Abstract
Background
Translocation of a free autologous graft consisting of retinal pigment epithelium (RPE), Bruch's membrane, choriocapillaris and choroid in patients with exudative age‐related macular degeneration is currently being evaluated in clinical practice. Angiographic studies in these patients suggest that their grafts become revascularised.
Aim
To investigate the histological evidence of revascularisation of the graft in a porcine model.
Methods
In 11 pigs (11 eyes), an RPE–choroid graft was translocated from the mid‐periphery to an intact or an intentionally damaged RPE and Bruch's membrane at the recipient site. The eyes were enucleated 1 week or 3 months after surgery. Tissue sections were evaluated using immunohistochemistry.
Results
Bridging vessels between recipient layer and graft were identified from 1 week to 3 months after surgery. This reconnection occurred regardless of whether the Bruch's membrane of the recipient site was left intact or intentionally damaged at the time of transplantation. The vasculature of the graft appeared open and perfused. Vessels with transcapillary pillars and conglomerates of small new vessels were present in the graft.
Conclusions
This study showed histological evidence for revascularisation by angiogenesis of a free autologous RPE–choroid graft.
Age‐related macular degeneration (AMD) is the leading cause of blindness in elderly people in industrialised countries.1 The exudative or neovascular form is responsible for the majority of AMD patients with severe visual loss.2,3 In exudative AMD, new choroidal blood vessels cross the Bruch's membrane, and invade the space underneath the retinal pigment epithelium (RPE) and/or retina. This precedes the loss of macular photoreceptor cells and a decrease in visual acuity.4,5 Laser coagulation or photodynamic therapy aims to occlude the choroidal neovascularisation (CNV), but just as in surgical removal of the CNV membrane,6,7 invariably causes loss of RPE, with subsequent dysfunction of both choriocapillaris and overlying photoreceptors.8,9,10,11 Therefore, recovery of RPE function is desirable. Even promising new treatments that neutralise growth factors such as vascular endothelial growth factor (VEGF) only affect active neovascularisation, and are unable to replace lost RPE cells.12 Therefore, surgical strategies aim to reconstitute functioning RPE. Cell suspensions and sheets of homologous fetal RPE, suspensions of autologous iris pigment epithelium or RPE have failed to form a functional monolayer.13,14,15,16
Translocation of the macula to an adjacent healthy extramacular area of RPE can preserve central vision and sometimes even improve macular function.17,18,19,20 Drawbacks are its restriction to smaller CNVs, the challenging surgical technique, the confinement to second eyes, diplopia, and other complications, especially proliferative vitreoretinopathy.21,22
Transplantation of an autologous full‐thickness graft consisting of RPE, Bruch's membrane, choriocapillaris and choroid was first suggested in 1991.23,24,25 Later, the feasibility of an RPE–choroid graft taken from the mid‐periphery was shown in a larger group of patients.26,27,28 Angiographic data strongly suggest that revascularisation of the graft occurred with survival and function of the graft for >3 years.29
As there is currently no histological evidence for revascularisation, this study investigated the revascularisation of a free autologous RPE–choroid graft in a pig model.
Materials and methods
Animals
This animal study was performed in accordance with the recommendations of the Association for Research in Vision and Ophthalmology and approved by the Bezirksregierung of Cologne (Number 50.203.2‐K26 14/03), Germany.
In total, 11 pigs (11 eyes), (6 Berlin minipigs and 5 domestic pigs aged 3–8 months) were pretreated with intramuscular azaperone (2 mg/kg) and ketamine (15–20 mg/kg), and anaesthetised with an intravenous injection of propofol 1% (1–2 mg/kg). General anaesthesia was maintained by normal ventilation at physiological blood gas values (partial pressure of carbon dioxide 40 mm Hg) with isoflurane 3%. Mydriasis was achieved using topical 0.5% tropicamide and 2.5% phenylepinephrine‐HCl. Postoperatively, the pigs received buprenorphin (Temgesic; 0.02 mg/kg).
