Abstract
Goals
To review the literature on chondrocyte movements and to develop plausible hypothesis for further work.
Design
Chondrocyte movements are herein defined as translocations of the cell body. To set the stage for a discussion of chondrocyte moves, a brief overview of cell migration in other cell types is presented, including a discussion of the challenges that cells find when moving within tissues. Reports of isolated chondrocyte migration in vitro (isolated cell systems) and ex vivo (cartilage organ cultures) are then summarized, followed by a discussion of recent studies that infer chondrocyte movements in vivo.
Results
Investigators from different laboratories have observed chondrocyte motility in vitro. I became interested in the question of whether articular chondrocytes retained their phenotype during their migratory excursions. We devised a simple method to separate migratory and stationary chondrocytes and then showed that migratory chondrocytes synthesized collagen II but not I—consistent with a differentiated phenotype. Our time-lapse video microscopy studies showed that the cells displayed appropriate movement kinetics, albeit with low speed and directionality. Similarly, others have presented data consistent with slow movement of chondrocytes out of cartilage explants. It is important to decipher whether these in vitro movements reflect physiological states and if so, which events are simulated. Examples of in vivo studies that have inferred chondrocyte movements include those describing rotational or gliding movements of chondrocytes in the proliferative zone of the growth plate and its importance in the growth process; and the notion that chondrocytes move from the cartilage endplates to the nucleus pulposus in the spine of rabbits and rats during development. Such studies are consistent with the hypothesis that chondrocytes exhibit highly controlled and specialized movements during tissue growth and remodeling in vivo. On the other hand, the cartilage explant studies elicit interest in the possibility that matrix injuries resulting in disruption of the collagen network of adult cartilages provide a permissive environment for chondrocyte motility.
Conclusions
The case for in vivo chondrocyte motility remains to be proven. However, the in vitro and in vivo data on chondrocyte movements present an argument for further thought and studies in this area.
Keywords: chondrocyte movement, migration, cell processes
Design
The overall aim of this review is to describe representative findings in the relatively unexplored area of chondrocyte movements. I start by presenting an overview of knowledge in the fascinating and rapidly evolving area of cell migration to provide a general context for discussion of chondrocyte moves. The problem posed by the concept of chondrocyte movements within their tissue environment is then presented, followed by a presentation of selected papers reporting on chondrocyte movements in various experimental systems. A general criterion used for the inclusion of papers for discussion was the evidence for central cell body displacement (movement of the cell center from point A to B). Examples of the elaboration of chondrocytic pseudopods or extensions are also presented since the deployment of cell extensions is a critical aspect of cell motility.
Introduction
Cell Migration Overview
Cell motility is a fascinating multistep process, which underlies a variety of physiological processes. Indeed, it has been noted that cell migration accompanies us from conception through life. Examples include the modeling of new tissues in the embryo, as well as angiogenesis, immune responses, and wound repair in the adult. On the other hand, deregulated cell migration drives the progression of mental retardation, atherosclerosis, and tumor metastasis. Excellent reviews covering multiple topics on cell migration are available1-4 (the reader may also enjoy visiting the cell migration consortium web site to view up to date information: www.cellmigration.org). The exact mechanisms that a particular cell uses to initiate and maintain motility are governed by the cell's genetic machinery and the sum of external stimuli. Both the extracellular matrix and the growth factor milieu provide motility signals, which the cells coordinate through associations of their signaling receptors, and by coupling of downstream intracellular effectors5-8. Major signaling pathways, including the phosphoinositol 3 phosphate (PI3K), mitogen activated protein kinase (MAPK) and phospholipase Cλ can be selectively used by cells-- along with a myriad of allied signaling intermediates-- to feed into central relay systems that promote physicochemical changes in the cell. For example, activation of myosin II (a central molecular event) can occur downstream of PI3K via the Rho kinase or as a result of ERK activation and downstream activation of the myosin light chain kinase. Activation of myosin governs cell contractility via the actinomyosin skeleton 3,4,9,10. The classic model of cell migration on planar surfaces describes various physicochemical events, including: (1) Polarization of the cell body in direction of the stimulus (this means that the molecular processes at the front and back of a moving cell are different); (2) Protrusion formation: Cells typically elaborate a dominant lamellipodia at the leading edge to sense the environment and anchor to the substratum while associated lamellae help to pull it forward, and multiple filopodia sense, grip and bend the surrounding matrix; (3) Regulation of adhesion to the substratum: Cells form focal complexes to stabilize the lamellipodia, to serve as traction sites over which the cell moves, and to act as mechanosensors that help to regulate cytoskeletal dynamics; (4) Contractility of the cell body: this transmits force to the adhesive complexes and supports cell deformation and; (5) Detachment of the rear to pull the cell forward. Cell migration is indeed complex; it requires that events such as those described above and other allied functions be highly coordinated in time and space to keep the cell moving forward.
Cell Migration on 3D Surfaces
To operate in vivo, cells require the ability to move in 3-dimensional environments. The vast majority of cell migration studies have been conducted by following movements of cells and/or cell lines on planar surfaces. These investigations have provided an impressive and rapidly growing literature. There is evidence11,12 that the information emanating from these studies can shed light into regulatory mechanisms of cell movement within model 3D and in vivo environments. For example, in the study of Wang et al12, green fluorescent protein (GFP)-labeled carcinoma cells were collected from tumors growing under rat mammary fat pads. Cell harvesting involved the use of microinjection needles that delivered a chemoattractant while migratory cells were monitored via multiphoton microscopy. In this manner invasive cells could be aspirated by microneedles and separated from the general tumor cell populations. Strong differential upregulation of genes coding for the “minimum motility machine” was observed in the invasive cells. The biochemistry and organization of the proteins of the “minimum motility machine” had been deciphered using simpler in vitro models of motility13-15 and it was known that this molecular set regulates β-actin polymerization at the leading edge. Thus, the simpler models aided interpretation of the results of the carcinoma study. On the other hand, as intuitively sound, important differences have been noted between 2D and 3D systems12,16. Indeed, cell movement within dense connective tissues requires mechanisms to allow the cells to overcome the barriers imposed by the tightly woven collagen networks, which often have orifices considerably smaller than a cell diameter. In this review, I will use the terms cell movement, motility or migration to signify cell displacement on any surface while invasiveness will be used to selectively indicate movement through model 3D matrices or tissue barriers. In their amazing adaptability and flexibility, cells have evolved at least 2 strategies for tissue invasion: proteolytic removal of barriers and amoeboid locomotion. These are described below with the aid of examples.
