Abstract
Aim
To evaluate the distribution of fundus autofluorescence in patients with age‐related macular degeneration and choroidal neovascularisation (CNV).
Methods
Colour fundus photographs, fundus fluorescein angiograms (FFA) and fundus autofluorescence images were obtained from a group of 40 patients (43 eyes) with age‐related macular degeneration and purely classic or occult CNV. Only patients with newly diagnosed CNV and in whom autofluorescence images were obtained within 2 weeks from FFA were included. The distribution of autofluorescence was qualitatively evaluated, and the findings compared with those from colour fundus photographs and FFA.
Results
29 (67%) eyes had classic CNV and 14 (33%) had occult CNV. In 26 (90%) eyes with classic CNV, a low autofluorescence signal was detected at the site of the CNV; in 7 (50%) eyes with occult CNV, multiple foci of low autofluorescence signal were detected. Outside the area affected by the lesion, homogeneous autofluorescence was observed in most of the cases (n = 33, 77%). Similarly, homogeneous autofluorescence was commonly observed in fellow eyes (62%). A pattern of focal increased autofluorescence was rarely seen in eyes with CNV (n = 4, 9%) or in fellow eyes (n = 4, 15%). In 11 of 43 (25%) eyes, areas of increased autofluorescence, other than a pattern of focal increased autofluorescence, were detected. In four patients, autofluorescence images had been obtained before the development of CNV; in none was any increased autofluorescence detected before the formation of CNV.
Conclusions
Distinct patterns of autofluorescence were observed in eyes with pure classic and occult CNV. Increased autofluorescence was rarely seen in eyes with CNV and in fellow eyes, suggesting that increased autofluorescence, and thus, retinal pigment epithelium lipofuscin, may not play an essential part in the formation of CNV.
Choroidal neovascularisation (CNV) is the primary cause of visual loss in patients with age‐related macular degeneration (AMD). On the basis of fundus fluorescein angiography (FFA), CNV has been classified as classic (early, well‐defined hyperfluorescence and late leakage blurring the CNV margins) or occult (ill‐defined and irregular stippled hyperfluorescence or late leakage of undetermined source).1 Histopathological studies have shown that most classic CNV grows through the retinal pigment epithelium (RPE) into the subretinal space, whereas most occult CNV remains underneath the RPE.2 These histopathological findings have been replicated using optical coherence tomography (OCT) imaging.3 Although visual loss occurs more rapidly in patients with classic CNV compared with those with occult CNV,4,5 classic CNV has been shown to respond better to different modalities of treatment.6
The pathogenesis of AMD is complex. To date, accumulating evidence suggests an important role of lipofuscin in RPE cell dysfunction and cell loss and, thus, in the development of geographical atrophy (reviewed by Zarbin7). RPE lipofuscin seems to derive predominantly from incomplete digestion of outer segments of photoreceptors.8,9 Lipofuscin accumulates in the RPE with age8,10 and in a variety of retinal diseases, including AMD.11,12,13 The main fluorophore of lipofuscin is N‐retinylidene N‐retinyl ethanolamine (A2E).14 A2E inhibits lysosomal digestion of proteins, causes blue light‐mediated cell damage and may play a part in the induction of RPE apoptosis.15,16,17 The role of increased RPE lipofuscin in the development of CNV has not been investigated.
It is now possible to obtain images of fundus autofluorescence using a confocal scanning laser ophthalmoscope.18 The autofluorescence signal is believed to derive predominantly from lipofuscin in the RPE.19 Autofluorescence images have been used to study the role of RPE lipofuscin in the development of geographical atrophy.20 Limited data exist on fundus autofluorescence findings in eyes with CNV. Dandekar et al21 have recently published their findings on the distribution of autofluorescence in patients at various stages of the evolution of CNV and those with disciform lesions. They found that patients with CNV of a “recent onset” had normal areas of autofluorescence, implying that, at least initially in the course of the disorder, the RPE may be viable. Reduced autofluorescence was found in most patients with longstanding lesions at the site of the CNV.
