the management of patients with Graves' ophthalmopathy. Clinical Endocrinology 1997479–14. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson A J, Perros P. Controversies in the clinical evaluation of thyroid‐associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol (Oxf). 2001 Sep 55283–303. [DOI] [PubMed] [Google Scholar]
- 13.Bahn R S, Gorman C A. Choice of therapy and criteria for assessing treatment outcome in thyroid‐associated ophthalmology. Endocrinol Metab Clin North Am 198716(2)391–407. [PubMed] [Google Scholar]
- 14.Aroichane M, Pieramici D J, Miller N R.et al A comparative study of Hardy‐Rand‐Rittler and Ishihara colour plates for the diagnosis of nonglaucomatous optic neuropathy. Can J Ophthalmol. 1996 Dec 31350–355. [PubMed] [Google Scholar]
- 15.Trobe J D, Glaser J S, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Archives Ophthalmology 1978961199–1209. [DOI] [PubMed] [Google Scholar]
- 16.Mourits M P, Lombardo S H, van der Sluijs F A.et al Reliability of exophthalmometry value distribution in a healthy dutch population and in Graves' patients. An exploratory study. Orbit. 2004 Sep 23161–168. [DOI] [PubMed] [Google Scholar]
- 17.Tsaloumas M D, Good P A, Burdon M A.et al Flash and pattern visual evoked potentials in the diagnosis and monitoring of dysthyroid optic neuropathy. Eye 19948638–645. [DOI] [PubMed] [Google Scholar]