Abstract
Anterior segment imaging is a rapidly advancing field of ophthalmology. New imaging modalities, such as rotating Scheimpflug imaging (Pentacam‐Scheimpflug) and anterior segment optical coherence tomography (Visante OCT and Slit‐Lamp OCT), have recently become commercially available. These new modalities supplement the more established imaging devices of Orbscan scanning slit topography and ultrasound biomicroscopy (UBM). All devices promise quantitative information and qualitative imaging of the cornea and anterior chamber. They provide a quantitative angle estimation by calculating the angle between the iris surface and the posterior corneal surface. Direct angle visualisation is possible with the OCT devices and UBM; they provide images of the scleral spur, ciliary body, ciliary sulcus and even canal of Schlemm in some eyes. Pentacam‐Scheimpflug can measure net corneal power, a feature particularly useful for cataract patients having undergone previous corneal surgery. Anterior segment OCT can measure corneal flap depth following LASIK and anterior chamber width prior to phakic intraocular lens implantation. The arrival of the new imaging devices may herald the dawn of a new era for ophthalmic diagnosis, particularly in view of the ease and non‐contact nature of examination.
Assessment of anterior segment structures is an integral part of ophthalmic examination. In clinical practice imaging of the anterior segment has traditionally been carried out with slit lamp biomicroscopy. Objective quantitative assessment of anterior segment structures is limited and direct iridocorneal angle visualisation can only be carried out with the use of diagnostic contact lenses. New anterior segment imaging instruments promise to overcome these limitations. They aim to improve imaging of the anterior segment and to enhance clinical practice and research in ophthalmology. Anterior segment imaging has become a rapidly advancing field of ophthalmology. New modalities such as rotating Scheimpflug imaging (Pentacam, Oculus Inc, Lynnwood, WA, USA) and anterior segment Optical Coherence Tomography (Visante OCT, Carl Zeiss Meditec Inc, Dublin, CA, USA, and SL‐OCT, Heidelberg Engineering GmbH, Heidelberg, Germany) have recently become available. They promise quantitative information and qualitative imaging of the cornea, anterior chamber, iris, iridocorneal angle and lens.
In the following article we describe the newer instruments, Pentacam‐Scheimpflug, Visante OCT and SL‐OCT (Slit‐Lamp OCT), and compare them to more established imaging devices such as ultrasound biomicroscopy (UBM) (P60 UBM, Paradigm Medical Industries Inc, West Salt Lake City, UT, USA) and Orbscan scanning‐slit topography (Orbscan IIz, Bausch & Lomb Surgical Inc, San Dimas, CA, USA) (table 1). We also review the literature on their imaging capabilities and clinical applications, with an emphasis on the newer instruments.
Table 1 Imaging devices in review.
| Imaging instruments | Manufacturer and website | Approximate cost: Pound Sterling/Euro/US Dollar* |
|---|---|---|
| Pentacam‐Scheimpflug | Oculus Inc (Lynnwood, WA, USA) www.oculususa.com | £26800–30200/40000–45000€/$51000–57400 |
| Visante OCT | Carl Zeiss Meditec Inc (Dublin, CA, USA) www.meditec.zeiss.com | £50000/74500€/$95000 |
| SL‐OCT | Heidelberg Engineering GmbH (Heidelberg, Germany) www.HeidelbergEngineering.com | £30000/44700€/$57000 |
| Paradigm P60 UBM | Paradigm Medical Industries Inc (West Salt Lake City, UT, USA) www.paradigm‐medical.com | £34200/51000€/$65000 |
| Orbscan IIz | Bausch & Lomb Surgical Inc (San Dimas, CA, USA) www.bausch.com | £26000/38700€/$49400† |
*cost excludes local taxes and based on exchange rate on 25th September 2006.
†cost does not include Shack‐Hartmann aberrometer.
Imaging devices
Rotating scheimpflug imaging: pentacam‐scheimpflug
Pentacam‐Scheimpflug uses the Scheimpflug principle in order to obtain images of the anterior segment. The Scheimpflug principle describes the optical properties involved in the photography of objects when their plane is not parallel to the film of the camera. It requires that the plane containing the slit beam and the image plane intersect at one point, with the corresponding angles equal.1 It has been used for many years in commercial units, such as the Nidek EAS‐1000 and the Topcon SL‐45. The Pentacam‐Scheimpflug is a non‐contact optical system that has specifically been designed to image the anterior segment of the eye. It has a rotating Scheimpflug camera that takes up to 50 slit images of the anterior segment in less than 2 seconds.2 Software is then used to construct a three‐dimensional image. A second camera captures eye movements and makes appropriate corrections. It calculates data for corneal topography (anterior and posterior corneal surface) and thickness, anterior chamber depth (ACD), lens opacification and lens thickness. It also provides data on corneal wavefront of the anterior and posterior corneal surface using Zernike polynomials (Oculus Inc, www.oculususa.com/prd_comp.php, accessed October 2006). A newer version has recently become available, the Pentacam HR. In addition to a higher resolution camera, it has phakic intraocular lens (IOL) software that simulates the position of the proposed lens. The quality of the lens data depends on the pupil size, as only part of the lens can be examined through the pupillary aperture.2 The Pentacam‐Scheimpflug requires minimal experience for image acquisition. It has a function that automatically starts the scan when correct alignment and focus with the patient's cornea has been achieved.2 It also has a feature that calculates the corrected intraocular pressure (IOP) based on the corneal thickness of the individual patient.