The refined involvement of proteolytic enzymes in the process of cell migration is well documented (for review, please refer to 17). Importantly, there is good evidence that in a number of cases, proteases involved in cell movement and matrix invasion are membrane-bound and polarized to leading edges18-22. Studies of the invasion of a fibronectin-gelatin substratum23 by melanoma cells give us an impressive example of the importance of polarization. The locomotion of these cells involves the localization of MT1-MMP (a membrane-bound member of the metalloproteinase family) to invasive leading edges (“invadopodia”). The importance of this segregation is shown in the study by treatment of the cells with concanavelin A, which localizes the protease on the cell membrane without targeting it to the invadopodia. This results in a proteolytically active MT1-MMP, but the cells become non-invasive.
Interestingly, cells appear to have evolved protease-independent mechanisms to overcome tissue barriers. In fact, it is worth noting that while the extracellular matrix can present a formidable barrier to cell movements, the other side of the coin is that --within certain permissive environments—cell migration is aided by “contact guidance” and directionality provided by collagen fibers24,25. Further, the tensile strength of collagen provides a relatively nondistensible surface that allows the cells to get a “good grip”24-26. Studies using fluorescent confocal microscopy to monitor differentially tagged cells (neutrophils) and matrix (chorioallantoic membrane) provide a very clear visualization of neutrophil-matrix interactions during locomotion27. The majority of cells are seen migrating along the length of collagen fibers, changing from one fiber to the other now and then. Occasionally they are seen “grabbing and pulling” on the collagen, promoting some movement of the fibers. Extension of lateral pseudopods and their insertions into “footholds” in the matrix appear to help to pull the cells forward towards the constrictions. Strikingly, the neutrophils can squeeze and crawl through narrow openings aided by constriction of their cell bodies. Clearly, this dynamic depends not only on the cell type but also on the nature of the matrix: recent quantitative studies of cell migration on model 3D matrices directly relate speed of locomotion to matrix stiffness when other parameters are held constant16.
The flexibility of some cells in their selection of locomotive “style” is directly demonstrated by observations of fibrosarcoma cells (transfected with MT1-MMP) and of carcinoma cell lines moving through collagen lattices28. Normally, these cells move in a typical protease-aided fashion, displaying elongated (fibroblast-like) morphology, integrin clustering, and integrin/MT1-MMP co-localization at the leading edge. Surprisingly, in the presence of a mix of protease inhibitors, the “protease-challenged” cells resort to a more rounded morphology, use deformation of their cell bodies to enter restricted tissue spaces and display propulsive “amoeboid”-type movements28,29. These studies and others11,12 suggest that certain cells are able select their mobile “equipment” in response to their make up or environment: a thoroughly sensible strategy.
The Problem: Movement Within Cartilage
Despite the amazing cellular feats described above, we need to ask how chondrocytes could move within the apparently impenetrable cartilage matrix at any time during their life cycle. It is well known that when we get a cut in the skin, neighboring cells divide and move towards the wound site to lay down repair tissue. On the other hand, post-natal articular cartilage does not heal partial depth defects effectively (i.e. defects that do not penetrate the underlying bone), likely due to lack of sufficient reparative cells at the defect site. Except for the thin superficial zone of articular cartilage, adult chondrocytes usually maintain a spheroid shape throughout the depth of the tissue30. Within these vast areas, the chondrocytes are surrounded by the proteoglycan-rich pericellular matrix and by the territorial (or capsular) basket-like matrix characterized by the presence of a network of fibrillar collagen (chondrons). The interterritorial matrix, also enriched in collagens and proteoglycans, comprises vast expanses of the tissue. Furthermore, the cartilage matrix contains the large proteoglycan aggrecan, which binds to long chains of hyaluronan creating enormous polyanionic complexes. Aggrecans exist within cartilage in a partially compressed state because of their entrapment within the interstices of the highly tensile collagen network31,32; this state of partial compression imposed by collagen is opposed by the swelling tendency of the polyanionic aggrecans resulting in a highly pressurized matrix. Thus, the problem of chondrocyte migration is an extremely challenging one, which requires an explanation as to how the cells could overcome the density and pressure of the surrounding matrix to migrate to other sites. Also, it is intriguing whether cells could accomplish this under non-pathological conditions, i.e. without adversely affecting tissue structure and function.
Chondrocyte Extensions and Movements Observed In Vitro
Chondrocyte Processes
The in vitro work of Lee and Loeser33 gave provocative insights into the relationship between chondrocytes and their fibrillar environment. Video-enhanced differential interference contrast (DIC) microscopy showed that isolated chondrocytes could interact with a surrounding collagen II lattice and “wrap” the collagen fibrils around their bodies. Further, sharp bending of the collagen fibrils in close proximity to the cells was well documented; in one dramatic capture, a cell extended two cell processes to contact a collagen fibril that was subsequently moved and bent. Interestingly, rounded cells were fully capable of interacting with collagen in this manner. Vautier et al34 explored the effect of substratum charge on pseudopod formation. These authors coated titanium beads with negatively or positively charged films and seeded cells from a human-chondrosarcoma derived cell line (HCS-2/8) on the beads. It was noted that the negatively charged beads encouraged pseudopod formation and that these processes contained actin and tubulin. This paper also included impressive capture of cell dynamics: for example, cells located on adjacent beads could be seen projecting pseudopods towards one another. In other photos, long processes (100-160 μm) breached the space between beads and connected cell-to-cell or cell-to-bead (consider that the cell lengths ranged between 10-17 μm). The influence of the sarcoma nature of the cell line on protrusion formation is not known. In fact, these in vitro studies may lead some to ask if either type of cell extension-- capable of sensing the distant environment34 or of bending stiff collagen fibers33 -- is an artifact of cell culture. However, as discussed in the next section, long cell processes have also been observed in intact cartilage in vivo35 suggesting that the in vitro observations may well simulate or reflect aspects of physiology.