Surgery
A standard three‐port vitrectomy including a posterior hyaloid detachment and lensectomy was performed. Subsequently, the anterior border venule and retinal vessels were coagulated. After a retinal detachment was created in the temporal quadrants by subretinal injection of balanced salt solution through a 42×21‐gauge rigid microinjection cannula (Synergetics, St Louis, Missouri, USA), a 180° peripheral retinotomy was made. With the use of perfluorodecalin (Fluoron, Ulm, Germany), the temporal half of the retina was flapped over to the nasal periphery to allow direct access to the underlying RPE. To prepare the graft, a fluid–air exchange was performed and a mid‐peripheral donor area of about 16 mm2 was surrounded by laser or diathermy coagulation. The borders of the graft were cut with 20‐gauge vitreous scissors (Geuder, Heidelberg, Germany). Subsequently, the full‐thickness RPE–choroid graft (mean size 9 mm2) was carefully separated from the sclera. In five pigs, RPE and Bruch's membrane of the recipient bed were intentionally damaged with a soft‐tipped silicone loop to mimic the damage after CNV extraction in patients. The graft was translocated to the recipient bed in the macular area using retinal forceps or a specially designed aspiration‐reflux spatula (Dutch Ophthalmic, Zuidland, The Netherlands). Care was taken to grasp the graft from the choroidal side to avoid damage to the RPE. The retina was repositioned and refixated by a reinjection of decalin, resulting in a graft covered by retina.
To complete the operation, decalin was exchanged for silicone oil (5000 Cst, Siluron, Fluoron).
Enucleation
The eyes were enucleated 1 week or 3 months after surgery. The pigs were sedated with azaperone (2 mg/kg) and ketamine (20 mg/kg), and killed using pentobarbital sodium. The non‐operated fellow eye served as a control.
Tissue preparation
The anterior segment of the eye was dissected posterior to the ora serrata, and the fundus was inspected for location and macroscopic features of the graft, retinal detachment or bleeding.
The posterior cups were fixed with 4% buffered paraformaldehyde at 4°C overnight. Subsequently, the graft was cut out as a small block of tissue, including retina and sclera. For light microscopy, the fixed tissue was embedded in paraffin wax, processed for sectioning (4‐μm thick sections), and stained with haematoxylin and eosin and periodic acid–Schiff (PAS).
Immunohistochemistry
The sections were dewaxed, rehydrated, and washed in three changes of phosphate‐buffered saline (PBS). To eliminate endogenous peroxidase activity, specimens were incubated for 30 min in 3% hydrogen peroxide. After rinsing in water for 5 min and three changes of PBS, sections were pretreated for antigen retrieval with citrate buffer pH 6 for 15 min at 100°C. After three changes of PBS, incubation with the primary antibody (dilution 1:400) was performed at 4°C overnight. Primary antibodies used in the study were rabbit antihuman von Willebrand factor (VWF; DakoCytomation, Glostrup, Denmark) and rabbit antihuman antibody directed against VEGF receptor‐1 (flt‐1; Santa Cruz, California, USA).
After three washes with PBS‐Tween 0.5%, specimens previously incubated with the anti‐VWF were incubated with goat antirabbit Cy3 (1:400, 30 min at room temperature; Jacksons ImmunoResearch, Westgrove, Pennsylvania, USA) for immunofluorescent evaluation. For specimens incubated with flt‐1, polyalkaline phosphatase‐GAM/R IgG (ImmunoLogic, Duiven, The Netherlands) was applied for 30 min, followed by washes with PBS and TRIS/HCl. New fuchsin was used to develop the alkaline phosphatase‐chromogen. All specimens were counterstained with haematoxylin.
Results
Complications of surgery
In most pigs, complications were encountered during surgery, mainly consisting of choroidal and retinal haemorrhage (n = 8). Despite an uncomplicated operation, pig 3 had to be killed 18 h after surgery because of postoperative lung oedema.
Revascularisation of the graft
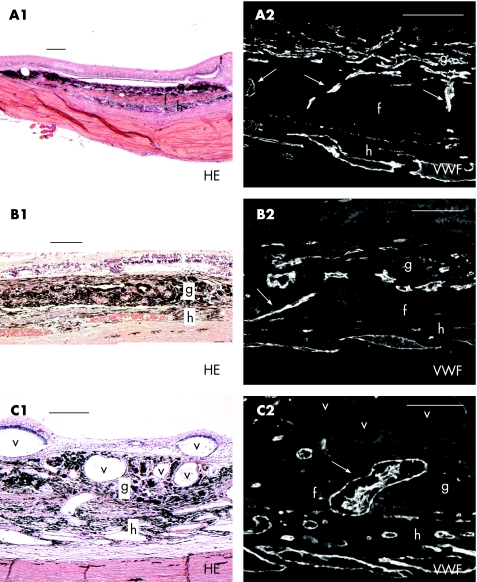
All RPE–choroid grafts had continuous or partial contact with the macular recipient bed (fig 1A1–C1). In 6 of 11 pigs, the graft vasculature appeared open and perfused (presence of erythrocytes) 1 week and 3 months after transplantation. Moreover, in these six grafts, vertical bridging vessels between recipient area and graft were found after 1 week (pigs 1, 8 and 10) and 3 months (pigs 5, 7 and 11; fig 1A2–C2). On serial sectioning, no obvious differences in the number of bridging vessels were seen between 1 week and 3 months after surgery. This vessel infiltration into the graft was detected irrespective of whether the recipient Bruch's membrane was intentionally damaged or left intact as much as possible at the time of transplantation, and irrespective of whether intraoperative haemorrhages had occurred or not. In addition, bridging vessels were spread equally over the graft and recipient interface.