Optical coherence tomography: visante OCT and SL‐OCT
The principle of OCT is analogous to ultrasound but with the emission and reflection of light instead of sound. Low coherence interferometry measures the delay and intensity of backscattered light by comparing it to light that has travelled a known reference path length and time delay by using a Michelson‐type interferometer.3 Concerning ophthalmic use, this technology was applied initially to the retinal OCT; the first OCT became commercially available in 1995 by Carl Zeis Meditec. The anterior segment OCT is an evolution of the retinal OCT. It uses a longer wavelength (1310 nm) than the retinal OCT (820 nm).4,5 This allows greater penetration through tissues that highly scatter light such as sclera and limbus, allowing for visualisation of the iridocorneal angle.5 It provides images of anterior segment structures, including the cornea, iris, angle and anterior lens. Visualisation of retro‐iris lens, ciliary body and ciliary sulcus is also possible.5 The ocular media absorb about 90% of the 1310 nm light before it reaches the retina. Therefore, the anterior segment OCT can use higher power than the retinal OCT. This allows near video rate imaging acquisition and elimination of motion artefacts.4 Two currently available anterior segment OCT devices are the Visante OCT and the SL‐OCT.
Visante OCT
Like the Pentacam‐Scheimpflug, the Visante OCT it is a non‐contact optical system. It provides anterior segment scans, high‐resolution corneal and angle scans and pachymetry maps at a rate of up to 2048 A‐scans per second. It can also be used to calculate the depth, width and angle of the anterior chamber (Carl Zeiss Meditec Inc, www.meditec.zeiss.com, accessed October 2006). According to the manufacturer, it has an optical axial resolution of up to 18 μm and optical transverse resolution of up to 60 μm. It can scan through an opaque cornea and minimal experience is required for image acquisition.
Slit‐lamp OCT
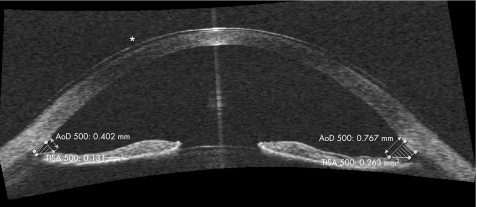
The SL‐OCT is an alternative OCT imaging device. It is a non‐contact system that is incorporated into a modified slit‐lamp biomicroscopy system, a feature that may prove time and space saving in clinical practice. This may also guide image acquisition, as set‐up and use are very similar to the conventional slit‐lamp. However, manual rotation of the scanning beam is required in order to image a meridian other than the vertical. According to the manufacturer, it has an optical axial resolution of less than 25 μm and transverse optical resolution between 20–100 μm (personal communication with Michael Cordes, Heidelberg Engineering at 2006 Annual Meeting of European Society of Cataract and Refractive Surgery, London, UK). Software can automatically calculate the central corneal thickness (CCT), the central ACD, the volume of the anterior chamber (AC) and the inter‐spur distance. As demonstrated in fig 3, it also provides gonioscopy with automated quantification of iridocorneal angle parameters, such as the angle opening distance at 500 μm (AOD 500) and the trabecular‐iris spur area at 500 μm (TISA 500). Compared to the Visante OCT, greater operator skill is required for image acquisition but incorporates more automated software.
Figure 3 Anterior chamber image with SL‐OCT. Direct angle visualisation is possible; software provides automated quantification of angle parameters. The values for the angle opening distance at 500 μm (AOD 500) and the trabecular‐iris spur area at 500 μm (TISA 500) can be seen. The asterisk (*) shows the presence of a contact lens.
