Abstract
Aim
To evaluate the influence of hydrophilic and hydrophobic acrylic material and haptic angulation on anterior capsule opacification (ACO).
Methods
Prospective study on 53 patients with bilateral age‐related cataract. Patients underwent standard cataract surgery by the same surgeon and randomly received a hydrophilic acrylic intraocular lens (IOL) in one eye and a hydrophobic acrylic IOL in the other eye. Forty five of these patients completed the one‐year follow‐up. The following parameters were assessed: decentration, buttonholing, anterolenticular gap (ALG), ACO, outgrowth and refractive outcome.
Results
At the one‐year follow‐up, ACO was seen in 80% of the hydrophilic and 100% of the hydrophobic IOLs. ACO was more intense in the hydrophobic IOLs (p<0.001). Outgrowth was seen in 42% of the hydrophilic and 2% of the hydrophobic IOLs (p = 0.0003). No case of persisting ALG was seen in the hydrophobic IOLs, but in 42% of the hydrophilic IOLs. The refractive outcome was −0.29 (SD 0.56) dioptres for the hydrophilic and 0.003 (SD 0.44) dioptres for the hydrophobic IOLs (p<0.001).
Conclusion
These results suggest that there is less ACO in hydrophilic acrylic than in hydrophobic acrylic IOLs. Although material properties might play a role, the angulated haptics of the hydrophilic IOLs exert an additional effect by the persisting ALG and a lack of contact between the IOL and the anterior capsule.
Keywords: acrylic intraocular lens, anterior capsule opacification, ACO, haptic angulation
Anterior capsule opacification (ACO) generally occurs early after surgery, sometimes within the first month.1,2 Excessive ACO can be accompanied by contraction of the capsulorhexis or even capsular phimosis3,4,5,6 and can lead to decentration of the intraocular lens (IOL), partial or total buttonholing of the rhexis, and IOL optic and axial shift of the IOL leading to ametropia.
Additionally, the whitening of the anterior capsule covering the IOL optic can hinder the visualisation and treatment of the peripheral retina. Extended capsular contraction has been reported in eyes with high myopia, pseudo‐exfoliation, uveitis, pars planitis, diabetes mellitus and retinitis pigmentosa.7,8,9 However, it also occurs to a certain extent in normal eyes after standard cataract surgery. The major factors that have been discussed as contributing to ACO and contraction of the capsulorhexis are: IOL material and surface properties,10 and size and overlap of the continuous curvilinear capsulorhexis.11,12
The purpose of this study is to evaluate the influence of IOL material and haptic design on ACO with hydrophilic and hydrophobic acrylic IOLs.
Materials and methods
The ACR6D SE (Laboratoires Cornéal®, Paris, France) is a single‐piece IOL made from a hydrophilic acrylic copolymer of hydroxyl‐ethyl methacrylate (HEMA) and ethyl methacrylate (EMA) with a water content of 26%. It has an optic with a diameter of 6.0 mm and an overall length of 12.0 mm. The optic has a sharp posterior edge. The ACR6D SE has 10° angulated haptics.
The AcrySof® SA60AT (Alcon® Fort Worth Tx, USA) is a single‐piece non‐angulated IOL made of hydrophobic acrylic material, comprising a biconvex optic with a diameter of 6,0 mm and an overall length of 13.0 mm. The optic has a sharp anterior and posterior edge.
Fifty three consecutive patients with age‐related cataract were included in this prospective study at the Department of Ophthalmology, Medical University of Vienna. Inclusion criteria were bilateral age‐related cataract. Exclusion criteria were a history of other ocular diseases or prior intraocular surgery, laser treatment, diabetes requiring medical control, glaucoma and retinal pathology that would make a postoperative visual acuity (VA) of 20/40 (decimal equivalent: 0.5) or better unlikely. All the research and measurements followed the tenets of the Helsinki Declaration. The approval of the ethics committee of the Medical University of Vienna and Vienna General Hospital was secured.
