Abstract
Objective
Hepatic steatosis occurs in up to 78% of patients with type 2 diabetes. Studies evaluating the effect of metformin on hepatic steatosis are conflicting. Insulin is believed to be detrimental due to its lipogenic effect. Since insulin and metformin combination is commonly used for the treatment diabetes, it is important to assess the effect of this combined therapy on hepatic steatosis. We evaluated the change in hepatic steatosis following initiation of insulin and metformin in patients with type 2 diabetes.
Methods
Newly-diagnosed, treatment-naïve patients with type 2 diabetes had their hepatic triglyceride (TG) content measured by magnetic resonance spectroscopy at baseline and after 3 months of treatment with BiAsp 30 insulin in combination with metformin. Insulin was administered twice daily and titrated to achieve normal capillary blood glucose levels. Metformin was titrated during the first month from 500 mg daily to 1000 mg twice-daily.
Results
The average hepatic TG content in the 19 subjects enrolled was 11.83%±7.61% (range 0.93% to 23.16%)] and correlated with BMI (r=0.567). Three months of treatment reduced hepatic steatosis by 45%, with 75% of the study subjects achieving a normal level. The change in hepatic TG content was partially explained by the change in HbA1c (p=0.006) and change in cholesterol level (p=0.003).
Conclusions
The combined treatment with insulin and metformin reduced significantly hepatic steatosis in patients with newly-diagnosed type 2 diabetes.
Keywords: type 2 diabetes, hepatic steatosis, insulin treatment, metformin
Non-alcoholic fatty liver disease (NAFLD) is characterized by fatty infiltration of the liver. This diagnosis represents a disease spectrum from simple steatosis to steatohepatitis. The prevalence of NAFLD has risen in the last decade parallel with the obesity and type 2 diabetes epidemics. (1). Previous studies suggest that one-third of the population has NAFLD (2). The prevalence of NAFLD in patients with type 2 diabetes is even higher. In the Dallas Heart Study, 63% of subjects with known diabetes or an elevated fasting plasma glucose level had abnormally elevated hepatic triglyceride (TG) content, similar with previous estimates (2, 3).
NAFLD carries a significant risk of morbidity and even mortality. Approximately 50% of patients with steatohepatitis develop fibrosis, 15% develop cirrhosis, and 3% develop liver failure (4). If the prevalence of type 2 diabetes will grow as predicted in the next 50 years, NAFLD will likely become a prominent public health issue and an important cause of cirrhosis and liver failure. Since there is no established treatment for NAFLD, there is an urgent need for one.
Metformin, which is thought to improve insulin sensitivity and decrease hepatic TNF-alpha(5), has been proposed as an effective agent for treatment of NAFLD, but data supporting its efficacy, especially in patients with type 2 diabetes, are controversial. Several uncontrolled studies using volunteers without diabetes showed improvement in ALT values, liver histology, or qualitative measurements of hepatic fatty infiltration (5-8). The two randomized studies supporting the effectiveness of metformin in the treatment of NAFLD excluded patients with diabetes (9, 10). In the only randomized trial that studied patients with type 2 diabetes, metformin was found to have a neutral effect on liver fat infiltration (11).
The effect of insulin on human liver steatosis has not been studied. Insulin is considered lipogenic and is thought to increase liver fat content. Anderwald et al. reported that 3 days of insulin infusion mediated near-normal glycemia stimulated lipid accumulation in the liver (12). However, this effect has not been clinically observed in patients with type 1 or type 2 diabetes who are initiated on insulin therapy.
Insulin and metformin are commonly used to treat patients with type 2 diabetes. Given the high prevalence of NAFLD in this population and the possibility of negative effects of these agents on hepatic fat accumulation, a study of the safety of these agents is important. This pilot study evaluates the effect of insulin and metformin on the degree of hepatic steatosis in patients with newly diagnosed type 2 diabetes.
Research Design and Methods
Study overview
This is a prospective, single-group study evaluating the effects of a 3-month treatment with insulin and metformin on hepatic steatosis in patients with type 2 diabetes.
Study participants
Patients with type 2 diabetes diagnosed within the previous 2 months were recruited from the Parkland Memorial Hospital Inpatient and Outpatient Services. Inclusion criteria were as follows: diabetes treatment naïve, weight under160 kg (the limit of the magnet size), absence of any metallic implants or claustrophobia. Exclusion criteria included age <21 years, HbA1c<7%, creatinine level over 1.5 mg/dl, women seeking pregnancy or not willing to practice contraception, history of chronic liver disease, lactic acidosis, recent illicit drug use or alcoholism. The study protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and all participants provided written informed consent prior to the study.