The purpose of this study was to evaluate the distribution of autofluorescence in a selected group of patients with AMD and CNV, to gain knowledge on the pathogenesis of this condition and to investigate the possible role of RPE lipofuscin in the formation of CNV.
Methods
Patients
All patients examined at the Medical Retina Clinic, Grampian University Hospital NHS Trust, Aberdeen, UK, between July 2004 and July 2005 with 100% classic or 100% occult CNV, who had no prior treatment and in whom autofluorescence images had been obtained within 2 weeks from the FFA that had shown the presence of the CNV, were included in this study. All patients were either pseudophakic, had clear media or minimal nuclear sclerosis. A total of 75 patients with newly diagnosed CNV were imaged during this period. A total of 43 eyes from 40 patients were eligible on the basis of the above criteria and were included in this study.
This research followed the tenets of the Declaration of Helsinki and was approved by the Grampian University Hospital‐NHS Trust Local Research Ethics Committee. Informed consent was obtained from all patients after full explanation of the nature of the study.
Imaging studies
Colour fundus photographs, FFA (including stereo‐pairs) and autofluorescence images were obtained in all patients. FFA images were obtained using a digital fundus camera (Topcon IMAGEnet, Topcon, Rotterdam, The Netherlands). Autofluorescence images were obtained using a Heidelberg Retina Angiograph 2 (Haag‐Streit, Harlow, Essex), which uses an argon blue laser light with a wavelength of 488 nm for excitation and a barrier filter with a cut‐off at 500 nm to record fundus autofluorescence. Autofluorescence images were produced using a 30° field‐of‐view mode. A series of 20–25 digital images obtained and saved from each eye were averaged to reduce noise in order to produce the final autofluorescence image. Autofluorescence imaging was performed before FFA or at least 2 days after FFA. Autofluorescence images were obtained in the eyes with the CNV and in the fellow eye in each patient.
Findings on colour fundus photographs, FFA and autofluorescence images were compared. The distribution of autofluorescence was evaluated at the site of the CNV, around the CNV and outside the area affected by the CNV (background autofluorescence). Autofluorescence was defined as low (autofluorescence signal lower than background), high (autofluorescence signal higher than background) or unchanged compared with background (autofluorescence signal indistinguishable from background). The distribution of autofluorescence outside the area affected by the CNV (background autofluorescence) was classified, as previously described,22,23 as homogeneous, reticular (ill‐defined small areas of decreased autofluorescence surrounded by areas of increased autofluorescence), focal (focal areas of increased autofluorescence) or mixed (focal and reticular patterns of autofluorescence). Background autofluorescence in fellow eyes was also classified based on these criteria.
Autofluorescence images were also evaluated, and findings were described in patients in whom autofluorescence images were available before the development of CNV.
The method recommended by Altman et al24 was used to calculate the 95% confidence intervals (CI) for the proportion of eyes with different characteristics. Fisher's exact test was used to compare autofluorescence patterns in classic and occult CNV.
Results
In all, 29 women and 11 men with a median age of 78 years (mean 76 years, range 53–92 years) were included in this study. A total of 29 eyes had 100% classic CNV and 14 eyes had 100% occult CNV, on the basis of FFA findings. Three patients had bilateral CNVs (one patient with classic CNV in both eyes and two patients with classic CNV in one eye and an occult CNV in the fellow eye). Autofluorescence findings in these two groups of CNVs (100% classic and 100% occult) and in fellow eyes are described later.
Autofluorescence findings in eyes with classic CNV
In 26 eyes with classic CNV (90%; 95% CI 74 to 96), there was low autofluorescence signal at the site of the CNV (fig 1A). In 10 eyes (34%; 95% CI 20 to 53), a thin halo of increased autofluorescence signal was detected surrounding the CNV, which corresponded on FFA to a thin area of blocked fluorescence around the CNV (fig 1B). In a few cases (n = 3, 10%; 95% CI 4 to 26), autofluorescence at the site of the CNV was unchanged compared with background. Immediately surrounding the CNV, areas of variable size of low autofluorescence signal were detected in 19 eyes (66%; 95% CI 47 to 80). Only in 2 (7%) eyes were areas of high autofluorescence signal (other than the focal autofluorescence pattern) detected. These areas corresponded to areas of subretinal fluid observed on colour fundus photographs and FFA. Outside the area affected by the lesion, a homogeneous pattern of autofluorescence was observed in most of the cases (n = 21, 72%; 95% CI 54 to 85), followed by a reticular pattern of autofluorescence (n = 5, 17%, 95% CI 8 to 34). Focal increased autofluorescence (other than the ring of autofluorescence described above) was rarely detected in eyes with classic CNV (n = 3, 10%; 95% CI 4 to 26).