All patients underwent a preoperative examination and standard cataract surgery comprising peribulbar local anesthesia and a 3.2 mm limbo‐corneal tunnel incision. After filling the anterior chamber with a viscoelastic material, a continuous curvilinear capsulorhexis (CCC) was performed, followed by hydro‐dissection and phacoemulsification of the lens. All patients randomly received a hydrophilic acrylic IOL in one eye and a hydrophobic acrylic IOL in the other eye with an injector system.
The postoperative treatment consisted of prednisolone‐acetate (Ultracortenol® Novartis, Berne, Switzerland) and diclofenac‐sodium (Voltaren®, Novartis, Berne, Switzerland) eye‐drops four times a day for 4 weeks. At the time of surgery, the patients had a mean (SD) age of 76.9 (6.4) years with 39 (74%) female and 14 (26%) male patients. Patients were examined at baseline and 14 (4) months (one‐year follow‐up) after surgery. Of 53 patients at baseline, 45 were available for the one‐year follow‐up.
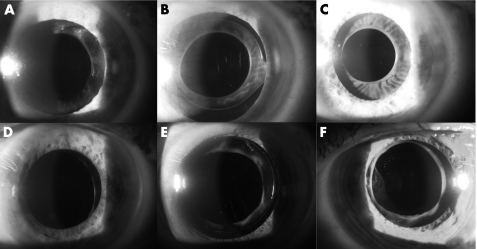
At the one‐year follow‐up, ACO intensity was subjectively assessed at the slit lamp (fig 1) using four categories:
Figure 1 Anterior capsule opacification (ACO) intensity and pattern: a diffuse mild; b diffuse moderate; c diffuse severe; d rhexis edge mild; e rhexis edge moderate; f rhexis edge severe.
none: clear (transparent) anterior capsule;
mild: mild whitening without capsular folding;
moderate: moderate whitening, sometimes with areas of capsular folding;
severe: intense whitening, with areas of capsular folding.
ACO was also divided into two morphological types: opacification of the “rhexis edge” and opacification of the entire capsule being in contact with the optic, i.e. “diffuse”. Gaps between the IOL‐optic and the capsular bag (anterolenticular gap, ALG) were subjectively categorised into (none, small, moderate, large) at the slit‐lamp (fig 2). Cases of outgrowth of lens epithelial cells (LECs) onto the IOL optic, decentration or buttonholing were documented. The IOL power and the targeted postoperative refractive error (spherical equivalent) were calculated using the SRK‐T formula with biometric data obtained with the IOL‐Master® (Carl Zeiss Meditec AG Jena, Germany). The postoperative refractive error was assessed at the follow‐up. The refractive outcome was calculated as the difference between measured refractive error and targeted refractive error.
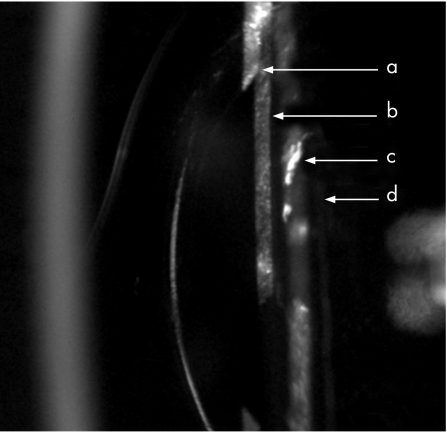
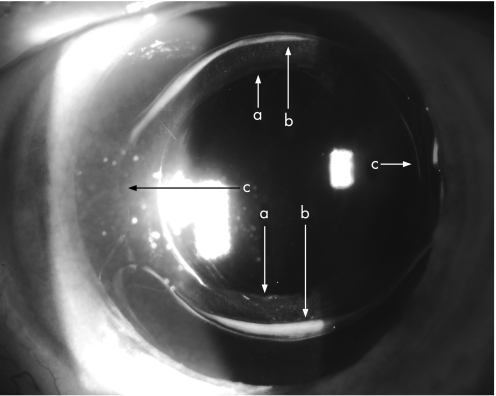
Figure 2 Hydrophilic intraocular lens (IOL) after one year: anterolenticular gap (ALG) small, ACO mild at rhexis edge. a rhexis edge ACO; b IOL anterior surface; c IOL posterior surface; d vitreus anterior limiting membrane.