Chondrocyte migration in model in vitro systems
A number of pioneering studies have shown that isolated chondrocytes are able to migrate under the direction of different stimuli on or within various planar and 3D matrices. For example, it has been reported that chondrocytes move in response to bone morphogenetic factors36, hepatocyte scatter factor37, urokinase plaminogen activator38, insulin-like growth factor-139, transforming growth factor-β39, platelet derived growth factor40, and fibroblast growth factor41,42. Further, the cells can move towards hyaluronic acid or on sulfated hyaluronic acid42,43, fibronectin38,39, fibrin44, collagen I38, and even polarize and move towards cathodic electrical fields45. Perhaps more importantly, various investigators have observed chondrocyte movement through 3D collagen I gels36,46 (also Morales, 2005, unpublished) or polymer scaffolds (ε-caprolactone-co-lactide)46. Most of these studies used primary chondrocytes and did not report on the phenotype of the cells. In 2003, we39 developed a straightforward method to phenotype migratory chondrocytes. The cells were attached on the fibronectin-coated porous membrane of a Boyden chamber and stimulated by a chemoattractant (IGF-I) in the lower chamber to migrate and squeeze through the 8 μm pores of the membrane to the underside. The stationary cells on top of the membrane were then removed and the migrated cells on the underside collected for analysis. The migrated cells synthesized collagen II but not collagen I, demonstrating for the first time that chondrocytes can move without loss of their differentiated collagen II phenotype. We also observed chondrocyte dynamics by time-lapse video microscopy on fibronectin-coated plates (Figure 1) in the presence of 10 ng/ml IGF-I. Under the experimental conditions, only ∼1/3 of the cells looked well attached the substratum and 20 fields enriched in well attached cells were selected for monitoring. Approximately 25-35% of the cells in these fields displayed significant movements. The speed (‘rate’ at which a cell moves) and directional persistence (measure of the average time between ‘significant’ (60°) changes in direction) were used to define motility as >1 μm/h or >1h respectively. Figures 1A and 1B show examples of a stationary and motile cell (representative of nearly 200 tracked cells): The former cells were rounded and showed few, very short cell protrusions, while the migratory cells were well spread, polarized and elaborated typical lamellipoida and filopodia. Within the population of motile cells, about half moved faster than 5μm/h, but only occasional cells moved faster than 50μm/h. These parameters indicate that under the experimental conditions, chondrocytes were slow compared to other cell types (movement speeds in the range of 20-720 μm/h have been recorded by others for various mammalian cells39). On the other hand, the majority of the migratory cells (∼2/3) exhibited directional persistence of 1-10 h, while the remaining one third showed persistence > 10 h. Path lengths (speed x directional persistence) for chondrocytes were found to mainly fall within 30-50 μm, i.e. on the average, the cells can move 30-50 μm before changing direction. Note that the cell path does not establish which direction the cells move in. In these experiments, the IGF-I stimulant was dissolved in the medium bathing the cells, i.e. it was not provided as a chemotactic stimulus. The later may be expected to help establish the orientation of the cells in the direction of the stimulus. Maniwa et al42 reported speeds of chondrocyte movement within the same range as we did. In their studies, chondrocytes were stimulated with 10 ng/ml basic fibroblast growth factor (bFGF) and displayed mean speeds of ∼10 μm/h; when stimulated by both bFGF and hyaluronan (1mg/ml) their speed increased to ∼15 μm/h. It is worth noting that while movement kinetics on planar substrates can provide insight into the abilities of a particular cell type, the kinetics of cell movement on complex 3D matrices can be expected to differ. Additional regulatory elements come into play, both positive and negative. For example, collagen fibers may improve directionality24,25. Also, recent modeling of 3D motility indicates a strong dependency of cell speed and directionality on matrix pore size, as well as on the ability of cells to deform their cell bodies and to deploy MMPs47.
Figure 1. Individual Chondrocyte Videotracking.
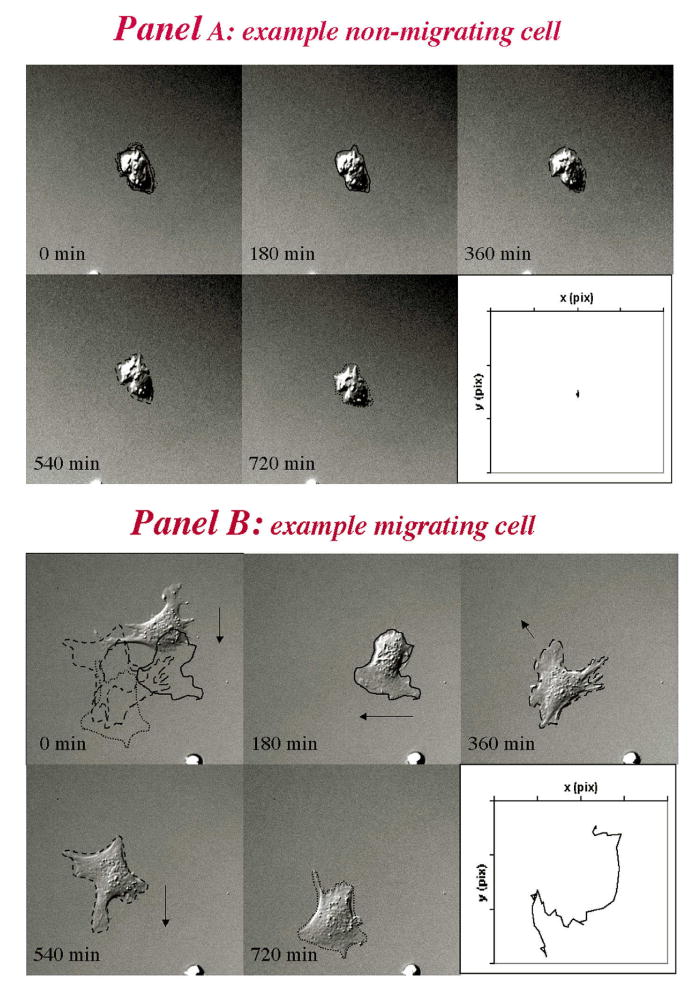
Example of a non-motile (panel A) and a motile cell (panel B). The cell images were captured at the times indicated. At each time, the shape of the cell is outlined. The cell at time 0 shows the superimposed outlines of the cell during subsequent movement captured from the individual frames shown in the different panels. The arrows show the direction of movement, and the inset on the lower right of each panel shows the cell trajectories. Copied from Chang C., Lauffenburger DA and Morales, T.I., OA and C, 2003.
Independent of migration kinetics, we now know that chondrocytes are able to deploy and organize the complex machinery required for locomotion. Given the energy and spatiotemporal “finesse” required for cell movement, it is not likely that the chondrocyte would acquire this capability de novo as a culture artifact under a variety of conditions36-46 and maintain it in their differentiated state39. The possibility that the movements observed in vitro reflect-- at least to some extent-- aspects of incompletely defined vivo functions needs to be considered.