Paradigm P60 ultrasound biomicroscopy
In 2005 the fourth generation ultrasound biomicroscope, the Paradigm P60 UBM, became available by Paradigm Medical Industries. Ophthalmic ultrasound imaging is based on the emission of an acoustic pulse and reception of the pulse after it has been reflected off ocular tissues. It has been used in the form of A and B‐scans for many decades.6,7 The P60 UBM offers flexibility in clinical use by incorporating four different probes with frequencies of 12.5, 20, 35 and 50 MHz (Paradigm Medical Industries Inc, www.paradigm‐medical.com, accessed October 2006). The best image quality and resolution are obtained by the 50 MHz transducer, but the scan field is limited to a 5×5 mm square. The 12.5, 20 and 35 MHz transducers can produce images of the entire anterior chamber with a single scan. The P60 UBM provides images of the cornea, iris and structures of the iridocorneal angle. Dual callipers are available for manual measurement of AC parameters, such as ACD, CCT and inter‐spur distance. Detailed anatomy of the posterior chamber with measurement of the sulcus‐to‐sulcus distance is also possible. (Paradigm Medical Industries Inc, www.paradigm‐medical.com, accessed October 2006) High frequency UBM provides high‐resolution images with an axial resolution of about 25 μm and transverse resolution of about 50 μm.8 It has a depth of penetration of 5 mm in tissues and can scan through opaque media. Image acquisition requires the eye to be immersed in a fluid with an eyecup or other holding device. This is uncomfortable and may potentially distort the eye anatomy and angle configuration.9 The contact nature of this instrument may make it impractical for many clinical situations, such as perforating ocular injuries. In addition, a highly skilled operator is needed to obtain high quality images.
Orbscan scanning‐slit topography
Orbscan is another non‐contact optical system. It is based on the principle of measuring the dimensions of a slit‐scanning beam projected on the cornea. Orbscan II and newer versions have a Placido disc attachment in order to obtain curvature measurements directly. The latest hardware upgrade, Orbscan IIz, can be integrated with a Shack‐Hartmann aberrometer in the Zyoptix workstation. This integrated system offers total wavefront analysis through the 5th order and identifies the total aberrations of the eye. The Orbscan IIz scans the entire surface of the cornea and acquires over 9000 data points in 1.5 seconds (Bausch & Lomb Surgical Inc, http://www.bausch.com/en_US/ecp/surgical/product/refractive/zyoptix.aspx, accessed October 2006). The curvature of the anterior and posterior surfaces of the cornea can be assessed along with the anterior surface of the lens and the iris. Mapping of the iris in conjunction with posterior surface corneal topography allows an estimation of the iridocorneal angle of the eye. Longitudinal assessment of these measurements may provide a role for Orbscan in glaucoma management.10 In addition, Orbscan IIz has a compensatory function for correcting post‐LASIK IOP readings; this has been shown to be accurate and useful.11 Image acquisition does not require a highly skilled operator.
Discussion of imaging capabilities
Corneal pachymetry
Central corneal thickness (CCT) had been shown to influence IOP measurements with Goldman applanation tonometry and to be an independent risk factor for the development of primary open‐angle glaucoma.12 Accurate knowledge of corneal thickness is essential prior to refractive surgery. The most commonly used technique for measuring corneal thickness is ultrasound pachymetry, a technique that provides a spot measurement. All the imaging instruments in discussion can be used to measure CCT; the Pentacam‐Scheimpflug, Visante‐OCT and Orbscan IIz also provide pachymetry maps. Pachymetry mapping may aid the diagnosis of corneal ectasias, such as keratoconus, and the may guide corneal refractive surgery.
Pentacam‐Scheimpflug has been shown to have good intraobserver repeatability in CCT measurements in healthy eyes and good correlation with ultrasound pachymetry (Allergan‐Humphrey 850); O'Donnell et al found that Pentacam‐Scheimpflug CCT values (528 μm) were slightly thinner than ultrasound pachymetry (534 μm) with a correlation coefficient of 0.96. Repeatability was slightly better with ultrasound pachymetry than Pentacam‐Scheimpflug.13 Another study that compared CCT measurements in healthy eyes also found thinner CCT values with Pentacam‐Scheimpflug compared to ultrasound pachymetry (Pachymeter SP‐2000; Tomey, Erlangen, Germany); mean CCT with Pentacam‐Scheimpflug was 542 vs. 552 μm with ultrasound.14 In the same study Orbscan values were the thickest (576 μm), whereas application of the ‘acoustic factor' resulted in the thinnest values (530 μm). Pentacam‐Scheimpflug showed the best between observer reproducibility of all modalities. Pentacam‐Scheimpflug CCT values were closer to ultrasound pachymetry and differences showed less variability than those observed with (corrected and uncorrected) Orbscan.14 A practical drawback of the Orbscan is that examination of grossly distorted or scarred corneas is limited.15 Long wavelength OCT measurements of CCT have also shown good correlation with ultrasound pachymetry (CorneoGage 2, Sonogage, Cleveland, OH). In a study of 42 myopic eyes the correlation coefficient was 0.97 and the mean OCT CCT value was slightly less than ultrasound (546.9 vs. 553.3 μm, p<0.001). OCT showed excellent repeatability; within a central 7 mm zone the overall repeatability of mean corneal thickness was 2 μm.16 UBM can also be used to measure CCT. In a study of 60 eyes a strong correlation (r = 0.859) was found between UBM and ultrasound pachymetry (Biocomp AP3 Optikon) CCT measurements.17 Although CCT measurements with UBM have high intraobserver reproducibility, they have poor interobserver reproducibility, as there is considerable difference between observers in selection of reference points.18,19
Anterior chamber depth
ACD is an important parameter to consider prior to cataract surgery and phakic IOL implantation. Measurement of ACD has also been used to detect occludable angles in screening programmes for primary angle closure (PAC).20 In routine clinical practice, measurement of the ACD has traditionally been carried out by applanation ultrasound and more recently with the IOL Master.