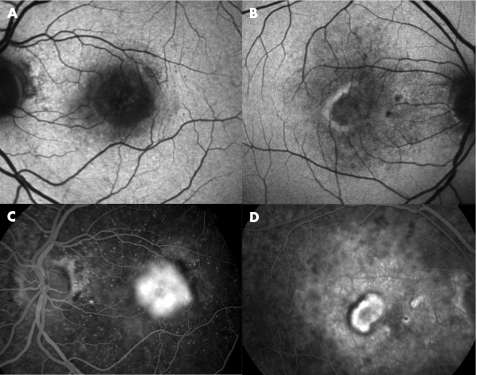
Figure 1 Fundus autofluorescence images (A,B) and corresponding fundus fluorescein angiograms (FFAs; (C,D) of two eyes with classic choroidal neovascularisation (CNV). A low autofluorescence signal is observed at site of CNV on autofluorescence images (A). A thin “halo” of increased autofluorescence surrounding the CNV is also seen (B) which corresponded to a thin area of blocked fluorescence on FFA (D).
Autofluorescence findings in eyes with occult CNV
In 7 eyes (50%; 95% CI 27 to 73) with occult CNV, there were multiple foci of low autofluorescence signals where the CNV was present (fig 2) and in 4 eyes (29%; 95% CI 12 to 55), there was a low autofluorescence signal at the site of the CNV. In the remaining eyes with occult CNV, autofluorescence was unchanged compared with background in 2 eyes (14%; 95% CI 4 to 40) and there were multiple foci of high and low autofluorescence signals in 1 eye (7%; 95% CI 1 to 31). In 9 eyes (64%; 95% CI 39 to 84), variable areas of high autofluorescence signal were detected surrounding the CNV. These areas corresponded to areas of subretinal fluid observed on colour fundus photographs and FFA in 8 of these eyes (89%; 95% CI 56 to 98). Outside the area affected by the lesion, a homogeneous pattern of autofluorescence was observed in most of the cases (n = 12, 86%; 95% CI 60 to 96). A reticular pattern of autofluorescence and focal increased autofluorescence were rarely detected in eyes with occult CNV (n = 1 for each, 7%; 95% CI 1 to 31).
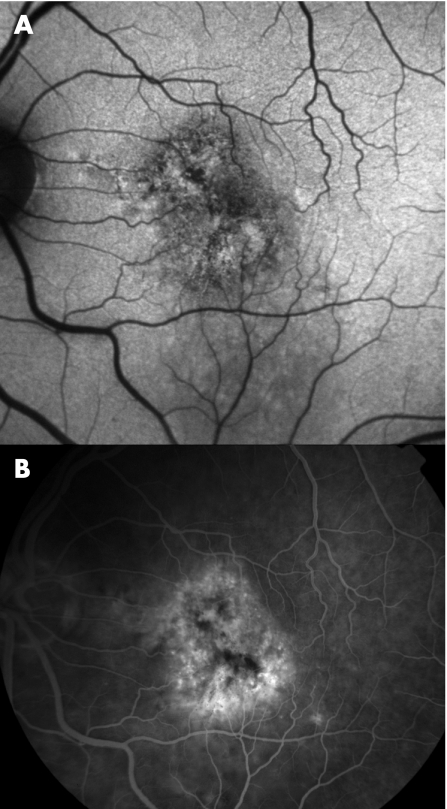
Figure 2 Fundus autofluorescence image (A), and corresponding fundus fluorescein angiograms (B) of an eye with occult choroidal neovascularisation (CNV). Foci of low autofluorescence signal are seen at the site of the CNV.