Chondrocyte Extensions and Inferred Movements in 3D
In vivo cell processes
The existence of a primary cilium in chondrocytes and other finger-like processes in chondrocytes within native cartilage has been well established30,48,49. Normally, these are relatively small (5 μm or less) and confined inside the chondron. The primary cilium—presumably involved in mechanotransduction-- is the best characterized chondrocyte process, with recent data indicating that this cell extension expresses α2, α5 and β1 integrin receptors while apparently excluding other receptors detected in the cell body (annexin V and CD44)49. Interestingly, a recent study shows that the presence of cilia in mesenchyme cells is critical for endochondral formation during limb development50. It is unknown if or how cilia of any type could aid chondrocyte motility. Other types of cell extensions have also been described in cartilage. Bush and Hall35 reported on intriguing microscopy data capturing extremely long processes in vivo. These investigators carried out confocal scanning laser microscopy to determine the morphology of living, fluorescently labeled chondrocytes in situ within non-eroded and degenerate cartilage from human tibial plateaus. This technique revealed fine cytoplasmic processes extending from the cell body of a large proportion of chondrocytes of all OA grades. Indeed processes were present within cells of all zones and for cartilage of all grades from normal to severely degenerated in a relatively large proportion of the total cells: 31-41%. The authors noted that the processes were of variable length and that in some cases (<8 μm) they probably remained within the chondron while in other cases (>8 μm) they could transverse their fibrous confines. Indeed, in extreme cases, processes as long as 80 μm were observed. Neither the composition nor the functions of these cell processes are known, but it may be instructive to examine their possible dedication to cell movements and/or matrix remodeling51. It is interesting that very long cell processes have also been observed in the chondrocyte-like cells of the nucleus pulposus and inner annulus of the intervertebral disc, as well as in the fibroblastic cells of the outer annulus52.
Human Cartilage Organ Cultures
Lyman et al (Abstract53) prepared osteochondral disks from hip or knee arthroplasties and made central partial depth defects prior to culture. During the culture period, the authors noted that some of the cells in the vicinity of the wound polarized and extended processes, sometimes long enough (∼50 μm) to contact the wounded margin. Process extension was observed by DIC, confocal and transmission electron microscopy (TEM). These investigators quantified the number of cells in the vicinity (350 μm) of the wound edge at different time points in culture and showed that cells were slowly and progressively depleted from the superficial zone and from the floor of the defect. After 2 weeks, only ∼ 10% of the original cell density remained in these zones relative to areas more distal from the wound. By contrast, ∼60% of the cells remained in the area adjacent to the wound in the middle cartilage zone. After several weeks in culture, cells could be seen lining the defect site. The investigators noted that the apparent cell translocation took place predominantly in areas where the collagen fibril orientation led into the wound. Fibrillation of collagen in these areas may have released the cells from their matrix entrapment and enabled them to use the collagen fibers for traction and directionality. Possibly, the culture conditions contributed to the activation & polarization of chondrocytes. Nonetheless, this study provides interesting insights that may help to understand some aspects of the poor repair response of articular cartilage usually seen in vivo: (1) the lack of effectiveness of cells to polarize and move towards the wound site in areas where the collagen fibers are parallel to the edge of the wound (i.e. most of the cartilage depth); and (2) their inferred slow movements even in sites with the appropriate collagen architecture.
Other studies have likewise shown outgrowth of cells from human cartilage explants in culture54, 55. The above cited studies have in common the finding that disruption of the collagen network- by extensive cutting54, collagenase digestion55 or defect drilling53-is accompanied by chondrocyte outgrowth. It is unclear whether in some cases, the exit of chondrocytes from the cartilage is solely due to a robust proliferative response of cells close to or on the edge of the wounds56 followed by their attachment to outer planar surfaces and subsequent migration. However, the electron microscopy of Lyman et al53 indicates that in their experimental model (partial depth defects in human articular cartilage in culture) cells polarize and elaborate extensions while still within cartilage, a finding consistent with a motile phenotype.
Growth Plates
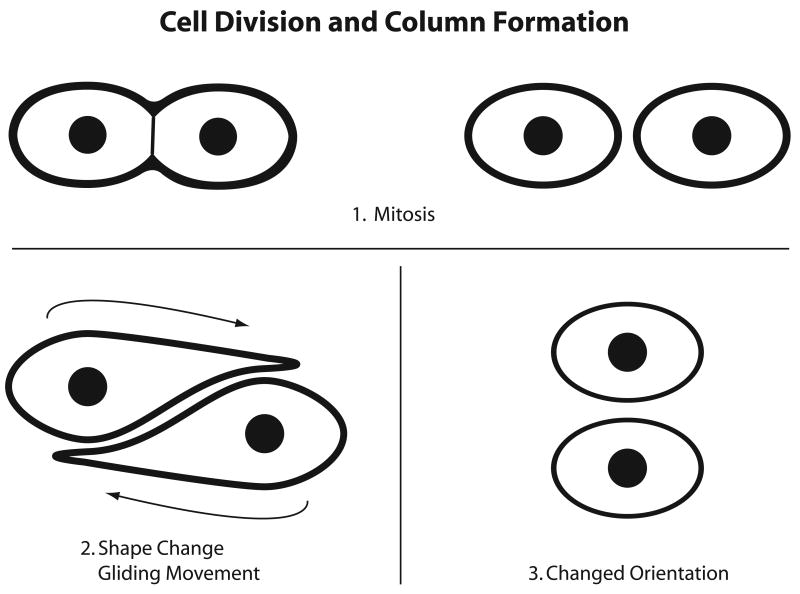
The growth plate is a specialized region of cartilage that sustains the longitudinal growth of bones during development. In the normal growth plates, the resting cell zone contains chondrocytes dispersed in the matrix without an apparently ordered organization. This reservoir zone maintains the continuous expansion of the proliferative zone, which is comprised of longitudinal columns of cells. At the lower end of the growth plate, the proliferative cells hypertrophy. The hypertrophic cells are eventually replaced by bone. As early as 1930, Doods57 suggested that chondrocytes must undergo extensive movements to arrange in longitudinal columns. Aszodi et al.58 recently presented elegant data that suggest to us that chondrocytes in the growth plate may indeed be capable of such mobility. These investigators show that mice carrying a cartilage-specific deletion of the β1 integrin gene present with a severe chondrodysplasia and a high rate of perinatal lethality. In the few survivors, the pathology progresses after birth and is characterized by distorted growth plates. To understand the abnormality in the mutants, it is important to consider the model for mitotic and post mitotic events in the normal growth plate. In the proliferative zone, the mitotic figures are aligned perpendicular to the long axis of the underlying bone. The daughter cells are semicircular and initially lie side by side in the lacuna but then they become discoid and presumably undertake a gliding movement with one cell moving on top of the other (Figure 2). The cells then pull away from each other to give rise to the typical columnar arrangement of the proliferative zone. In the mutant mice, the daughter cells fail to change shape following division and to glide on top of one another, as judged by lack of formation of columns and by the presence of a disorganized, broader plate with reduced longitudinal growth. Consistent with their inability to move in vivo, when the mutant cells are excised from the growth plates, they show reduced attachment on collagen II, fibronectin and laminin as well as a reduced number of focal adhesions and disorganized cytoskeletal organization. These changes are consistent with impaired locomotive ability, although cell migration in the isolated cells was not directly assessed. It is tempting to speculate that the abnormalities exhibited by the MT1-MMP null mouse59 are related in part to the process of cell “gliding or rotation” in the proliferative cell zone. In the MT1-MMP mutants59, growth plate abnormalities included decreased cell proliferation and loss of the normal columnar arrangement of the cells in the proliferative zone.