Treatment
All patients received a combination of BiAsp 30 insulin and metformin. Insulin was initiated at 0.2 units/kg/day divided in 2 daily injections. It was titrated based on the capillary blood glucose levels obtained by patients 2-4 times daily, targeting a fasting blood glucose level of <110 mg/dl. Metfomin was started at 500 mg/day, and titrated at weekly intervals by 500 mg to the final dose of 1000 mg twice daily. This titration schedule was chosen to minimize gastrointestinal side effects and maximize compliance and tolerability. Study participants were seen in the clinic monthly. Phone contacts were only patient-initiated if questions occurred. Since statins or other lipid lowering agents might also have an effect on hepatic steatosis (13), such therapy was not initiated during the 3-month study, but it was continued at same dose if already taking it.
Measurements
Clinical evaluations were performed at baseline and monthly thereafter. At baseline we completed a history (including weight and alcohol use history) and physical examination. At the follow-up visits we assessed weight, history of side effects, compliance, the use of additional medications, and performed insulin dose adjustments as needed.
Biochemical evaluations, performed at baseline and the end of the study, included blood glucose level, HbA1c, liver function tests, and lipid profile. All blood samples were obtained in a fasting state (10-14 hrs), in the morning, processed immediately, stored appropriately and analyzed within 24 hrs. HbA1c was measured using high performance liquid chromatography at the Diabetes Laboratory at the University of Texas Southwestern Medical Center at Dallas, that is certified by the National Glycohemoglobin Standardization Program. The HbA1c inter-assay coefficient of variability is <2% and intra-assay variability <0.3%. All other laboratory measurements were performed by standard assays at a local clinical commercial laboratory (Quest Diagnostics - Irving, TX).
Hepatic TG content was measured by localized MRS at baseline and the end of the study. MRS was chosen as it is non-invasive and provides an accurate and reliable quantification of the hepatic TG content. Liver biopsy is considered the gold standard for diagnosis and quantification of hepatic steatosis, but it is invasive and carries a significant risk of morbidity and mortality. Hepatic MRS results highly correlate with the biochemical results obtained from biopsy specimens in animal studies (14) and human studies (15). The coefficient of variation of the MRS method is 8.5% (16), which is superior to the reproducibility of the liver biopsy to determine hepatic fat content (17-19).
Hepatic TG content was measured as described earlier (16). In short, measurements were conducted with subjects in prone position using a 1.5 Tesla Gyroscan INTERA MR clinical system (Philips Medical Systems, The Netherlands). The volume of interest (27 cm3) was selected on sagittal, coronal and axial images through the upper, right liver lobe avoiding major blood vessels, intra-hepatic bile ducts and the lateral margin of the liver. The voxel size and position were optimized to prevent a contamination of signal from liver by signal from abdominal adipose fat. In localized spectroscopy, only signals from the selected element of volume are collected. Signals from tissues out of volume are not collected. We used a relatively large voxel size to collect good quality data in a short time to minimize the time the patient spent in the magnet. Spectra were collected using a Q-body coil and a PRESS sequence for spatial localization. Data were acquired with the following parameters: interpulse delay Tr = 3 s, spin echo Te = 25 ms, number of acquisitions per spectrum16, and 1024 data points over a 1000 Hz spectral width. Areas of resonances from protons of water and methylene groups in fatty acid chains of the hepatic TG were calculated with line-fit procedure and commercial software (NUTS – ACORNNMR, Freemont, CA). Signal decay due to spin-spin relaxation was calculated using mean T2 relaxation times for water and fat of 50 ms and 60 ms, respectively. (14). The unit of measurement is a ratio of signal from fat (f) to total signal from fat (f) and water (w) [f/(f+w)] and is expressed as %. The upper limit of normal is considered to be 5.56% (16).
Statistical analysis
The change in the outcome variables before and after the intervention was analyzed using a paired t-test. Non-normally distributed data were log transformed before analysis. The analysis was also confirmed with a non-parametric method (Sign test for paired data). The hepatic TG content was analyzed as a dependent variable in a linear regression model to evaluate predictors of its change. All data are shown as mean +/− standard deviation (SD). P<0.05 was considered significant. Since this was an exploratory single-group pilot study a power calculation was not performed a priori. Analyses were conducted using SPSS for Windows versions 14.0 (SPSS, Inc., Chicago, IL) and SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Baseline hepatic TG content was measured in 19 participants with newly diagnosed type 2 diabetes. Three volunteers no longer met inclusion criteria after enrollment and did not complete the study, 4 volunteers completed the treatment but due to scheduling conflicts the second MRS study was not performed. These volunteers' baseline characteristics did not differ significantly from those who completed the study. Twelve patients completed the treatment and had all evaluation repeated at the end of the study. The characteristics of these participants are shown in Table 1. All participants reported alcohol intake <6 drinks/week in the prior 6 months. The average BMI was in the obese range (34.9±4.7 kg/m2) and everyone had a BMI >25 kg/m2. The average weight loss in the 6 months prior to diagnosis of diabetes and enrollment in the study was 8.7 kg. A weight gain of 2.1 kg occurred during the study. This gain was expected and in most part represented a regain of the weight lost prior to the diagnosis of diabetes and initiation of treatment.