The difference in the autofluorescence pattern at the site of the CNV in eyes with classic (low autofluorescence signal in 26 of 29 cases) and occult CNV (low autofluorescence signal in 4 of 14 cases) was significant (p<0.001).
In four eyes (four patients), autofluorescence images were available before the development of CNV. In these cases, increased autofluorescence was not detected at the site where the CNV later developed (fig 3A,B), or around it.
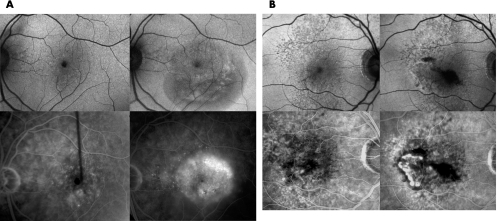
Figure 3 Fundus autofluorescence images (A,B top) and corresponding fundus fluorescein angiograms (FFAs; A,B bottom) of two eyes before (A,B left) and after (A,B right) the development of choroidal neovascularisation (CNV). Patient 1 (A): In November 2004, although a small area of blocked fluorescence was observed on FFA, which corresponded to a small retinal haemorrhage, no CNV was detected (A bottom, left). Fundus autofluorescence images at this time showed no major abnormalities (A top, left). After 3 months, an occult CNV developed (A bottom, right) and an irregular area of low autofluorescence signal was observed at the site of the CNV (A top, right). Patient 2 (B): Areas of increased fluorescence, corresponding to small window defects were observed on FFA, but no CNV was detected in November 2004 (B bottom, left). Fundus autofluorescence images at this time showed small foci of decreased autofluorescence signals (B top, left). After 5 months, a classic CNV developed (B bottom, right) and associated subretinal blood was observed. An irregular area of low autofluorescence signal was observed at the site of the CNV (B top, right) and a low autofluorescence signal corresponded to the subretinal blood.
Autofluorescence findings in fellow eyes
There were 37 fellow eyes; of these, background autofluorescence could be classified in only 26 eyes. In 11 eyes, background autofluorescence could not be classified because of the presence of large disciform scars (n = 8) and poor‐quality images (n = 3).
In fellow eyes, homogeneous autofluorescence was the most commonly observed pattern of autofluorescence (fig 4A), and was detected in 16 eyes (62%; 95% CI 43 to 78). Reticular autofluorescence (fig 4B) and areas of focal increased autofluorescence (fig 4C) were seen in 6 (23%; 95% CI 11 to 42) and 4 eyes (15%; 95% CI 6 to 33), respectively.
Figure 4 Fundus autofluorescence images of fellow eyes. Homogeneous autofluorescence (A), mixed homogeneous and reticular autofluorescence (B) or focal increased patterns of autofluorescence (C) are observed.
Discussion
A characteristic pattern of fundus autofluorescence was detected in eyes with purely classic CNV, with a well‐defined area of low autofluorescence signal at the site of the CNV. On the basis of recent histopathological and OCT studies,2,3 the low autofluorescence signal observed at the site of the classic CNV was probably related to blockage of autofluorescence caused by the CNV growing in the subretinal space, rather than being related to severe damage to the RPE by the neovascularisation process. The damage to the RPE by the neovascularisation process would be unlikely given that all patients included in this study had newly diagnosed CNV. In addition, it would be unlikely that the neovascularisation process would have destroyed the RPE as evenly as seen and shown by the autofluorescence images obtained in this study. A ring of increased autofluorescence was observed around the CNV in some cases. As it corresponded to an area of blocked fluorescence on FFA, the increased autofluorescence signal probably corresponds to proliferation of RPE cells around the CNV. Although a more variable pattern of autofluorescence was observed in eyes with purely occult CNV, which was not surprising given the more heterogeneous nature of this lesion, foci of decreased autofluorescence were commonly seen overlying occult CNV. Given that most occult CNV seems to grow underneath the RPE,2,3 and considering that occult CNV often has a chronic and more indolent course than classic CNV,4,5 the small foci of low autofluorescence signals at the site of occult CNV probably correspond to small areas of RPE damage or loss.