All statistical analysis was performed using NCSS® software. Analytical statistics were performed with paired tests as fellow eyes of each patient were compared (intrapatient design) using McNemar's test for categorial data and paired t‐tests for ordinal and scaled data. p values are results of two‐sided tests if not explicitly stated otherwise. Results were deemed to be statistically significant at p<0.05. Spearman rank correlation coefficients were calculated with one‐sided p values.
Results
In general, we found ACO in 67% of the hydrophilic and 100% of the hydrophobic acrylic IOLs (table 1). Hydrophilic IOLs had less intense ACO than hydrophobic IOLs (p<0.05).
Table 1 Anterior capsule opacification (ACO) in hydrophilic and hydrophobic acrylic intraocular lenses (IOLs): detailed results after one year.
| Hydrophilic | Hydrophobic | p | |
|---|---|---|---|
| ACO intensity | 67% (30) | 100% (45) | 0.0000016 |
| None | 33% (15) | 0% (0) | |
| Mild | 51% (23) | 33% (15) | |
| Moderate | 16% (7) | 56% (25) | |
| Severe | 0% (0) | 11% (5) | |
| ACO rhexis edge | 33% (15) | 13% (6) | 0.088 |
| ACO diffuse | 33% (15) | 87% (39) | 0.000016 |
| Outgrowth | 40% (18) | 2% (1) | 0.00034 |
| Refractive outcome (SD) | −0.29 (0.56) | 0.03 (0.44) | 0.0001 |
| Decentration | 2%(1) | 0% (0) | — |
| ALG | 0.00013 | ||
| None | 58 (26) | 100% (45) | |
| Small | 27 (12) | 0% (0) | |
| Moderate | 9 (4) | 0% (0) | |
| Large | 7 (3) | 0% (0) | |
| Buttonholing | 2%(1) | 0% (0) | — |
Diffuse ACO (fig 1a–c) was seen in 33% of the hydrophilic acrylic IOLs and 87% of the hydrophobic acrylic IOLs (p<0.05). Rhexis edge ACO (fig 1d–f) was found in 33% of the hydrophilic acrylic IOLs and 13% of the hydrophobic acrylic IOLs but the difference of this distribution was not significant (p = 0.088). Outgrowth was seen in 40% of the hydrophilic acrylic IOLs and 2% of the hydrophobic acrylic IOLs.
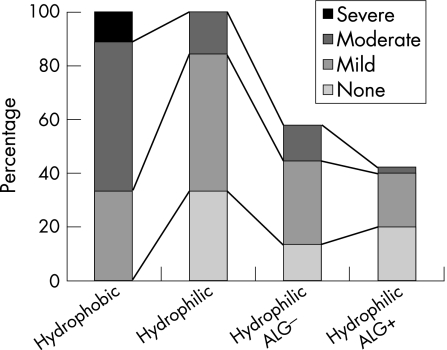
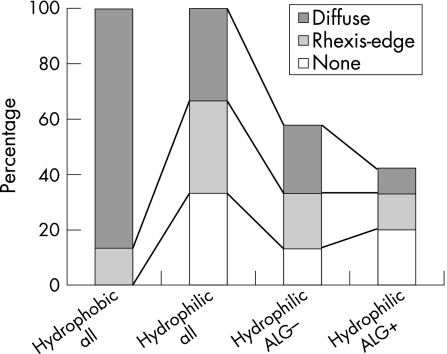
In the hydrophilic acrylic IOLs (table 2), the intensity as well as the ACO type and outgrowth inversely correlated with the persistence of an ALG. The correlation was weak but significant for ACO intensity (fig 3; c = −0.31, p = 0.018), diffuse ACO (c = −0.27, p = 0.035) and outgrowth (c = −0.33, p = 0.013). In hydrophilic IOLs, there was no significant correlation found for rhexis edge type ACO (fig 4; c = 0.022, p = 0.44) and ALG. As there were no persisting ALGs in hydrophobic acrylic IOLs, no correlation could be computed.