All imaging devices in review can measure ACD. Meinhardt et al compared ACD measurements obtained with Pentacam‐Scheimpflug, IOL Master, AC Master and slit‐lamp pachymetry by Jaeger (Haag‐Streit).21 In this study ACD was measured largest with the Pentacam‐Scheimpflug with a median value of 3.915 mm compared to 3.802 mm with the AC Master and 3.63 mm with the IOL Master. Pentacam‐Scheimpflug showed less intraobserver variation than the IOL Master (SD 12.7 vs. 24.5 μm), but the AC Master (that uses partial coherence interferometry) showed the best intraobserver repeatability (SD 5.4 μm). Reddy et al compared ACD with Orbscan II, IOL Master and non‐immersion ultrasound (Ocuscan, Alcon).22 Orbscan II and IOL Master produced almost identical mean values (3.32 and 3.33 mm respectively), whereas ultrasound produced a significantly lower value of 2.87 mm. The authors attributed this difference to the effect of applanation when using the hand‐held contact ultrasound probe. The anterior segment OCT has also been shown to measure ACD with very high precision. In a study by Goldsmith et al the variability between images was zero, and the variance between raters was 2.01% with a SD of 47 μm.4 UBM also shows high intraobserver reproducibility in ACD measurement with a variance coefficient less that 1.3%.18,19 However, it has poor interobserver reproducibility, as selection of reference points is not an automated process.19
Iridocorneal angle and iris
Assessment of the iridocorneal angle is important in routine ocular examination and essential in glaucoma patients. Currently this is carried out with gonioscopy, an examination that requires the use of a diagnostic contact lens. Grading of the angle is subjective and depends on visualisation of specific angle structures. Alternatively, the Van Herick technique may be used to assess peripheral ACD in relation to corneal thickness.
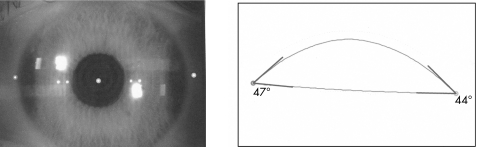
All imaging devices discussed in this article provide quantitative angle estimation but only OCT and UBM provide angle visualisation. Angle estimation is a calculation of the angle between the iris and the posterior surface of the cornea (fig 4), whereas angle visualisation includes anatomical details such as the iris root, the angle recess, the anterior ciliary body, the scleral spur and the canal of Schlemm (figs 2, 3 and 5).5
Figure 4 Anterior chamber angle estimation with Orbscan IIz.
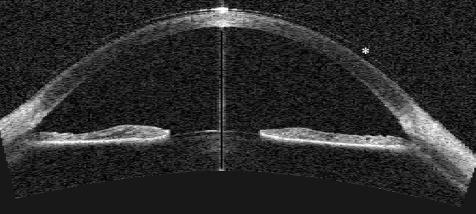
Figure 2 Anterior segment image with Visante OCT. Direct angle visualisation is possible, with details of the angle morphology visible. The iris can be visualised in full thickness and the anterior lens imaged. The asterisk (*) shows the presence of a contact lens in this patient.
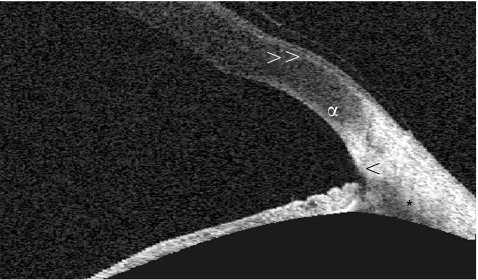
Figure 5 High‐resolution scan of the anterior chamber angle with the Visante OCT demonstrating angle visualisation. The arrowhead (<) shows the scleral spur and the asterisk (*) the anterior ciliary body. The letter α marks the limbal transition from cornea to sclera and the double arrowhead (>>) points to Bowman's membrane of the cornea with the overlying epithelium.