Figure 2. Simplified Diagram of Proposed Proliferative Chondrocyte Movements in the Growth Plate.
This diagram was prepared by the author (T.I.M.) with the aid of the MGH photolab from information the video 1 and discussion of the paper by Aszodi A, Hunziker EB, Brakebusch C and Fassler R, Genes and Development 2003, 17:2465-2479, after consultation with Drs Aszodi and Hunziker.
Clearly, the “gliding” movements that have been ascribed to proliferative cells appear to take place over relatively short distances and may include highly coordinated interactions between daughter cells. In this respect, the cell translocations may be fairly unique and further studies could reveal novel mechanisms. On the other hand, the apparent dependence of cell translocation on cell-matrix adhesion, cytoskeletal organization, and possibly MMP activity suggest that some of the underlying mechanisms may-at least in part-be shared with those of active cell movement28,47.
Osteoarthritic Cartilage
Clusters or clones of proliferating cells surrounded by newly synthesized matrix molecules constitute one of the histological hallmarks of the chondrocytic response to the degeneration of cartilage during osteoarthritis (OA) 60,61. On the other hand, it is also possible that cells initially dispersed within the matrix can move towards one another, clustering together and contributing to the clinical picture. This was suggested in an early study by Kouri et al62. These authors carried out an ultra structural study of human cartilage chondrocytes in samples from normal and OA subjects. The number of chondrocytes per lacunae as well as the total cells per unit area of OA cartilage in fibrillated and non-fibrillated regions was assessed in a careful morphometric study. The findings revealed that the fibrillated areas contained the highest percentage of cell clusters Vs single cells. Interestingly, the total number of cells per unit area in these fibrillated, highly cloned regions were not statistically different from that of cells in non-fibrillated regions. In addition, the authors made the observation that many of the cells displayed changes in cytoskeletal arrangement, presence of abundant filopodia and a primary cilium. These observations lead the authors to suggest “the possibility that chondrocytes form aggregates by active cell migration needs to be considered”.
Intervertebral Dics
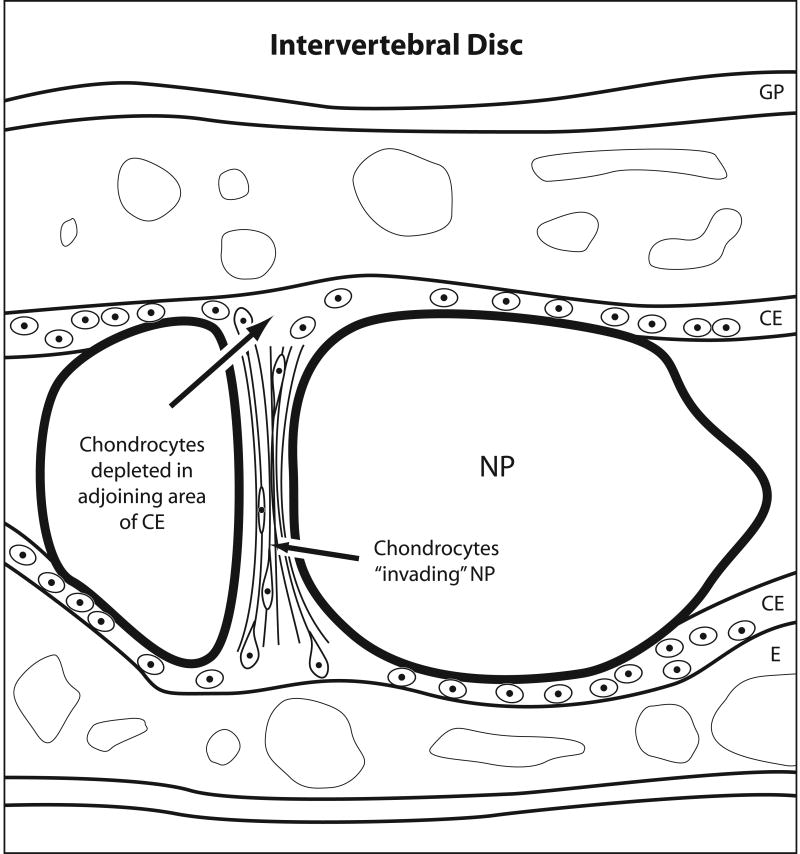
Kim et al63 carried out a histologic investigation of 125 rabbit intervertebral discs (IVDs) to investigate the mechanisms whereby the nucleus pulposus (NP) makes a transition from a vacuolated, notochordal tissue to a fibrocartilage during development. They noted that fibrocartilage fibers and lamellae progressively invaded the nucleus pulposus from the superior and inferior cartilage endplates (CEs) and traced the origin of the collagen fibers to the deep regions of the endplates. These lamellae were studded with chondrocytes, some of which appeared elongated and polarized in the direction of the NP (Figure 3). Consistent with the concept of chondrocyte migration from the CE, the formation of a fibrocartilaginous NP was reportedly accompanied by loss of cells from the CE and thinning of the later. The authors commented that this loss of CE structure was seen in areas neighboring the fibrocartilaginous growth in the NP, but not in more central areas. Precise quantitation of these seemingly correlated cell changes would provide further evidence for these interesting observations. In a second study, rat IVDs were maintained in organ culture64. Based on previous observations with the rabbit, a set of the IVDs was fractured to accelerate the invasion of the chondrocytes into the NP. IHC of the “invading” chondrocytes showed positive staining in many of the cells for MT1-MMP, suggesting that this enzyme could be responsible for burrowing a trail in the matrix of the CE to allow cell exodus and colonization of the NP. In addition, many cells expressed the Ki-67 protein marker indicative of cell proliferation and importantly, were surrounded by collagen II. The authors concluded that resting chondrocytes in the endplate are activated to migrate towards the NP. It is possible that mechanical pressures may have initially activated chondrocytes and/or that they moved due to their release by micro fractures of the collagen; elucidation of the exact nature of the initiating stimulus will be of great interest. While histology is insufficient to prove the case for active motility in any system, the results of the intervertebral disc studies are interesting and raise potentially important questions that invite further thought and experimentation.
Figure 3. Simplified Diagram of Hypothetical Chondrocyte Movements in the Intervertebral Disc.