Table 2 ACO intensity and types in hydrophilic IOLs with and without anterolenticular gap (ALG).
| ALG − | ALG + | Correlation coefficient | p (one‐sided) | |
|---|---|---|---|---|
| Intensity | −0.31 | 0.018 | ||
| No | 13% (6) | 20% (9) | ||
| Mild | 31% (14) | 20% (9) | ||
| Moderate | 13% (6) | 2% (1) | ||
| Severe | 0% (0) | 0% (0) | ||
| Rhexis edge | 20%(9) | 13%(6) | −0.022 | 0.44 |
| Diffuse | 24%(11) | 9% (4) | −0.27 | 0.035 |
| Outgrowth | −0.33 | 0.013 | ||
| No | 27% (12) | 33% (15) | ||
| Yes | 31% (14) | 9% (4) |
Figure 3 ACO intensity in hydrophilic and hydrophobic IOLs and ALG after one year.
Figure 4 ACO types in hydrophilic and hydrophobic IOLs and ALG after one year.
During examination, we also found fibrosis in four hydrophilic IOLs at the IOL edge, which, to our knowledge, has not been described in the literature (fig 5). We could not reliably assess the true number of hydrophilic IOLs affected by this type of fibrosis as the necessary strong mydriasis could not be achieved in many eyes.
Figure 5 Fibrosis of the IOL edge in a hydrophilic IOL: a rhexis edge; b IOL‐edge fibrosis; c haptic insertion.
The refractive outcome for the hydrophilic acrylic IOLs was −0.29 (SD 0.56) dioptres (median:−0.16) and for the hydrophobic acrylic IOLs 0.026 (SD 0.44) dioptres (median: 0). The difference was significant (p = 0.0001).
Discussion
In this study, hydrophobic and hydrophilic acrylic IOLs were found to have a different induction of ACO one year postoperatively. In general, hydrophilic IOLs induced less intense ACO. The proportion of ACO of the rhexis edge was higher in the hydrophilic than in the hydrophobic IOLs as well as the proportion of outgrowth. The hydrophilic IOLs more often had persisting ALG, which inversely correlated with ACO intensity, diffuse ACO and outgrowth but not with ACO of the rhexis edge.
ACO and IOL material
ACO is understood to be the result of a fibrous dysplasia of residual anterior lens epithelial cells (LECs) coming into contact with the anterior surface of the IOL.13,14 Hydrophilic acrylic material is said to be a better matrix for migration of cells,15,16,17 i.e. has worse capsular biocompatibility, but adheres less to inflammatory cells,10,18 that is has better uveal biocompatibility than hydrophobic acrylic material or silicone.19,20,21
Saika et al22 suggest that the biocompatible hydrophilic acrylic material allows more postoperative LEC proliferation, maintaining an active wound‐healing reaction with continuous cell proliferation and an epithelial‐cell phenotype. On the contrary, in hydrophobic IOLs, cells would switch to a fibroblast‐like phenotype.
Whereas the ACO of hydrophobic acrylic single‐piece IOLs is already well described in the literature, no studies for hydrophilic acrylic IOLs with angulated haptics are available. The hydrophilic acrylic material (HEMA/EMA) used in the ACR6D and ACR6D SE is different from other hydrophilic IOL materials described so far and has a water content of 26%. For the ACR6D, the predecessor of the ACR6D SE, a hydrophilic acrylic IOL made of the same material but without angulated haptics, Tognetto et al23 found ACO in 100% after 2 years. The ACR6D had higher ACO rates than AcrySof® and Hydroview® IOLs. High ACO rates for the ACR6D were also found by Park et al.24
ACO and haptic angulation
Compared with Tognetto's and Park's findings, less ACO was found in the hydrophilic IOLs in our study. As the inverse correlation of ACO intensity and ALG suggests, this could be explained by the haptic angulation, a new feature of the ACR6D SE. The haptic angulation could have prevented permanent contact of the anterior capsule and the IOL. This would lead to less intense ACO than one could have expected from the ACR6D SE's material and the broad fenestrated haptics, which per se would have offered more contact area with the anterior capsule leading to more intense ACO.25 Therefore, haptic angulation and its influence on the contact between the anterior capsule and the IOL seem to be an important determinant for ACO.