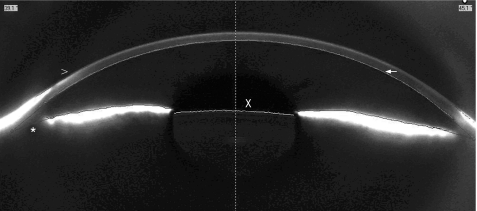
Pentacam‐Scheimpflug and Orbscan provide angle estimation, but no direct angle visualisation (figs 1 and 4). The angle estimation measurements obtained with Orbscan have shown high reproducibility and significant correlation with clinical parameters.23 In the absence of visualisation of the angle, however, important morphological information, such as plateau iris may be missed.
Figure 1 Anterior chamber image with Pentacam‐Scheimpflug. The arrowhead (>) shows the anterior surface of the cornea, as delineated by software. The arrow points to the posterior surface of the cornea, as delineated by software. The asterisk (*) shows that direct anterior chamber angle visualisation is not possible. The x shows the anterior lens surface, delineated again by software.
Anterior segment OCT and UBM provide angle visualisation (figs 2, 3 and 5) and excellent discriminative value for the detection of narrow angles.5 In this study by Radhakrishnan et al, the two devices showed equal reproducibility and similar mean values of quantitative angle parameters.5 According to the authors, visualisation of the ciliary body with OCT was not as complete as with UBM, but the scleral spur was more distinct in OCT images. It has been reported that UBM measurements of specific angle parameters, such as the angle opening distance at 500 μm (AOD 500), show high intraobserver and interobserver variation.18,19,24 This is mainly due to difficulty in the exact identification of the scleral spur for placing the measurement calliper. UBM also requires contact with the ocular surface in the presence of a coupling agent; this may potentially distort the eye anatomy and angle configuration.9
Screening for primary angle closure
The imaging devices in discussion may prove useful screening tools for PAC. Primary angle‐closure glaucoma (PACG) is a significant cause of visual morbidity. It accounts for half the cases of primary glaucoma worldwide and is the commonest type of glaucoma in eastern Asia.25 Measurement of axial ACD and assessment of limbal chamber depth has been shown to detect occludable angles in East Asians; assessment of these parameters may therefore have a role in population screening for PACG.20,26
All the imaging devices in review measure ACD and provide quantitative angle estimation, making them potential tools for screening programmes. In addition, the anterior segment OCT and UBM provide direct angle visualisation. Examination with Pentacam‐Scheimpflug, OCT and Orbscan can readily be carried out in darkness in order to simulate conditions that may provoke angle closure. Pentacam‐Scheimpflug and SL‐OCT provide a novel measure of the AC, the AC volume. This measure may prove useful for detecting individuals at risk of developing PACG; further studies are required to look into this. Gonioscopy is considered the definitive method of assessing the characteristics of the drainage angle.26 It allows visualisation of angle morphology but is limited by the considerable skill and the time required for examination. The use of UBM as a screening tool is limited by similar drawbacks. On the other hand, anterior segment OCT offers non‐contact examination, is relatively quick and does not require extensive experience for image acquisition. It is a very promising tool in screening for PAC and studies are needed to define its role. However, the considerable cost of this new modality may inhibit its widespread use for this purpose.
Table 2 Examination of patient with different imaging devices.
| Pentacam‐Scheimpflug | Visante OCT | SL‐OCT | P60 UBM | Orbscan IIz | |
|---|---|---|---|---|---|
| Image source | Optical | Optical | Optical | US | Optical |
| Position | Sitting | Sitting | Sitting | Supine | Sitting |
| Contact | No | No | No | Yes | No |
| Operator skill | Low | Low | Medium | High | Low |
US: ultrasound
Table 3 Features offered by different imaging devices.
| Features | Pentacam‐Scheimpflug | Visante OCT | SL‐OCT | P60 UBM | Orbscan IIz |
|---|---|---|---|---|---|
| Topography | Yes | No | No | No | Yes |
| IOP correction | Yes | No | No | No | Yes |
| Lens densitometry | Yes | No | No | No | No |
| Wavefront analysis | Yes | No | No | No | Yes* |
*with Shack‐Hartmann aberrometer integrated
Table 4 Summary of imaging capabilities.
| Imaging capabilities | Pentacam‐Scheimpflug | Visante OCT | SL‐OCT | P60 UBM | Orbscan IIz |
|---|---|---|---|---|---|
| Optical axial resolution | N/A | 18 μm | <25 μm | 25 μm | N/A |
| Pachymetry | Yes | Yes | Yes | Yes | Yes |
| Angle visualisation | No | Yes | Yes | Yes | No |
| Angle estimation | Yes | Yes | Yes | Yes | Yes |
| Ciliary sulcus visible | No | No | No | Yes | No |
| Opaque media | No | Yes | Yes | Yes | No |
N/A: not applicable
Refractive surgery
The Orbscan has been used in refractive surgery for many years. It provides both anterior and posterior corneal elevation maps and evaluates corneal thickness across the entire surface. It is useful for identifying pre‐operative corneal pathology, such as posterior keratoconus, and post‐operative problems, such as corneal ectasia. However, as summarised by Cairns and McGhee, there are issues concerning the disparity of Orbscan and ultrasound pachymetry values, and the inability to compare Orbscan posterior corneal surface topography against a gold standard.10 The newer imaging modalities aim to overcome these limitations and promise safer and more accurate refractive surgery.