The diagram was prepared by the author (T.I.M.) with the aid of the MGH photolab from information in the papers from Kim K-W, Lim T-H, Kim JG, Jeong S-T, Masuda K et al, Spine 2003 and Kim K-W, Ha k-Y, Park J-B, Woo Y-K, Chung H-N et al, Spine 2005 following consultation with Dr. An. NP=nucleus pulposus; CE=cartilage endplate; E= epiphysis; GP=growth plate. Note that the rabbit CE differed from what is reported for human CE. In the human, both the articular region and the growth plate are continuous in the CE. However, in rabbits, the articular region is separated gradually from the growth plate with the development of a secondary ossification center, an epiphysis62. Please see ref. 77 for an up to date review on the human intervertebral disc.
In vivo meniscal repair in Canine Model
In an in vivo study of meniscus repair in a canine model65, the authors monitored changes in the wounded cartilage up to a year. In this model, plugs were excised from the avascular zone of the meniscus, freeze thawed to kill cells and then re-inserted in the wound site to provide a scaffold for repair activities. A new zone of matrix containing cells was seen to develop on the margins of the wounded meniscal cartilage after a week. Interestingly, these cells were positive for α smooth muscle actin as early as 3 weeks post-injury. By contrast, the uninjured cartilage areas had positive cells only in the superficial zone, suggesting-but certainly not proving-that superficial cells close to the wound may have moved downwards to line the defect margins. After a year, the freeze-thawed plug re-inserted into the wound site had been recolonized by live cells. While their origin could not be determined, many of the resident cells were also positive for the α smooth muscle protein marker. A subpopulation of chondrocytes expressing the α smooth muscle marker has also been noted in articular cartilages66. A canine model with an articular cartilage injury to the level of the tidemark showed some reparative activity and both chondrocytic and fibroblastic cells staining for the SMA marker were noted in the repair tissue. These findings suggest that the contractile α-smooth muscle actin positive chondrocytes should be further studied for their motile and reparative potential. The authors of these studies did not conclude that there was active chondrocyte motility in their models. This is certainly appropriate. However, it may be considered that in future studies, co-localization of SMA with molecular markers of the chondrocyte and/or fibroblast phenotypes in reparative cells at various stages of healing may be instructive.
Summary
There is growing evidence that in several in vitro models chondrocytes are able to migrate under the stimulation of various chemoattractants and on and within several matrices. It will be important in future work to extend such observations by careful phenotyping of the cells in question. It may also be interesting and instructive to explore the possibility that early-differentiated chondrocytes could contribute to some of the observations of cartilage cell motility, given the findings that chondroprogenitor cells are present in cartilage67, 68. Early studies of mesenchymal stem cell differentiation along the chondrocyte lineage indicated that there is an early stage (lasting about 6 days in pellet cultures) where cells express some chondrocyte matrix components (e.g. COMP, fibromodulin and aggrecan core protein) but not collagen II69. It is presently unknown if and how the progenitors in cartilage differentiate to mature chondrocytes or if they can move within the tissue. In our previous study on planar models, the migratory chondrocytes expressed collagen II and not collagen I indicating that fully differentiated chondrocytes are motile39, but it is not possible at this time to rule out the presence of a small number of cells in our migrated cell populations that do not express collagen II (or detectable collagen I). It is also possible that different conditions are required to mobilize immature chondrocytes. In addition, while movements have not been directly visualized (nor proven) in vivo, descriptive yet provocative evidence suggests critical chondrocyte movements during development and/or pathology. Taken together, the in vitro and in vivo data invite further investigations of chondrocyte movements and of their underlying mechanisms.
Working Models for 3D Moves in Cartilage
The aforementioned studies of chondrocyte movements in vivo raise the important technical question of how the case for active cell movement within cartilage can be directly proven and captured. Another intriguing question is how the cells could move at all. A major roadblock to their movement is undoubtedly the stiff cartilage matrix closely surrounding and entrapping the cell. As discussed in the introduction, these problems are not insurmountable if the cells are able to express proteases, a possibility well within the chondrocyte purview. Indeed, a precedent establishing that chondrocytes are probably able to rapidly and effectively remodel the matrix around them was set by studies of hypertrophic chondrocytes 70,71. Consider that in the growing rat, hypertrophic cells are removed from the growth plate every 3 hours and to maintain steady state new ones must replace them every 3 hours. In this situation, there is a 10-fold increase in mean cell volume as the cell transitions between a proliferative and hypertropic stage. This process is accompanied by an increase in matrix synthesis rates, likely reflecting extensive remodeling. It is striking that during this time there is maintenance of the highly ordered histological structure of the various compartments (pericellular, territorial and inter-territorial). Clearly, the hypertrophic cell data does not establish the same dynamic for proliferative or other chondrocyte types, or for moving cells, but supports the plausibility of rapid matrix remodeling even within a complex cartilage matrix.
Altogether, the in vitro and in vivo data on cartilage raises the possibility that chondrocytes may display regulated movements during growth and remodeling, i.e. times of high energetic states when the remodeling machinery of the cell is revved up sufficiently to temporally and spatially reduce the matrix density and stiffness to allow cell passage. In addition, high synthetic rates may aid repair of any damage inflicted on the matrix during cell transit. This may be optimal during fetal life when the cartilage matrix is relatively soft. This was suggested by the observation that fetal lambs were able to fully repair defects inflicted on cartilage in utero72, as judged by the finding that repair matrix was morphologically identical to the surrounding matrix. It is premature to present detailed mechanistic hypothesis for the apparent movements that have been reported in different in vivo cartilages. The spirit of this review is to invite thought-not to present foregone conclusions. In this spirit, I will limit this initial discussion to a presentation of a plausible general working hypothesis, as follows. Chondrocytes may translocate in vivo by active migration. For example, they may respond to motility cues by weakening their attachment to the surrounding matrix and polarizing their catabolic machinery in order to “open” a way through the thick matrix ahead. The cells may then propel their bodies forward by use of cell extensions to “grip” and pull on the collagen fibers and use their contractile actinomyosin skeleton to generate force for detachment and/or deformation. The in vitro findings described in this review are consistent with this hypothesis, e.g. that chondrocyte can use their extensions to bend collagen fibers33 and that they display active, measurable motility39,42. Highly synchronized secretion of matrix primarily at the rear of the moving cell (or by a partner cell) could theoretically repair the damaged matrix. Another view that has been put forward to explain chondrocyte movements is that the putative secretion of a pressurized proteoglycan matrix in the rear of the moving cell may help to propel the cell body forward73. It seems reasonable to think that this mechanism would also require some form of regulated proteolysis of matrix. In any event, it is not presently clear that the forces required for effecting cell displacement could be generated exclusively in this manner. For example, it is worth considering that many cell types, even primitive cells such as amoeba and parasites that use rudimentary cell gliding movements reportedly rely on active myosin motors74-76.