Dandekar et al21 suggested that the presence of normal or near normal autofluorescence over areas of CNV would indicate normal or near normal RPE and a better prognosis. On the basis of our findings, it may be difficult to establish whether or not the RPE is healthy over the CNV on the basis of autofluorescence images alone, especially if low levels of autofluorescence are found (see comments above). In this regard, imaging studies combining autofluorescence and OCT may provide important clues on the interpretation of the autofluorescence images and on the understanding of the pathogenesis of AMD.
Increased autofluorescence (other than a thin ring of increased autofluorescence around the CNV) either as a focal pattern of autofluorescence (detected in 9% of eyes) or as areas of variable size (detected in 25% of eyes) was not often observed in this study on eyes with AMD and pure classic or occult CNV. Similarly, increased autofluorescence was not detected before the development of CNV in the four patients examined. This contrasts with findings in eyes with geographical atrophy, in which areas of increased autofluorescence are commonly seen.20 In a review of autofluorescence images of 28 consecutive eyes with AMD and early geographical atrophy detected clinically, areas of increased autofluorescence were often found (20 eyes, 71%; data not shown; fig 5). Furthermore, in eyes with geographical atrophy, areas of increased autofluorescence may become areas of low autofluorescence signal over time, suggesting that increased autofluorescence levels in the RPE may presage the development of geographical atrophy.20,22 The above findings suggest that increased autofluorescence and, thus, RPE lipofuscin, may be implicated in the development of geographical atrophy, but may not play an essential part in the occurrence of CNV. The fact that CNV has a tendency to grow towards the centre of the fovea, where levels of autofluorescence and RPE lipofuscin are the lowest in the fundus,25,26 and that in some of our cases and those reported previously,21 the distribution of autofluorescence was normal at the site of the CNV also seems to support this concept. Moreover, in other retinal diseases characterised by an increased content of lipofuscin in the RPE, such as Stargardt macular dystrophy‐fundus flavimaculatus,12 the occurrence of CNV is extremely rare. Recent data suggest that changes in the alternative complement pathway may be implicated in the pathogenesis of AMD,27,28,29,30 especially in the neovascular form of AMD.30 Thus, other pathogenic mechanisms, in addition to or other than lipofuscin‐related RPE damage, may be involved in the formation of CNV.7
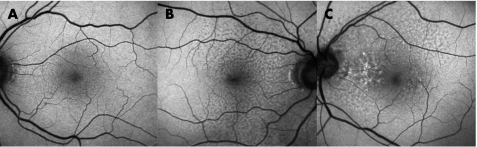
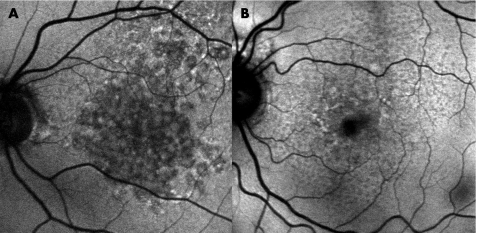
Figure 5 Fundus autofluorescence images of two eyes (A,B) with early geographical atrophy detected clinically. Focal areas of increased autofluorescence are seen.
By using a fundus camera‐based system with a longer wavelength for excitation (580 nm) than that used in this study, Spaide31 found higher levels of autofluorescence in fellow eyes of patients with exudative AMD than in those from patients with non‐exudative AMD. Spaide's findings do not necessarily contradict ours, given the differences in the study design and methods. In this study, images were obtained with a confocal scanning laser ophthalmoscope, using a shorter wavelength (488 nm) than that used by Spaide. Furthermore, we evaluated qualitatively, rather than quantitatively, the distribution of autofluorescence in eyes with newly developed CNV and fellow eyes, and looked for changes in autofluorescence (ie, large or small areas of increased or decreased autofluorescence compared with background). Thus quantitative values of autofluorescence were not available in this study. However, on the basis of our previous findings, it is unlikely that an overall, smooth and evenly distributed increased in autofluorescence (known to occur with age) would occur associated with and be causative of a CNV.32,33 If increased autofluorescence (and thus RPE lipofuscin) were to play an important part in CNV formation, areas of increased autofluorescence would probably be seen at the site of the CNV and/or around it, and especially before the formation of CNV.