Table 1.
Clinical and biochemical characteristics of volunteers at baseline and after 3 months of treatment. (n=12) Results are mean+/−SD.
| Parameter | Baseline | 3-month | P value |
|---|---|---|---|
| Age (years) | 43.67±7.8 | ||
| Gender: | |||
| Male N(%) | 10 (83) | ||
| Female N(%) | 2 (17) | ||
| Race: | |||
| White N(%) | 3 (25) | ||
| Hispanic N(%) | 6 (50) | ||
| African American N(%) | 3 (25) | ||
|
| |||
| Weight (kg) | 106.21±16.0 | 108.28±15.8 | 0.313 |
| HbA1C (%) | 11.21±2.1 | 6.07±0.4 | <0.0001 |
| Total Cholesterol (mg/dl) | 177.3±53.2 | 162.8±40.8 | 0.281 |
| Triglyceride (mg/dl) | 165.3±77.5 | 134.1±58.8 | 0.210 |
| AST (IU/l) | 31.2±18.7 | 17.4±4.6 | 0.031 |
| ALT (IU/l) | 44.7±32.3 | 20.2±6.8 | 0.015 |
|
| |||
| Dose of insulin (U/day) | 55.4±20.02 | ||
|
| |||
| Hepatic TG content (%) | 11.12±7.7 | 6.1±6.6 | <0.0001 |
During the course of the study HbA1c improved by 5.1%, achieving recommended targets in all patients (range 5.2-6.6%). The average dose of insulin required was 0.51 units/kg/day (55.42±20.02 units/day).
Cholesterol and triglyceride levels improved from baseline, but the change was not statistically significant (p=0.281 and 0.210, respectively).
The most common treatment related side effects were gastrointestinal in nature, related to the initiation of metformin treatment. Symptoms were self-limited in all volunteers and titration to the target dose was achieved without exception. Minor hypoglycemic episodes were rare and mostly occurred in the first month after the initiation of insulin treatment. There were no severe hypoglycemic episodes. Compliance with the treatment was excellent (over 95% for both insulin and metformin).
Hepatic TG content
At baseline 79% of patients had hepatic steatosis (i.e. hepatic TG content >5.56%), with the average content of 11.83±7.6% (range 0.93 to 23.16). Hepatic TG content was reduced by 45% (p<0.001 compared with no change) after treatment, and it normalized in 75% of volunteers (Figure 1). Liver transaminases (AST and ALT) improved significantly, as well (Table 1).
Figure 1.
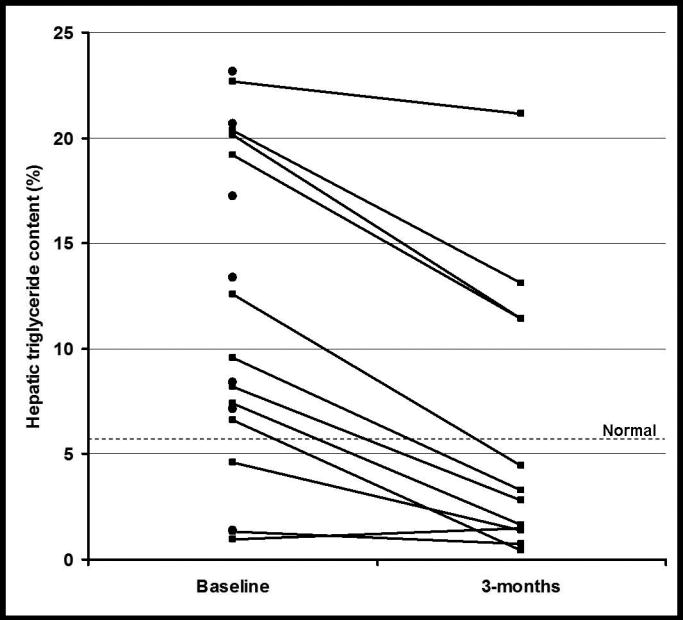
Individual hepatic triglyceride levels at baseline and after 3 months of treatment. Black lines = data on volunteers who completed the study (n=12), Black circles = data on volunteers who only had a baseline evaluation (n=7).