It is possible to speculate that motility may become dormant in mature articular chondrocytes as they become encased within chondrons and/or engage in matrix maintenance. However, mechanical and/or other injury to the matrix such as may occur during normal wear and tear, injury and inflammation, and/or osteoarthritis can be expected to change the matrix “pore size” and density and present a more permissive environment for cell movements. Additionally cell injury could generate chemoattractants to mobilize neighboring cells. Various cartilage explant studies and one of the in vivo studies discussed in this review are consonant with this view53,54,55, 62.
Clearly, the general models discussed above are hypothetical and will require much experimental scrutiny. Information in the area of cartilage cell movements may open avenues for design of protocols to engineer chondrocyte mobility in lieu of cartilage repair and/or to correct cartilage pathologies.
Acknowledgments
I would like to gratefully acknowledge the helpful comments by some authors of the papers cited in this review, including Drs. Greta Lee, Scott Kelley and Jeff Lyman who have extensively discussed their work with me; Attila Aszodi, K.W. Kim and Howard S. An also provided helpful comments. In particular, I appreciate the careful discussions and comments by Dr. Ernst B. Hunziker. Dr. Douglas A. Lauffenburger contributed many discussions of cell migration and sparked my initial interest in this subject. Clearly the vast literature on cell movements in general or the more select papers on chondrocyte movements and related subjects cannot be fully covered in this short review and I sincerely apologize to the authors that were not quoted due to lack of sufficient space.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lauffenburger DA, Horowitz AF. Cell Migration: A Physically Integrated Molecular Process. Cell. 1996;34:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Stossel TP. On the Crawling of Animal Cells. Science. 1993;260:1086–94. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 3.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, et al. Cell Migration: Integrating signals from front to back. Science. 2003;302:1704–09. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 4.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Science. 2005;118:4917–19. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 5.Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin – like growth factor I receptor to regulate intracellular signaling. Endocrinology. 2003;144:1664–70. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- 6.Lebrun P, Baron V, Hauck CR, Schlaepfer DD, Van Obberghen E. Cell adhesion and focal adhesion kinase regulate insulin receptor substrate-1 expression. J Biol Chem. 2000;275:38371–377. doi: 10.1074/jbc.M006162200. [DOI] [PubMed] [Google Scholar]
- 7.Schneller M, Vouri K, Rouslathi E. αvβ3 Integrin associates with activated insulin and PDGF receptors and potentiates the biological activity of PDGF. The Embo Journal. 1997;16:5600–07. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodard AS, Garcia-Cardena G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of αvβ3 integrin PDGF receptors increases cell migration. J Cell Sci. 1998;111:469–78. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- 9.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–74. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Research. 2002;62:6278–88. [PubMed] [Google Scholar]
- 12.Wang W, Goswami S, Lapaidus K, Wells AL, Wyckoff JB, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Research. 2004;64:8585–94. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 13.Loisel TP, Boujemaa R, Pantaloni D, Cartier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–16. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 14.Svitkina TM, Borisy GG. Arp 2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biology. 1999;145(5):1009–26. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machesky LM, Insal RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the arp2/3 complex. Curr Biol. 1998;8:1347–56. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 16.Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. PNAS. 2006;103:10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–42. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol. 1999;144:1285–94. doi: 10.1083/jcb.144.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollanen J, Hedman K, Nielsen LS, Dano K, Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1998;106:87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estreicher A, Mulhauser J, Carpentier JL, Orci L, Vassalli JD. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990;111:783–92. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yebra M, Parry GCN, Stromblad S, Mackman N, Rosenberg S, et al. Requirement of receptor-bound urokinase-type plasminogen activator for integrin αVβ5 directed cell migration. J Biol Chem. 1996;271:29393–99. doi: 10.1074/jbc.271.46.29393. [DOI] [PubMed] [Google Scholar]
- 22.Mignatti P, Robbins E, Rifkin DB. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986;47:487–98. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- 23.Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proceedings of the National Academy of Sciences. 1997;94:7959–64. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn GA, Ebendall T. Contact guidance on oriented collagen gels. Exp Cell Res. 1978;111:475–9. doi: 10.1016/0014-4827(78)90196-9. [DOI] [PubMed] [Google Scholar]
- 25.Brown AF. Neutrophil granulocytes: Adhesion and locomotion on collagen substrata and in collagen matrices. J Cell Sci. 1982;58:455–67. doi: 10.1242/jcs.58.1.455. [DOI] [PubMed] [Google Scholar]
- 26.Kuntz RM, Saltzman WM. Neutrophil motility in extracellular matrix gels: Mesh size and adhesion affect speed of migration. Biophysical Journal. 1997;72:1472–80. doi: 10.1016/S0006-3495(97)78793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandeville JTM, Lawson MA, Maxfield FR. Dynamic imaging of neutrophil migration in 3 dimensions: Mechanical interactions between cells and matrix. J Leukocyte Biol. 1997;61:188–200. doi: 10.1002/jlb.61.2.188. [DOI] [PubMed] [Google Scholar]
- 28.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, et al. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–77. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T Lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize β1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. European Journal of Immunology. 1998;28:2331–43. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Hunziker EB. In: Articular cartilage structure in humans and experimental animals in Articular Cartilage and Osteoarthritis. Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC, editors. Raven Press; New York, N.Y.: 1992. pp. 183–199. [Google Scholar]
- 31.Maroudas A, Bannon C. Measurement of swelling pressure in cartilage and comparison with the osmotic pressure of constituent proteoglycans. Biorheology. 1981;18:619–32. doi: 10.3233/bir-1981-183-624. [DOI] [PubMed] [Google Scholar]
- 32.Urban JPG, Maroudas A, Bayliss MT, Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16:447–64. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- 33.Lee GM, Loeser RF. Cell surface receptors transmit sufficient force to bend collagen fibrils. Exp Cell Res. 1999;248:294–305. doi: 10.1006/excr.1999.4418. [DOI] [PubMed] [Google Scholar]
- 34.Vautier D, Hemmerle J, Vodouhe C, Koenig G, Richert L, et al. 3-D surface charges modulate protrusive and contractile contact of chondrosarcoma cells. Cell Motility and the Cytoskeleton. 2003;56:147–58. doi: 10.1002/cm.10140. [DOI] [PubMed] [Google Scholar]
- 35.Bush PG, Hall AC. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis Cartilage. 2003;11:242–51. doi: 10.1016/s1063-4584(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 36.Frenkel SR, Clancy RM, Ricci JL, DiCesare PE, Rediske JJ, et al. Effects of nitric oxide on chondrocyte migration, adhesion and cytoskeletal assembly. Arth Rheum. 1996;39:1905–12. doi: 10.1002/art.1780391118. [DOI] [PubMed] [Google Scholar]