ACO of the rhexis edge and IOL edge
Only a few studies have discerned diffuse ACO and ACO of the rhexis edge.21 Although Werner et al25 defined rhexis‐edge‐related ACO as a more minor degree of ACO than diffuse ACO, we chose to define the rhexis edge ACO as a category of its own. In this study, in the hydrophilic acrylic IOLs, the incidence of ACO of the rhexis edge did not correlate with a persisting ALG. Several reasons might explain this.
As Saika et al22 indicated in a histopathologic study, LEC proliferation was more marked at the edge of the anterior capsulotomy with a hydrogel IOL than with IOLs of other materials. Although the phenotype of LECs in hydrophilic acrylic IOLs seems to be less fibroblastic, there is expression of α‐smooth‐muscle actin, a contractile protein expressed in myo‐fibroblasts, collagen I and III, both usually expressed in fibroblastic cell types but not in epithelial cells.22 Perhaps marked proliferation of LECs at the rhexis edge in hydrophilic establishes contact of the rhexis edge and the IOL. It also seems possible that the trauma of the capsulorhexis can, per se, induce fibrosis of the rhexis edge or that the haptic angulation prevents permanent and/or entire contact of the anterior capsule and the IOL.
Fibrosis of the IOL edge in the hydrophilic IOLs in this study might be induced by the angulated haptics. These angulated haptics seem to exert a tangential stress onto the whole capsule. The originally round capsular bag could be deformed and pressed against the IOL in a 90° angle to the haptic insertion zones.
Currently, we have no data about how IOL‐edge fibrosis affects the barrier effect of the sharp optic edge. Further studies are needed to clarify this form of fibrosis.
Capsular contraction
In our study, the CCC sizes were not significantly different between hydrophilic and hydrophobic IOLs one year postoperatively. In vitro studies indicate that α‐smooth‐muscle actin can be detected in LECs regardless of the hydrophilicity of the acrylic material22 these LECs are in contact with.
Clinically, Hayashi et al26 found a more pronounced contraction of the anterior capsule in hydrophilic (Hydroview® H60M) IOLs than in hydrophobic acrylic IOLs (MA60BM) of the same material as used for the hydrophobic IOLs in our study. The hydrophobic acrylic IOL in their study, however, had 10° angulated haptics. Park et al24 found no significant reduction of the area of the CCC in the ACR6D and the MA60BM.
Outgrowth
There was practically no outgrowth in the hydrophobic acrylic IOLs at the one‐year follow‐up but outgrowth was found in 40% of the hydrophilic IOLs in this study.
Outgrowth is frequent in all types of IOLs immediately after surgery. It is known to degenerate over time.21 The degeneration of outgrowth is more pronounced in hydrophobic acrylic materials, whereas hydrophilic acrylic IOLs are frequently found to have persisting outgrowth. It is generally believed that this is an effect of the hydrophilicity of the material which is promoting and prolonging an active wound‐healing process.17,21 Therefore, LEC proliferation is more increased at the edge of the CCC with a hydrophilic acrylic IOL.21
Koch et al16 found outgrowth in 33% of hydrophilic acrylic IOLs (Hydroview®). Georgopoulos et al27 found outgrowth in 62% one year after surgery and in 90% 2 years after surgery in the same IOL (Hydroview®).28 For the ACR6D, outgrowth was found to be even higher than in other hydrophilic IOLs (Stabibag® and Hydroview®).18,23 In one of our previous studies29 we only found 15% outgrowth in the same hydrophilic IOLs (ACR6D SE) as used for this study. One can expect lower outgrowth rates for (hydrophilic acrylic) IOLs when the anterior capsule is not in contact with the IOL optic.
Refractive error
For the hydrophobic IOLs in this study, the targeted spherical equivalent was not significantly different from the postoperatively measured spherical equivalent one year after surgery. However, the postoperatively measured spherical equivalent of the hydrophilic acrylic IOLs was lower than targeted. The difference was small (0.29 (SD 0.56) dioptres) but significant.
In part, an A‐constant of 120.0 was used for the power calculation with the IOL‐Master® whereas the recommended A‐constant is 119.8. Further, in angulated IOLs a postoperative forward shift was detected,30,31 which was attributed to a decay of memory of the haptics. The weak intensity of ACO in the hydrophilic IOLs could add to a postulated anterior shift, as the anterior capsule could be a weak bearing for the haptics resulting in a weaker pressure towards the posterior.