Figure 1 (A1–C1) Haematoxylin and eosin (HE) staining with accompanying von Willebrand factor (VWF) staining (A2–C2) of three retinal pigment epithelium–choroid grafts: vertically bridging vessels between recipient and graft. Arrows indicate bridging vessels; g, graft; f, fibrovascular layer; h, host choroid; t, transition healthy‐degenerated retina; v, intraretinal or intrachoroidal vacuoles filled with silicone oil. Scale bars: A1–C1, 200 μm and A2–C2, 100 μm.
Owing to impaired visualisation, the graft was accidentally placed upside down in two animals (pigs 8 and 11) as shown by histological evaluation; however, bridging vessels were found centrally between the recipient and graft and not at the edges.
Furthermore, vessels with transcapillary pillars and conglomerulates of small vessels in the graft were observed (pig 6). In all grafts, the large majority of vessels stained positive for flt‐1 (VEGF receptor‐1) and VWF, as did the choroid of the recipient bed. There was no difference in flt‐1 or VWF staining between the early and late postoperative course.
Tissue responses
The graft consisted of choroid, choriocapillaris, Bruch's membrane and RPE cells. Macroscopically, the graft had a dark grey appearance. Numerous macrophages were morphologically identified on haematoxylin and eosin‐stained sections in the graft after 1 week and 3 months. In the fellow eye, macrophages were not observed.
In the grafts harvested at 18 h (n = 1) and 1 week (n = 4) after surgery, large areas of haemorrhages were macroscopically apparent, which were microscopically noted subretinally, and between the graft and recipient tissue. Organisation of these haemorrhages had started at 1 week with fibroblastic and capillary ingrowth. At 3 months, a fibrovascular scar underneath the retina (pigs 2 and 6) or graft (pigs 5 and 11) was present (fig 1A1). Bridging vessels crossed the fibrovascular layer connecting the recipient and graft (fig 1A2).
Proliferative vitreoretinopathy (PVR) was present in six pigs as observed macroscopically and microscopically from 1 week after surgery.
In three grafts, giant cells and histiocytes surrounded intraretinal or intrachoroidal vacuoles filled with silicone oil (fig 1C1).
Retinal pigment epithelium
RPE cells covered the graft up to 3 months after surgery (fig 1). In pigs with traumatic surgery, stretches of graft RPE were degenerated or absent.
The RPE cells of the recipient bed underneath the graft were scarcely present or had degenerated, both in pigs where RPE was intentionally removed or was left intact as much as possible.
Bruch's membrane
PAS staining for basement membrane proteins showed the presence of the recipient Bruch's membrane underneath the graft 18 h after surgery (pig 3). In contrast, 1 week and 3 months after surgery, the recipient Bruch's membrane had largely disappeared underneath the graft. This was restricted to the transplantation area and occurred regardless of whether the recipient bed was intentionally damaged during surgery or not.
Retina
There was an obvious transition from relatively healthy retina, which had remained attached during the operation, to degenerated retina, which had been detached during the 180° retinotomy (fig 1A1).
The presence of subretinal haemorrhage in some grafts prevented RPE–retina contact, with loss of photoreceptors. In pigs (pigs 1, 4 and 5) where stretches of RPE monolayer covered the graft with a direct contact between the retina and graft, normal photoreceptors were observed up to 3 months after surgery.
Discussion
This study shows histological evidence for revascularisation of a free autologous RPE–choroid graft, which was apparent as early as 1 week and persisted up to at least 3 months after surgery. Vertically bridging vessels between the macular recipient bed and graft could be observed, suggesting revascularisation by vessel invasion from the underlying choroid. The vasculature of the graft appeared open, perfused and viable, as shown by intravascular blood cells and VEGF receptor‐1 expression. There was evidence of angiogenesis in the graft, as indicated by vessels with transcapillary pillars and conglomerates of small vessels.