- 37.Takebayashi T, Iwamoto M, Jikko A, Matsumura T, Enomoto-Iwamoto M, Myoukai F, et al. Hepatocyte growth factor/scatter factor modulates cell motility, proliferation, and proteoglycan synthesis of chondrocytes. J Cell Biol. 1995;129:1411–19. doi: 10.1083/jcb.129.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fibbi G, Pucci M, Serni U, Cerinic MM, Del Rosso M. Antisense targeting of the urokinase receptor blocks urokinase-dependent proliferation, chemoinvasion, and chemotaxis of human synovial cells and chondrocytes in vitro. Proceedings of the Association of American Physicians. 1998;110:340–50. [PubMed] [Google Scholar]
- 39.Chang CC, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11:603–12. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 40.Fujita T, Azume Y, Fukuyama R, Hattori Y, Yoshida C, et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hidaka C, Cheng C, Alexandre D, Bhargava M, Torzilli PA. Maturational differences in superficial and deep zone articular chondrocytes. Cell Tissue Research. 2006;323:127–135. doi: 10.1007/s00441-005-0050-y. [DOI] [PubMed] [Google Scholar]
- 42.Maniwa S, Ochi M, Motomura T, Nishikori T, Chen J, et al. Effects of hyaluronic acid and basic fibroblast growth factor on motility of chondrocytes and synovial cells in culture. Acta Orthop Scand. 2001;72(3):299–303. doi: 10.1080/00016470152846664. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton DW, Riehle MO, Rappuoli R, Monagham W, Barbucci R, et al. The response of primary articular chondrocytes to micrometric surface topography and sulphated hyaluronic acid-based matrices. Cell Biol Int. 2005;29:605–15. doi: 10.1016/j.cellbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Kirilak Y, Pavlos NJ, Willers Cr, Han R, Feng H, et al. Fibrin sealant promotes migration and proliferation of human articular chondrocytes: possible involvement of thrombin and protease-activated receptors. Int J Mol Med. 2006;17(4):5512–8. [PubMed] [Google Scholar]
- 45.Chao PHG, Roy R, Mauck RL, Liu W, Valhmu WB, et al. Chondrocyte translocation response to direct current electric fields. J Biomech Eng. 2000;122:261–7. doi: 10.1115/1.429661. [DOI] [PubMed] [Google Scholar]
- 46.Gosiewska A, Rezania A, Dhanaraj S, Vyakarnam M, Zhou J, et al. Development of a three-dimensional transmigration assay for testing cell-polymer interactions for tissue engineering applications. Tissue Engineering. 2001;7:267–77. doi: 10.1089/10763270152044134. [DOI] [PubMed] [Google Scholar]
- 47.Zaman MH, Matsudaira P, Lauffenburger DA. Understanding effects of matrix protease and matrix organization on directional persistence and translational speed in three –dimensional cell migration. Annals of Biomedical Engineering. 2007;35:91–100. doi: 10.1007/s10439-006-9205-6. [DOI] [PubMed] [Google Scholar]
- 48.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annual Rev Physiol. 2007;69:14.1–14.2. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 49.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J of Histochem and Cytochem. 2006;54(9):1005–14. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 50.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–314. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 51.Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, et al. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. JBC. 2006 doi: 10.1074/jbc.M607581200. in press. [DOI] [PubMed] [Google Scholar]
- 52.Errington RJ, Puustjary K, White IRF, Roberts S, Urban JPG. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;107:369–78. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyman JR, Kelley SS, Lee G. Chondrocyte process extension and migration response to partial thickness cartilage injuries in human explants. Proceedings of the Orthopaedic Research Society. 2000;46:0929. [Google Scholar]
- 54.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Ortho Res. 2006;24:1261–70. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 55.Qiu W, Murray MM, Shortkroff S, Lee CR, Martin SD, et al. Outgrowth of chondrocytes from human articular cartilage explants and expression of alpha-smooth muscle actin. Wound Repair Regen. 2000;8(5):383–91. doi: 10.1111/j.1524-475x.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 56.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267(2):416–25. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 57.Doods GS. Row formation and other types of arrangement of cartilage cells in endochondral ossification. Anat Record. 46:385–399. [Google Scholar]
- 58.Aszodi A, Hunziker EB, Brakebusch C, Fassler R. β1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes and Development. 2003;17:2465–79. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. PNAS. 2000;97(8):4052–57. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quintavalla J, Kumar C, Daouti S, Slosberg E, Uziel-Fusi S. Chondrocyte cluster formation in agarose cultures as a functional assay to identify genes expressed in osteoarthritis. J Cell Physiol. 2005;204(2):560–6. doi: 10.1002/jcp.20345. [DOI] [PubMed] [Google Scholar]
- 61.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II: Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg. 1971;53A:523–7. [PubMed] [Google Scholar]
- 62.Kouri JB, Jimenez SA, Quintero M, Chico A. Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthritis Cartilage. 1996;4:111–25. doi: 10.1016/s1063-4584(05)80320-6. [DOI] [PubMed] [Google Scholar]
- 63.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–90. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 64.Kim KW, Ha KY, Park JB, Woo YK, Chung HN, et al. Expressions of membrane type I matrix metalloproteinase, Ki-67 protein and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs. Spine. 2005;30:1373–78. doi: 10.1097/01.brs.0000166155.48168.0e. [DOI] [PubMed] [Google Scholar]
- 65.Kambic HE, Futani H, McDevitt CA. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8:554–61. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang Q, Breinan HA, Hsu HP, Spector M. Healing of defects in canine articular cartilage: distribution of non-vascular alpha-smooth muscle actin-containing cells. Wound Repair Regen. 2000;8(2):145–58. doi: 10.1046/j.1524-475x.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- 67.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoartrhitic human articular cartilage. Arth Rheum. 2004;50(5):1522–32. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 68.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Science. 2004;117:889–97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 69.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Experimental Cell Research. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 70.Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone and Joint Surg. 1987;69-A:162–73. [PubMed] [Google Scholar]
- 71.Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Namba RS, Martin M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lam model. J Bone and Joint Surg. 1998;80-A:4–10. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Hunziker EB. Personal Communication. 2006.
- 74.Hakansson S, Morisaki H, Heuser J, Sibley LD. Time-lapse video microscopy of gliding motility in toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Molecular Biology of the Cell. 1999;10:3539–47. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaskins E, Gilk S, DeVore N, Mann T, Ward G, et al. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J of Cell Biology. 2004;165:383–93. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manahan CL, Iglesias PA, Long Y, Devreotes PN. Chemoattractant signaling in Dictyostelium discoideum. Annu Rev Cell Dev Biol. 2004;20:223–53. doi: 10.1146/annurev.cellbio.20.011303.132633. [DOI] [PubMed] [Google Scholar]
- 77.Roberts S, Evans H. Histology and pathology of the human intervertebral disc. J Bone and Joint Surg. 2006;88A:10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]