The baseline hepatic TG correlated with BMI (r=0.567, p=0.011), but did not correlate with age, ALT, HbA1c, cholesterol or triglyceride levels (Figure 2).
Figure 2.
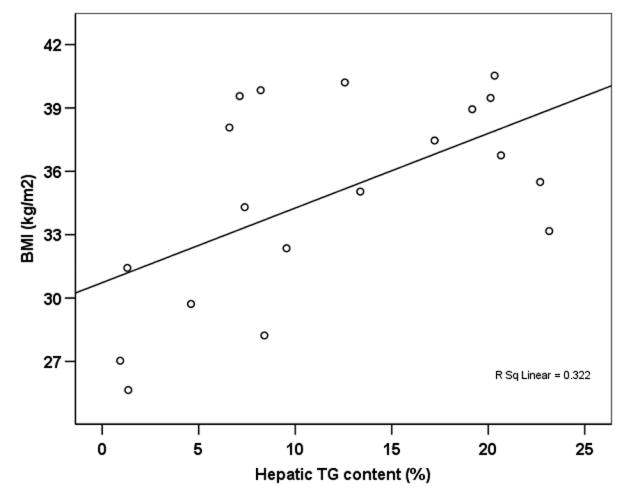
Scatter plot showing the correlation between baseline hepatic TG content and BMI (n=19).
The change in hepatic TG content (percent change from baseline) was analyzed as a dependent variable in a linear regression model with the changes in HbA1c and cholesterol level as covariates. This model had R2=0.717, and the change in HbA1c (beta= −0.13+/−0.04, p=0.006) and cholesterol level (beta= 0.007+/−0.002, p=0.003) were significant predictors of the change in hepatic TG content. The change in weight or ALT level did not correlate with the change in hepatic TG content.
Discussion
We found that individuals with newly diagnosed type 2 diabetes and poor glycemic control have an elevated hepatic TG content (a measure of hepatic steatosis). After 3 months of treatment with insulin and metformin a 45% reduction in hepatic TG content was observed. These findings were partially explained by the change in HbA1c and serum cholesterol level. The decrease in hepatic TG content was associated with an improvement in hepatic function, as liver transaminase levels (AST and ALT) normalized in all patients.
Few studies have evaluated treatment effects on hepatic steatosis in patients with type 2 diabetes. The degree of improvement in this study (45%) is comparable with results seen with the most effective treatments for hepatic steatosis. Agents from the thiazolidinedione class (troglitazone, rosiglitazone, pioglitazone) are thought to be the most effective intervention for this condition. These agents have produced improvements in the hepatic TG level ranging from 39% (20) to 51% (11) after 3 months of therapy, and 54% (21) after 6 months of therapy. Since insulin and metformin is a common combination treatment for patients with uncontrolled type 2 diabetes, we wanted to evaluate its effect on hepatic steatosis. Metformin activates pyruvate kinase and fatty acid beta oxidation and inhibits the expression of enzymes important in lipogenesis, at least in animal models (22). When studied in vivo, metformin's effect on hepatic steatosis has not been consistently beneficial, while insulin, although not specifically studied in humans, is thought to have a deleterious effect. In fact, when metformin was evaluated in patients with type 2 diabetes in a randomized double-masked study against rosiglitazone, it was found to produce no change in hepatic TG content (11). Our findings are unexpected in this context, but suggest that initiation of insulin therapy in combination with metformin is not deleterious to the liver and even improves steatosis by a comparable degree as the thiazolidinediones (11).
Because of the hypothesis that hyperinsulinemia is an etiological factor for fat accumulation in the liver and plasma insulin levels are elevated after subcutaneous insulin administration, our data seem contradictory. There are possible biological explanations for these observations. First, by providing the insulin required for maintenance of normoglycemia through a subcutaneous route, the first pass through the liver of a large amount of endogenously produced insulin is avoided. Thus more insulin is available at peripheral action sites, and less insulin passes through the liver to stimulate hepatic lipogenesis. Second, our volunteers had newly diagnosed type 2 diabetes with poor metabolic control (reflected by an average baseline HbA1c of 11.2%), and presented with uncontrolled hyperglycemia which is due to insulin deficiency. In the context of insulin deficiency, the lipogenic pathways in the peripheral tissues is decreased, lipolysis is increased, resulting in high levels of circulating free fatty acids. Hepatic lipogenesis is increased in the presence of high circulating free fatty acids which may explain the elevated intra-hepatic TG levels present at baseline in our patients. Following initiation of treatment, hyperglycemia was reversed, metabolic control improved, and insulin deficiency was no longer present. Thus the peripheral lipogenic pathways were activated in the presence of insulin and the fat transiently deposited in ectopic sites (i.e. liver) was now redirected to its natural storage place, the peripheral adipose tissue. This hypothesis is consistent with the correlation we found between the improvement in glycemic control (change in HbA1c) and change in hepatic TG content.