Revascularisation of free grafts is well known in skin transplants. Survival of a free skin graft is initially accomplished by plasmatic imibibition until definitive recirculation is achieved by anastomosis of host and graft vasculature (after 24–48 h) by neovascular ingrowth.30,31 In our study, the exact timing of revascularisation by localised choroidal neovascular ingrowth of the RPE–choroid graft was not determined. This might be established by repeated postoperative angiography in patients, starting as early as 1 day after surgery.
Interestingly, histologically, revascularisation of the graft did not result in the formation of subretinal neovascularisation, but was restricted to bridging vessels and vessel formation in the graft.
Several distinct processes seem to be involved in revascularisation of the graft. Potential stimuli include hypoxia, an inflammatory response and a breakdown of Bruch's membrane. Although we can only speculate as to whether relative hypoxia in the transplanted graft might be a stimulation for revascularisation, a striking finding was the ubiquitous presence of macrophages. These macrophages are either derived from the circulation or are migrated and dedifferentiated RPE cells from the recipient site. Macrophages have a central role in the normal chronic cellular inflammation response in wound healing. They release many growth factors that promote angiogenesis.32,33,34 The macrophage response was seen as early as 1 week and persisted during the 3 months of follow‐up.
The macrophage invasion probably occurs in the context of an immune reaction to tissue damage after surgical trauma (as proposed by Matzinger35 in the Danger model) and may contribute to neovascularisation, leading to revascularisation.
In patients, and in animal models of CNV, ruptures in Bruch's membrane are a prerequisite for the development of CNV.36,37,38 Therefore, it was initially hypothesised that an intact Bruch's membrane at the recipient site would act as a natural barrier, preventing infiltration of vessels into the graft. However, vascularisation of the graft was observed, irrespective of whether the recipient Bruch's membrane was intentionally damaged or left intact at the time of transplantation and even when the graft was placed upside down. PAS staining showed that the recipient Bruch's membrane had largely disappeared underneath the graft, whether or not the recipient site was left intact. This suggests that Bruch's membrane degenerates underneath the graft in the pig model. The macrophage reaction discussed above may contribute to this process. Joussen et al28 recently reported angiographic data on the revascularisation of an RPE–choroid graft in patients with atrophic AMD, which supports the finding that vessels can reconnect despite an initially intact Bruch's membrane.33
The investigations were carried out in pigs, as the morphology of the porcine choroid is very similar to that in humans.39,40 However, surgery in the pig proved to be much more difficult than in patients, mainly because a wide‐angle viewing system could not be used and the illumination was suboptimal. Moreover, porcine retina differs from human retina by the presence of a large anterior circumferential border venule and superficially located retinal vessels. Severe haemorrhages from these vessels complicated the 180° retinomy in some cases, preventing reattachment of the retina over the graft, with consequent loss of photoreceptors, and may have contributed to an excessive fibrovascular response under the graft. A small paramacular retinotomy in the temporal raphe could have improved the retinal reattachment rate over the graft, as in patients this approach is associated with fewer complications during and after surgery.27,28
Additionally, the 180° retinotomy with fluid–air exchange caused mechanical retinal trauma.41,42 There was a sharp border between relatively healthy retina (remained attached during the operation) and the degenerated retina, which had been in contact with air. This border was found over or just nasal to the graft, and should not be mistaken for degeneration caused by the graft itself. Moreover, the pig retina is not supplied by a central retinal artery, but by retinal arteries directly originating from the ciliary arteries with lower perfusion pressure, and therefore more vulnerable to intraoperative ischaemia during folding and a high bottle pressure.39,40 For these reasons, this animal model proved to be unsuitable to verify the clinical experience that RPE and retina can survive after a choroid–RPE graft transplantation.
This study, however, did serve to show histological evidence of revascularisation of a free autologous graft of RPE and choroid.
Acknowledgements
We thank Frank Lacina, Martina Becker, Tim Krohne, Norbert Kociok and the veterinary team of the Department of Experimental Medicine, University of Cologne, for their expert assistance.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascularisation
f lt‐1 - antibody directed against VEGF receptor‐1
PAS - periodic acid Schiff
PBS - phosphate‐buffered saline
RPE - retinal pigment epithelium
VEGF - vascular endothelial growth factor
VWF - von Willebrand factor
Footnotes
Funding: This study was funded by the RetinoVit Stiftung, Cologne, the Deutsche Forschungsgemeinschaft DFG Jo‐324/4‐1 (AMJ), DFG Jo‐324/6‐1 (Emmy Noether Grant to AMJ), and DFG Ki‐743/5‐1 (BK, AMJ) and the SWOO‐Flieringa Foundation, the Rotterdam Eye Hospital, Rotterdam, The Netherlands.