Pentacam provides anterior and posterior corneal surface topography and can directly measure the net corneal power. This may be particularly useful for calculating the required IOL power when patients who have undergone corneal refractive surgery require cataract extraction, as no reference to pre‐operative data is required.27 Orbscan II provides total optical power maps, but it has been suggested that their precision may be unsatisfactory.10
The anterior segment OCT is promising to be of particular use in refractive surgery. In can measure residual stromal thickness in LASIK patients who are candidates for re‐treatment and intraocular dimensions, such as anterior chamber width, prior to phakic IOL implantation.27 It has also been used to study the dynamic nature of phakic IOLs during accommodation and to demonstrate contact of the IOL with the anterior surface of the crystalline lens.28 UBM can also be used to measure intraocular dimensions prior to phakic IOL implantation. It is particularly useful for analysing the in vivo position of the IOL and its relationship to the iris and the crystalline lens.27
Other applications
Anterior segment tumours
UBM is useful in the management of suspected anterior segment tumours; it can characterise cystic lesions and detect growth of suspected tumours in serial examinations.29 OCT has also been described to have a role in differentiating cystic from solid lesions of the iris.30 Anterior segment imaging with UBM or OCT may allow for definitive diagnosis of certain lesions and, therefore, more conservative management.
Trauma
UBM has a valuable adjuvant role in the detection of small, anteriorly located, ocular foreign bodies.31 It has been used in the differential diagnosis of a nodular conjunctival mass by identifying the presence of an underlying small foreign body that was not visible on slit lamp examination.32 A similar role is envisaged for anterior segment OCT in trauma cases, particularly in view of the non‐contact nature of examination.
Glaucoma surgery
Anterior segment OCT and UBM may provide useful morphological information following surgical or laser procedures for glaucoma. UBM has been used to analyse the characteristics of filtering blebs after trabeculectomy and to show that iris‐lens contact distance increases after laser iridotomy for pupillary block angle closure.33,34 OCT has been used to demonstrate widening of the angles after iridotomy in a patient with narrow angles, and to visualise the anatomy and characteristics of the filtering bleb following non‐penetrating deep sclerectomy.35,36
Cataract surgery
Anterior segment OCT can image the position of the IOL and it has been used to assess a case of capsular block syndrome; posterior movement of the IOL was demonstrated after laser posterior capsulotomy.37 OCT has also been used to study features related to the development of posterior capsule opacification and the relationship of the IOL to the posterior capsule.38
Summary of advantages and disadvantages
The new imaging devices do not aim to replace conventional slit‐lamp biomicroscopy; they promise to supplement and augment clinical practice and to become invaluable tools for ophthalmic research. Anterior segment imaging instruments have been available for over a decade. UBM became available in 1990, but did not manage to become part of routine clinical practice. The most likely explanation for this is the contact and time‐consuming nature of examination. By contrast, Orbscan, a non‐contact device, has become much more widely utilised.
The major advantages of the newer devices are the non‐contact nature of examination, repeatability and range of quantitative and qualitative information they provide. Angle visualisation in a non‐contact, objective examination is a significant development in anterior segment imaging. In addition, extensive training is not required for image acquisition. Values and indices obtained from different instruments are rarely interchangeable; therefore, care is required when interpreting results and comparing them to established references and protocols. A potential issue with these devices is their cost. New technology is almost always expensive, but part of the expense is usually balanced by the accompanied increase in accuracy of diagnosis. In addition, there is often a trend for reduction in cost with increasing availability of new instruments.
The future clinical application of newer anterior segment devices can be compared to the clinical use of new posterior segment imaging devices. Retinal OCT, such as the Stratus OCT (Carl Zeiss Meditec Inc, Dublin, CA, USA) has had a significant impact on the detection and management of retinal disease.39 It has led to a better understanding of the role of vitreomacular traction in the pathogenesis of macular hole and to the detection of vitreoretinal changes that precede the development of a stage 1 macular hole.40 Cystoid macular oedema can be diagnosed easily and objectively with a non‐invasive procedure. It can now be readily quantified making longitudinal follow‐up and assessment possible.41 The outcome of treatment can be monitored without the discomfort and risks of fundus fluorescein angiography.