We do not believe that ACO‐induced capsular contraction3 or sharp‐optic edges of IOLs resulting in less posterior fibrosis31 have significantly influenced the IOL position of the IOLs, as this would have lead to a posterior shift and relative hyperopia.
Conclusion
In this study, we found less intense ACO in hydrophilic acrylic IOLs with haptic angulation than in hydrophobic acrylic IOLs without haptic angulation. Our results indicate that intense and diffuse ACO also occurs in hydrophilic acrylic IOLs when contact of the anterior capsule and the IOL is given. At least some of the ACO differences between the IOLs seem to be due to haptic angulation. Hence, haptic angulation and anterior capsule contact seem to be an important determinant of ACO. Future studies should examine this variable, rather than purely at optic material.
Abbreviations
ACO - anterior capsule opacification
ALG - anterolenticular gap
CCC - continuous curvilinear capsulorhexis
IOL - intraocular lens
LEC - lens epithelial cell
Footnotes
Competing interests: None.
References
- 1.Joo C K, Shin J A, Kim J H. Capsular opening contraction after continuous curvilinear capsulorhexis and intraocular lens implantation. J Cataract Refract Surg 199622585–590. [DOI] [PubMed] [Google Scholar]
- 2.Hara T, Azuma N, Chiba K.et al Anterior capsular opacification after endocapsular cataract surgery. Ophthalmic Surg 19922394–98. [PubMed] [Google Scholar]
- 3.Hansen S O, Crandall A S, Olson R J. Progressive constriction of the anterior capsular opening following intact capsulorhexis. J Cataract Refract Surg 19931977–82. [DOI] [PubMed] [Google Scholar]
- 4.Davison J A. Capsule contraction syndrome. J Cataract Refract Surg 199319582–589. [DOI] [PubMed] [Google Scholar]
- 5.Young D A, Orlin S E. Capsulorhexis contracture in phacoemulsification surgery. Ophthalmic Surg 199425477–478. [PubMed] [Google Scholar]
- 6.Spang K M, Rohrbach J M, Weidle E G. Complete occlusion of the anterior capsular opening after intact capsulorhexis: clinicopathologic correlation. Am J Ophthalmol 1999127343–345. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi H, Hayashi K, Nakao F.et al Area reduction in the anterior capsule opening in eyes of diabetes mellitus patients. J Cataract Refract Surg 1998241105–1110. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi H, Hayashi K, Nakao F.et al Anterior capsule contraction and intraocular lens dislocation in eyes with pseudoexfoliation syndrome. Br J Ophthalmol 1998821429–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi K, Hayashi H, Matsuo K.et al Anterior capsule contraction and intraocular lens dislocation after implant surgery in eyes with retinitis pigmentosa. Ophthalmology 19981051239–1243. [DOI] [PubMed] [Google Scholar]
- 10.Miyake K, Ota I, Miyake S.et al Correlation between intraocular lens hydrophilicity and anterior capsule opacification and aqueous flare. J Cataract Refract Surg 199622(Suppl 1)764–769. [DOI] [PubMed] [Google Scholar]
- 11.Auer C, Gonvers M. Implant intraoculaire monobloc en silicone et fibrose de la capsule antérieure. Klin Monatsbl Augenheilkd. 1995;206: 293–5, (English abstract. ) [DOI] [PubMed]
- 12.Kimura W, Yamanishi S, Kimura T.et al Measuring the anterior capsule opening after cataract surgery to assess capsule shrinkage. J Cataract Refract Surg 1998241235–1238. [DOI] [PubMed] [Google Scholar]
- 13.Pande M V, Spalton D J, Marshall J. In vivo human lens epithelial cell proliferation on the anterior surface of PMMA intraocular lenses. Br J Ophthalmol 199680469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishi O, Nishi K, Sakka Y.et al Intercapsular cataract surgery with lens epithelial cell removal. Part IV: capsular fibrosis by poly(methyl methacrylate). J Cataract Refract Surg 199117471–477. [DOI] [PubMed] [Google Scholar]