Competing interests: None.
References
- 1.Resnikoff S, Pascolini D, Ataya‘ale D.et al Global data on visual impairment in the year 2002. Bull World Health Organ 200482844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.van Leeuwen R, Klaver C C W, Vingerling J R.et al Epidemiology of age‐related maculopathy: a review. Eur J Epidemiol 200318845–854. [DOI] [PubMed] [Google Scholar]
- 3.Augood C A, Vingerling J R, de Jong P T V M.et al Prevalence of age‐related maculopathy. The European Eye Study (EUREYE). Arch Ophthalmol 2006124529–535. [DOI] [PubMed] [Google Scholar]
- 4.Gass J D M. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 1994118285–298. [PubMed] [Google Scholar]
- 5.Grossniklaus H E, Green W R. Choroidal neovascularization. Am J Ophthalmol 2004137496–503. [DOI] [PubMed] [Google Scholar]
- 6.Submacular Surgery Trials (SST) Research Group Surgery for subfoveal choroidal neovascularization in age‐related macular degeneration: ophthalmic findings. SST Report No.11. Ophthalmology 20041111967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Submacular Surgery Trials (SST) Research Group Surgery for subfoveal choroidal neovascularization in age‐related macular degeneration: quality of life findings. SST Report No.12. Ophthalmology 20041111981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossniklaus H E, Gass D M. Clinicopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovascular membranes. Am J Ophthalmol 199812659–69. [DOI] [PubMed] [Google Scholar]
- 9.Young R W, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 196942392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bok D, Hall M O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol 197149664–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korte G E, Repucci V, Henkind P. RPE‐destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci 1984251135–1145. [PubMed] [Google Scholar]
- 12.Gragoudas E S, Adamis A P, Cunningham E T.et al Pegaptanib for neovascular age‐related macular degeneration. N Engl J Med 20043512805–2816. [DOI] [PubMed] [Google Scholar]
- 13.Algvere P V, Berglin L, Gouras P.et al Transplantation of fetal retinal pigment epithelium in age‐related macular degeneration with subfoveal neovascularization. Arch Clin Exp Ophthalmol 1994232707–716. [DOI] [PubMed] [Google Scholar]
- 14.Binder S, Stolba U, Krebs I.et al Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age‐related macular degeneration: a pilot study. Am J Ophthalmol 2002133215–225. [DOI] [PubMed] [Google Scholar]
- 15.van Meurs J C, ter Averst E, van Hagen P M.et al Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol 200488110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lappas A, Foerster A M H, Weinberger A W A.et al Translocation of iris pigment epithelium in patients with exudative age‐related macular degeneration: long‐term results. Graefes Arch Clin Exp Ophthalmol 2004242638–647. [DOI] [PubMed] [Google Scholar]
- 17.Machemer R, Steinhorst U H. Retinal separation, retinotomy, and macular rerotation: II. A surgical approach for age‐related macular degeneration? Graefes Arch Clin Exp Ophthalmol 1993231635–641. [DOI] [PubMed] [Google Scholar]
- 18.Wolf S, Lappas A, Weinberger A W A.et al Macular translocation for surgical management of subfoveal choroidal neovascularizations in patients with AMD: first results. Graefes Arch Clin Exp Ophthalmol 199923751–57. [DOI] [PubMed] [Google Scholar]
- 19.Pertile G, Claes C. Macular translocation with a 360 degree retinotomy for management of age‐related macular degeneration with subfoveal choroidal neovascularization. Am J Ophthalmol 2002134560–565. [DOI] [PubMed] [Google Scholar]
- 20.Abdel‐Meguid A, Lappas A, Hartmann K.et al One year follow up of macular translocation with 360 degree retinotomy in patients with age related macular degeneration. Br J Ophthalmol 200387615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohji M, Fujikado T, Kusaka S.et al Comparison of three techniques of foveal translocation in patients with subfoveal choroidal neovascularization resulting from age related macular degeneration. Am J Ophthalmol 2001132888–896. [DOI] [PubMed] [Google Scholar]