Conclusions
It is envisaged that the new anterior segment imaging devices with have as significant an impact as the new posterior segment devices. Assessment of the irido‐corneal angle will become objective and quantitative; screening programs for primary angle closure glaucoma may become more feasible and less dependent on examiner skill. The new devices may improve our understanding of current limitations of surgery, such as astigmatism following penetrating keratoplasty, interface haze following deep lamellar keratoplasty and scarring of trabeculectomy blebs. They promise to improve the safety of phakic IOL implantation and overcome current problems with IOL power calculation in patients who have undergone prior corneal surgery. They may also lead to a new understanding of the changes the crystalline lens undergoes during ageing and accommodation. The potential clinical applications of these methods are only starting to be explored and the range of information they may yield has yet to be determined. Therefore, the use of the newer anterior segment imaging devices could well be the start of a new era for ophthalmic diagnosis.
Abbreviations
ACD - anterior chamber depth
CCT - central corneal thickness
IOL - intraocular lens
IOP - intraocular pressure
OCT - optical coherence tomography
PAC - primary angle closure
PACG - primary angle‐closure glaucoma
UBM - ultrasound biomicroscopy
References
- 1.Masters B R. Three‐dimensional microscopic tomographic imaging of the cataract in a human lens in vivo. Optics Express 19983332–338. [DOI] [PubMed] [Google Scholar]
- 2.Buehl W, Stojanac D, Sacu S.et al Comparison of three methods of measuring corneal thickness and anterior chamber depth. Am J Ophthalmol 20061417–12. [DOI] [PubMed] [Google Scholar]
- 3.Brezinski M E, Fujimoto J G. Optical coherence tomography: High‐resolution imaging in non‐transparent tissue. IEEE J Select Topics in Quantum Electron 199951185–1192. [Google Scholar]
- 4.Goldsmith J A, Li Y, Chalita M R.et al Anterior chamber width measurement by high‐speed optical coherence tomography. Ophthalmology 2005112238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radhakrishnan S, Goldsmith J, Huang D.et al Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 20051231053–1059. [DOI] [PubMed] [Google Scholar]
- 6.Mundt G H, Hughes W F. Ultrasonics in ocular diagnosis. Am J Ophthalmol 195642488–498. [DOI] [PubMed] [Google Scholar]
- 7.Baum G, Greenwood I. The application of ultrasonic locating techniques to ophthalmology‐part 2. Ultrasonic visualisation of soft tissues. Arch Ophthalmol 195860263–279. [DOI] [PubMed] [Google Scholar]
- 8.Ischikawa H, Liebmann J M, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol 200011133–139. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa H, Inazumi K, Liebmann J M.et al Inadvertent corneal indentation may cause artifactitious widening of the iridocorneal angle on ultrasound biomicroscopy. Ophthalmic Surg Lasers 200031342–345. [PubMed] [Google Scholar]
- 10.Cairns G, McGee C N J. Orbscan computerised topography: Attributes, applications and limitations. J Cataract Refract Surg 200531205–220. [DOI] [PubMed] [Google Scholar]
- 11.Lee D H, Seo S, Shin S C.et al Accuracy and predictability of the compensatory function of Orbscan II in intraocular pressure measurements after laser in situ keratomileusis. J Cataract Refract Surg 200228259–264. [DOI] [PubMed] [Google Scholar]
- 12.Gordon M O, Beiser J A, Brandt J D.et al Baseline factors that predict the onset of primary open‐angle glaucoma. Arch Ophthalmol 2002120714–720. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell C, Maldonado‐Codina C. Agreement and repeatability of central thickness measurement in normal corneas using ultrasound pachymetry and the OCULUS Pentacam. Cornea 200524920–924. [DOI] [PubMed] [Google Scholar]
- 14.Lackner B, Schmidinger G, Pieh S.et al Repeatability and reproducibility of central corneal thickness measurement with Pentacam, Orbscan and Ultrasound. Optom Vis Sci 200582892–899. [DOI] [PubMed] [Google Scholar]
- 15.Tam E S, Rootman D S. Comparison of central corneal thickness measurements by specular microscopy, ultrasound pachymetry, and ultrasound biomicroscopy. J Cataract Refract Surg 2003291179–1184. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Shekhar R, Huang D. Corneal pachymetry mapping with high‐speed optical coherence tomography. Ophthalmology 2006113792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierro L, Conforto E, Resti A G.et al High‐frequency ultrasound biomicroscopy versus ultrasound and optical pachymetry for the measurement of corneal thickness. Ophthalmologica 19982121–3. [DOI] [PubMed] [Google Scholar]