Areas of increased autofluorescence (other than those seen in a focal pattern of autofluorescence) were observed in eyes with occult CNV, but only exceptionally in eyes with classic CNV. These areas of increased autofluorescence corresponded clinically to a neurosensory retinal detachment in most cases. This increased autofluorescence signal may represent an increased content in RPE lipofuscin secondary to increased outer segment shedding at sites of chronic neurosensory retinal detachment.34,35 Although RPE damage may follow increased levels of autofluorescence, further longitudinal studies are being conducted to evaluate the significance of this finding with regard to anatomical and visual prognosis in these patients.
Abbreviations
A2E - N‐retinylidene N‐retinyl ethanolamine
AMD - age‐related macular degeneration
CNV - choroidal neovascularisation
FFA - fundus fluorescein angiograms
OCT - optical coherence tomography
RPE - retinal pigment epithelium
Footnotes
Funding: This research project was supported partly by a grant from Tenovus, Scotland.
Competing interests: None.
Presented partly at the 8th Michelson Symposium, 8–11 June 2005, Ghent, Belgium, and at the Association for Research in Vision and Ophthalmology, 30 April –4 May 2006.
Ethical approval: This research was approved by the Grampian Local Research Ethics Committee.
References
- 1.Macular Photocoagulation Study Group Subfoveal neovascular lesions in age‐related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol 19911091242–1257. [PubMed] [Google Scholar]
- 2.Lafaut B A, Bartz‐Schmidt K U, Vanden Broecke C.et al Clinicopathological correlation in exudative age related macular degeneration: histological differentiation between classic and occult choroidal neovascularisation. Br J Ophthalmol 200084239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes E H, Khan J, Patel N.et al In vivo demonstration of the anatomic differences between classic and occult choroidal neovascularization using optical coherence tomography. Am J Ophthalmol 2005139344–346. [DOI] [PubMed] [Google Scholar]
- 4.Bressler N M, Frost L A, Bressler S B.et al Natural course of poorly defined choroidal neovascularization associated with macular degeneration. Arch Ophthalmol 19881061537–1542. [DOI] [PubMed] [Google Scholar]
- 5.Bressler S B, Bressler N M, Fine S L.et al Natural course of choroidal neovascular membranes within the foveal avascular zone in senile macular degeneration. Am J Ophthalmol 198293157–163. [DOI] [PubMed] [Google Scholar]
- 6.Lois N. Neovascular age‐related macular degeneration. Comp Ophthalmol Update 20045143–161. [Google Scholar]
- 7.Zarbin M A. Current concepts in the pathogenesis of age‐related macular degeneration. Arch Ophthalmol 2004122598–614. [DOI] [PubMed] [Google Scholar]
- 8.Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci 197817583–600. [PubMed] [Google Scholar]
- 9.Feeney‐Burns L, Eldred G E. The fate of the phagosome: conversion to “age pigment” and impact in human retinal pigment epithelium. Trans Ophthalmol Soc UK 1983103416–421. [PubMed] [Google Scholar]
- 10.Wing G L, Blanchard G C, Weiter J J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 197817601–607. [PubMed] [Google Scholar]
- 11.Weingeist T A, Kobrin J L, Watzke R C. Histopathology of Best's macular dystrophy. Arch Ophthalmol 19821001108–1114. [DOI] [PubMed] [Google Scholar]
- 12.Eagle R C, Jr, Lucier A C, Bernardino V B., Jret al Retinal pigment epithelial abnormalities in fundus flavimaculatus: a light and electron microscopic study. Ophthalmology 1980871189–1200. [DOI] [PubMed] [Google Scholar]
- 13.Dorey C K, Wu G, Ebenstein D.et al Cell loss in the aging retina; relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 1989301691–1699. [PubMed] [Google Scholar]
- 14.Eldred G E, Lasky M R. Retinal age pigments generated by self‐assembling lysosomotropic detergents. Nature 1993361724–726. [DOI] [PubMed] [Google Scholar]