- 15.Müllner‐Eidenböck A, Amon M, Schauersberger J.et al Cellular reaction on the anterior surface of 4 types of intraocular lenses. J Cataract Refract Surg 200127734–740. [DOI] [PubMed] [Google Scholar]
- 16.Koch M U, Kalicharan D, van der Want J J L. Lens epithelial cell layer formation related to hydrogel foldable intraocular lenses. J Cataract Refract Surg 1999251637–1640. [DOI] [PubMed] [Google Scholar]
- 17.Schauersberger J, Amon M, Kruger A.et al Lens epithelial cell outgrowth on 3 types of intraocular lenses. J Cataract Refract Surg 200127850–854. [DOI] [PubMed] [Google Scholar]
- 18.Tognetto D, Toto L, Ballone E.et al Biocompatibility of hydrophilic intraocular lenses. J Cataract Refract Surg 200228644–651. [DOI] [PubMed] [Google Scholar]
- 19.Dahlhauser K F, Wroblewski K J, Mader T H. Anterior capsule contraction with foldable silicone intraocular lenses. J Cataract Refract Surg 1998241216–1219. [DOI] [PubMed] [Google Scholar]
- 20.Reeves P D, Yung C W. Silicone intraocular lens encapsulation by shrinkage of the capsulorhexis opening. J Cataract Refract Surg 1998241275–1276. [DOI] [PubMed] [Google Scholar]
- 21.Abela‐Formanek C, Amon M, Schauersberger J.et al Results of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in uveitic eyes with cataract—comparison to a control group. J Cataract Refract Surg 2002281141–1152. [DOI] [PubMed] [Google Scholar]
- 22.Saika S, Miyamoto T, Ohnishi Y. Histology of anterior capsule opacification with a polyHEMA/HOHEXMA hydrophilic hydrogel intraocular lens compared to poly(methyl methacrylate), silicone, and acrylic lenses. J Cataract Refract Surg 2003291198–1203. [DOI] [PubMed] [Google Scholar]
- 23.Tognetto D, Toto L, Sanguinetti G.et al Lens epithelial cell reaction after implantation of different intraocular lens materials. Ophthalmology 20031101935–1941. [DOI] [PubMed] [Google Scholar]
- 24.Park T K, Chung S K, Baek N H. Changes in the area of the anterior capsule opening after intraocular lens implantation. J Cataract Refract Surg 2002281613–1617. [DOI] [PubMed] [Google Scholar]
- 25.Werner L, Pandey S K, Apple D J.et al Anterior capsule opacification: correlation of pathologic findings with clinical sequelae. Ophthalmology 20011081675–1681. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi K, Hayashi H, Nakao F.et al Anterior capsule contraction and intraocular lens decentration and tilt after hydrogel lens implantation. Br J Ophthalmol 2001851294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgopoulos M, Menapace R, Findl O.et al Posterior continuous curvilinear capsulorhexis with hydrogel and silicone intraocular lens implantation—development of capsulorhexis size and capsule Opacification. J Cataract Refract Surg 200127825–832. [DOI] [PubMed] [Google Scholar]
- 28.Georgopoulos M, Menapace R, Findl O.et al After‐cataract in adults with primary posterior capsulorhexis—comparison of hydrogel and silicone intraocular lenses with round edges after 2 years. J Cataract Refract Surg 200329955–960. [DOI] [PubMed] [Google Scholar]
- 29.Vock L, Georgopoulos M, Sacu S.et al Eigenschaften der hydrophilen single‐piece Linse ACR6D SE. Spektrum Augenheilk 20046288–293. [Google Scholar]
- 30.Wirtitsch M G, Findl O, Menapace R.et al Effect of haptic design on change in axial lens position after cataract surgery. J Cataract Refract Surg 20043045–51. [DOI] [PubMed] [Google Scholar]
- 31.Petternel V, Menapace R, Findl O.et al Effect of optic edge design and haptic angulation on postoperative intraocular lens position change. J Cataract Refract Surg 20043052–57. [DOI] [PubMed] [Google Scholar]