We recognize several limitations to our study. First this is a single group study, designed as a pilot study to evaluate if an insulin-based treatment is detrimental on hepatic steatosis, so we cannot draw firm conclusions on the effectiveness of therapy in the absence of a control arm. Thus we are unable to discern if the beneficial effects seen were due to the effect of insulin, that of metformin, the improvement in metabolic control overall regardless of the intervention, or even chance alone. Changes of such magnitude due to chance alone are unlikely as the variability of the method is low (less than 10%) (16) and previous studies also report similar magnitude changes with other interventions which are considered effective (11, 20, 21, 23). In our study, the improvement in metabolic control was large, with an average decrease in HbA1c of 5.14%. Such results are not attainable in practice with any therapeutic combination except one that is insulin based. In patients with diabetes and poor metabolic control it would be inappropriate to withhold insulin treatment and monotherapy is no longer a desirable choice for these complex patients. Even though the mechanistic question is not answered by this study, from a practical standpoint we showed that treatment of patients with newly diagnosed type 2 diabetes with an insulin-based regimen has a beneficial effect on hepatic steatosis. It remains an open question if these results persist with continuous long term therapy. Further studies addressing these questions are needed.
Second, the MRS-based hepatic TG content measurement technique does not provide any information on the presence of coexistent hepatic structural damage (e.g. steatohepatitis, fibrosis), so we do not know if the observed improvement in hepatic steatosis is associated with histological improvement. Yet hepatic biopsies are not clinically recommended in our study population, and the method used has the advantage of being noninvasive, well tolerated by patients, as well valid and reproducible (14, 24).
Despite these limitations, this is the first study that reports on the effect of an insulin-based treatment regimen on the hepatic TG content in patients with type 2 diabetes.
In conclusion, combined therapy with insulin and metformin in patients with newly diagnosed type 2 diabetes and poor metabolic control had a beneficial effect on the hepatic TG content within 3 months of therapy. These results suggest that treatment with insulin does not worsen hepatic steatosis in patients with type 2 diabetes.
Acknowledgments
We would like to thank David Leonard, PhD and Beverley Adams-Huet, MS for assistance with the statistical analysis.
IL was supported by NIH Training Grant 5T3DK007307-25 and a Departmental Clinical Scholars Award; LSS by K25HL-68736 from NIH. NovoNordisk, Inc provided partial support of this project through an Investigator Initiated Trial Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luyckx FH, Lefebvre PJ, Scheen AJ. Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss. Diabetes & metabolism. 2000;26:98–106. [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology (Baltimore, Md. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes care. 2006;29:1845–1850. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 4.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Annals of internal medicine. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 6.Duseja A, Murlidharan R, Bhansali A, Sharma S, Das A, Das R, Chawla Y. Assessment of insulin resistance and effect of metformin in nonalcoholic steatohepatitis--a preliminary report. Indian J Gastroenterol. 2004;23:12–15. [PubMed] [Google Scholar]
- 7.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Alimentary pharmacology & therapeutics. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Alimentary pharmacology & therapeutics. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. The American journal of gastroenterology. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 10.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Alimentary pharmacology & therapeutics. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 11.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 12.Anderwald C, Bernroider E, Krssak M, Stingl H, Brehm A, Bischof MG, Nowotny P, Roden M, Waldhausl W. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes. 2002;51:3025–3032. doi: 10.2337/diabetes.51.10.3025. [DOI] [PubMed] [Google Scholar]
- 13.Siebler J, Galle PR. Treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2006;12:2161–2167. doi: 10.3748/wjg.v12.i14.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. The American journal of physiology. 1999;276:E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 15.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology (Baltimore, Md. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 16.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. American journal of physiology. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, Rybicki L, McCullough AJ. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560–565. [PubMed] [Google Scholar]
- 20.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A Placebo-Controlled Trial of Pioglitazone in Subjects with Nonalcoholic Steatohepatitis. New England Journal of Medicine. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 22.Comar KM, Sterling RK. Review article: Drug therapy for nonalcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2006;23:207–215. doi: 10.1111/j.1365-2036.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 24.Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Croce LS, Grigolato P, Paoletti S, de Bernard B, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]