- 18.Urbak S F. Ultrasound biomicroscopy. I. Precision of measurements. Acta Ophthalmol Scand 199876447–455. [DOI] [PubMed] [Google Scholar]
- 19.Urbak S F, Pedersen J K, Thorsen T T. Ultrasound biomicroscopy. II. Intraobserver and interobserver reproducibility of measurements. Acta Ophthalmol Scand 199876546–549. [DOI] [PubMed] [Google Scholar]
- 20.Devereux J G, Foster P J, Baasanhu J.et al Anterior chamber depth measurement as a screening tool for primary angle‐closure glaucoma in an East Asian population. Arch Ophthalmol 2000118257–263. [DOI] [PubMed] [Google Scholar]
- 21.Meinhardt B, Stachs O, Stave J.et al Evaluation of biometric methods for measuring the anterior chamber depth in the non‐contact mode. Graefe's Arch Clin Exp Ophthalmol 2005. Published online first 15th September 2005. DOI: 10, 1007/s00417–005–0103–7. [DOI] [PubMed]
- 22.Reddy R A, Pande M V, Finn P.et al Comparative estimation of anterior chamber depth by ultrasonography, Orbscan II, and IOL Master. J Cataract Refract Surg 2004301268–1271. [DOI] [PubMed] [Google Scholar]
- 23.Allouch C, Touzeau O, Borderie V.et al Orbscan: a new device for iridocorneal angle measurement. J Fr Ophthalmol 200225799–806. [PubMed] [Google Scholar]
- 24.Tello C, Liebmann J, Potash S D.et al Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability. Invest Ophthalmol Vis Sci 1994353549–3552. [PubMed] [Google Scholar]
- 25.Quigley H A. Number of people with glaucoma worldwide. Br J Ophthalmol 199680389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster P G, Devereux J G, Alsbirk P H.et al The detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol 200084186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'hEineachain R. ESCRS Symposium Report, Lisbon 2005. Anterior Segment Imaging. EuroTimes November 200524–27.
- 28.Baikoff G, Lutun E, Wei J.et al Contact between 3 phakic intraocular lens models and the crystalline lens: an anterior chamber optical coherence tomography study. J Cataract Refract Surg 2004302007–2012. [DOI] [PubMed] [Google Scholar]
- 29.Conway R M, Chew T, Golchet P.et al Ultrasound biomicroscopy: role in diagnosis and management in 130 consecutive patients evaluated for anterior segment tumours. Br J Ophthalmol 200589950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siahmed K, Berges O, Desjardins L.et al Anterior segment tumour imaging: advantages of ultrasound (10,20 and 50 Hz) and optical coherence tomography. J Fr Ophthalmol 200427169–173. [DOI] [PubMed] [Google Scholar]
- 31.Deramo V A, Shah G K, Baumal C R.et al The role of ultrasound biomicroscopy in ocular trauma. Tr Am Ophth Soc . 1998;vol XCVI355–367. [PMC free article] [PubMed]
- 32.Taherian K, MacKenzie J M, Atta H R. Ultrasound biomicroscopy: fisherman's tale. Br J Ophthalmol 2002861445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McWhae J A, Crichton A C. The use of ultrasound biomicroscopy following trabeculectomy. Can J Ophthalmol 199631187–191. [PubMed] [Google Scholar]
- 34.Caronia R M, Liebmann J M, Stegman Z.et al Increase in iris‐lens contact after laser iridotomy for pupillary block angle closure. Am J Ophthalmol 199612253–57. [DOI] [PubMed] [Google Scholar]
- 35.Chalita M R, Li Y, Smith S, Patil C.et al High‐speed optical coherence tomography of laser iridotomy. Am J Ophthalmol 20051401133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nozaki M, Kimura H, Kojima M.et al Optical coherence tomographic findings of the anterior segment after non‐penetrating deep sclerectomy. Am J Ophthalmol 2002133837–839. [DOI] [PubMed] [Google Scholar]
- 37.Baikoff G, Rozot P, Lutun E.et al Assessment of capsular block syndrome with anterior segment optical coherence tomography. J Cataract Refract Surg 2004302448–2450. [DOI] [PubMed] [Google Scholar]
- 38.Elgohary M A, Chauhan D S, Dowler J G. Optical coherence tomography of intraocular lens implants and their relationship to the posterior capsule: a pilot study comparing a hydrophobic acrylic to a plate‐haptic silicone type. Ophthalmic Research 200638116–124. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe G J, Capriolli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol 2004137156–169. [DOI] [PubMed] [Google Scholar]
- 40.Chan A, Duker J S, Schuman J S.et al Stage 0 macular holes. Observations by optical coherence tomography. Ophthalmology 20041112027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas D, Duguid G. Optical coherence tomography – a review of the principles and contemporary uses in retinal investigation. Eye 200418561–570. [DOI] [PubMed] [Google Scholar]