- 22.Pawlak D, Glacet‐Bernard A, Papp M.et al Limited macular translocation compared with photodynamic therapy in the management of subfoveal choroidal neovascularization in age related macular degeneration. Am J Ophthalmol 2004137880–887. [DOI] [PubMed] [Google Scholar]
- 23.Peyman G A, Blinder K J, Paris C L.et al A technique for retinal pigment epithelium transplantation for age‐related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg 199122102–108. [PubMed] [Google Scholar]
- 24.Stanga P E, Kychenthal A, Fitzke F W.et al Retinal pigment epithelium translocation and central visual function in age related macular degeneration: preliminary results. Int Ophthalmol 200123297–307. [DOI] [PubMed] [Google Scholar]
- 25.Stanga P E, Kychenthal A, Fitzke F W.et al Retina pigment epithelium translocation after choroidal neovascular membrane removal in age‐related macular degeneration. Ophthalmology 20021091492–1498. [DOI] [PubMed] [Google Scholar]
- 26.Van Meurs J C, van den Biesen P R. Autologous pigment epithelium and choroids translocation in patients with age‐related macular degeneration: short‐term follow up. Am J Ophthalmol 2003136688–695. [DOI] [PubMed] [Google Scholar]
- 27.Van Meurs J C. Retinal pigment epithelium and choroid translocation in patients with exudative age‐related macular degeneration. In: Kirchhof B, Wong D, eds. Essentials in ophthalmology: vitreo‐retinal surgery, 1st edn. Berlin: Springer‐Verlag, 200573–87.
- 28.Joussen A M, Heussen F M, Joeres S.et al Autologous translocation of the choroid and retinal pigment epithelium in age‐related macular degeneration. Am J Ophthalmol 200614217–30. [DOI] [PubMed] [Google Scholar]
- 29.Van Meurs J C, Maaijwee K J M. Long‐term results of submacular surgery combined with macular translocation or the retinal pigment epithelium in neovascular age‐related macular degeneration. Ophthalmology 20061131471. e1–2 author reply 14712. [DOI] [PubMed] [Google Scholar]
- 30.Converse J M, Rapaport F T. The vascularization of skin autografts and homografts. Ann Surg 1956120306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young C M A. The revascularization of pedicle skin flaps in pigs: a functional and morphologic study. Plastic Reconstr Surg 198270455–464. [DOI] [PubMed] [Google Scholar]
- 32.Takemura R, Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol 1984246C1–C9. [DOI] [PubMed] [Google Scholar]
- 33.Folkman J, Klagsbrun M. Angiogenic factors. Science 1987235442–447. [DOI] [PubMed] [Google Scholar]
- 34.Campochiaro P A, Soloway P, Ryan S J.et al The pathogenesis of choroidal neovascularization in patients with age‐related macular degeneration. Mol Vis 1999534. [PubMed] [Google Scholar]
- 35.Matzinger P. The danger model: a renewed sense of self. Science 2002296301–305. [DOI] [PubMed] [Google Scholar]
- 36.Ryan S J. Subretinal neovascularization after argon laser photocoagulation. Graefes Arch Clin Exp Ophthalmol 198021529–42. [DOI] [PubMed] [Google Scholar]
- 37.Ryan S J. Subretinal neovascularization. Natural history of an experimental model. Arch Ophthalmol 19821001804–1809. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi T, Miller H, Orr G.et al Morphologic observations on experimental subretinal neovascularization in the monkey. Invest Ophthalmol Vis Sci 1987281116–1130. [PubMed] [Google Scholar]
- 39.Simoens P, de Schaepdrijver L, Lauwers H. Morphologic and clinical study of the retinal circulation in the miniature pig. A: morphology of the retinal microvasculature, Exp Eye Res 199254965–973. [DOI] [PubMed] [Google Scholar]
- 40.De Schaepdrijver L, Simoens P, Pollet L.et al Morphologic and clinical study of the retinal circulation in the miniature pig. B: fluorescein angiography of the retina, Exp Eye Res 199254975–985. [DOI] [PubMed] [Google Scholar]
- 41.Hasamura T, Yonemura N, Hirata A.et al Retinal damage by air infusion during vitrectomy in rabbit eyes. Invest Ophthalmol Vis Sci 2000414300–4304. [PubMed] [Google Scholar]
- 42.Hirata A, Yonemura N, Hasumura T.et al Effect of infusion air pressure on visual field defects after macular hole surgery. Am J Ophthalmol 2000130611–616. [DOI] [PubMed] [Google Scholar]