- 15.Holz F G, Schutt F, Kopitz J.et al Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci 199940737–743. [PubMed] [Google Scholar]
- 16.Sparrow J R, Nakanishi K, Parish C A. The lipofuscin fluorophore A2E mediates blue light‐induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 2000411981–1989. [PubMed] [Google Scholar]
- 17.Suter M, Reme C, Grimm C.et al Age‐related macular degeneration. The lipofuscin component N‐retinyl‐N‐retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J Biol Chem 20001539625–39630. [DOI] [PubMed] [Google Scholar]
- 18.von Ruckmann A, Fitzke F W, Bird A C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol 199579407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delori F C, Dorey C K, Staurenghi G.et al In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 199536718–729. [PubMed] [Google Scholar]
- 20.Holz F G, Bellman C, Staudt S.et al Fundus autofluorescence and development of geographic atrophy in age‐related macular degeneration. Invest Ophthalmol Vis Sci 2001421051–1056. [PubMed] [Google Scholar]
- 21.Dandekar S S, Jenkins S A, Peto T.et al Autofluorescence imaging of choroidal neovascularization due to age‐related macular degeneration. Arch Ophthalmol 20051231507–1513. [DOI] [PubMed] [Google Scholar]
- 22.Lois N, Owens S L, Coco R.et al Fundus autofluorescence in patients with age‐related macular degeneration and high risk of visual loss. Am J Ophthalmol 2002133341–349. [DOI] [PubMed] [Google Scholar]
- 23.Bindewald A, Bird A C, Dandekar S S.et al Classification of fundus autofluorescence patterns in early age‐related macular disease. Invest Ophthalmol Vis Sci 2005463309–3314. [DOI] [PubMed] [Google Scholar]
- 24.Altman D G, Machin D, Bryant T N.et alStatistics with confidence. 2nd edn. London: BMJ Books, 2000
- 25.von Ruckmann A, Fitzke F W, Bird A C. Fundus autofluorescence in age‐related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci 199738478–486. [PubMed] [Google Scholar]
- 26.Dorey C K, Staurenghi G, Delori F C. Lipofuscin in aged and AMD eyes. In: Holyfield JG, Anderson RE, La Vail MM, eds. Retinal degeneration. New York: Plenum Press, 19933–14.
- 27.Klein R J, Zeiss C, Chew E Y.et al Complement factor H polymorphism in age‐related macular degeneration. Science 2005308385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haines J L, Hauser M A, Schmidt S. Complement factor H variant increases the risk of age‐related macular degeneration. Science 2005308419–421. [DOI] [PubMed] [Google Scholar]
- 29.Edwards A O, Ritter R I I I, Abel K J.et al Complement factor H polymorphism and age‐related macular degeneration. Science 2005308421–424. [DOI] [PubMed] [Google Scholar]
- 30.Hageman G S, Anderson D H, Johnson L V.et al A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age‐related macular degeneration. Proc Natl Acad Sci USA 20051027227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaide R F. Fundus autofluorescence in age‐related macular degeneration. Ophthalmology 2003110392–399. [DOI] [PubMed] [Google Scholar]
- 32.Lois N, Halfyard A S, Bird A C.et al Fundus autofluorescence in Stargardt macular dystrophy‐fundus flavimaculatus. Am J Ophthalmol 200413855–63. [DOI] [PubMed] [Google Scholar]
- 33.Lois N, Halfyard A S, Bird A C.et al Quantitative evaluation of fundus autofluorescence “in vivo” in eyes with retinal disease. Br J Ophthalmol 200084741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivert L, Kjeldbye H, Gouras P. Long‐term effects of short‐term retinal bleb detachments in rabbits. Graefes Arch Clin Exp Ophthalmol 2002240232–237. [DOI] [PubMed] [Google Scholar]
- 35.Framme C, Walter A, Gabler B.et al Fundus autofluorescence in acute and chronic‐recurrent central serous chorioretinopathy. Acta Ophthalmol Scand 200583161–167. [DOI] [PubMed] [Google Scholar]