Cancer is a major burden of disease worldwide. Each year, tens of millions of people are diagnosed with cancer around the world, and more than half of the patients eventually die from it. In many countries, cancer ranks the second most common cause of death following cardiovascular diseases. With significant improvement in treatment and prevention of cardiovascular diseases, cancer has or will soon become the number one killer in many parts of the world. As elderly people are most susceptible to cancer and population aging continues in many countries, cancer will remain a major health problem around the globe.
In this review, we summarized published data to describe the severity of the burden. We also analyzed the GLOBOCAN 2002 database to evaluate the morbidity and mortality of cancer in various geographic regions around the world. The GLOBOCAN 2002 database was put together using the huge amount of data available in the Descriptive Epidemiology Group of the International Agency of Research on Cancer (IARC), a World Health Organization agency in Lyon, France [1]. Incidence data are available from cancer registries. They cover either entire national populations or samples of such populations from selected regions. Cancer registries also provide statistics on cancer survival. Mortality data by cause are available for many countries through the registration of vital events.
Cancer data are always collected and compiled sometime after the events to which they relate, so the most recent statistics available are always “late.” GLOBOCAN 2002 was first made available in September 2005 and presented estimates for 2002. These estimates are based on the most recent incidence, mortality, and survival data available at IARC, but more recent figures may be available directly from local sources. The Age-Standardized Rate (ASR, world standard) is calculated using the five age groups of 0 to 14, 15 to 44, 45 to 54, 55 to 64, and ≥ 65 years. The weights of the world standard population for the five age groups were 0.31, 0.43, 0.11, 0.08, and 0.07, respectively.
Overall Burden of Cancer Worldwide
Based on the GLOBOCAN database, there were about 10,862,496 new cancer cases (excluding skin cancer) in the world in 2002. Of these, 5,801,839 (53.4 percent) were male and 5,060,657 (46.6 percent) were female. Nearly 45 percent of the new cases were diagnosed in Asia, 26 percent in Europe, 15 percent in North America, 7 percent in Latin America, and 6 percent in Africa. For males and females combined, the most common cancer site worldwide was lung (965,446 male and 386,875 female cases per year). The second most common site was colon (550,513 males and 472,743 females), followed by stomach (603,003 males and 330,290 females). Among women, the number one cancer site was breast (1,152,161 new cases per year), followed by cervix (493,100 cases), and colon (472,743 cases). Among men, the three most common cancer sites were lung (965,446 cases), prostate (679,060 cases), and stomach (603,003 cases).
The number of deaths caused by cancer worldwide in 2002 was 6,723,887, among which 3,795,991 were male and 2,927,896 were female. Lung cancer led to most cancer deaths in the world. In 2002, the total death toll due to lung cancer was 1,179,074, of which 848,321 were male and 330,753 were female. The second on the list was stomach cancer, which resulted in a total of 699,803 deaths, including 445,691 in males and 254,112 in females. Liver cancer was the number three cause of cancer mortality. A total of 598,412 deaths (416,926 male and 81,486 female) were attributed to liver cancer in 2002. For women, the top three sites for cancer mortality were breast (411,093 deaths), lung (330,753 deaths), and cervix uteri (273,449 deaths), while lung (848,321), stomach (445,691), and liver (416,926) constituted the top three sites for cancer mortality in men.
Incidence of Cancer by Geographic Regions
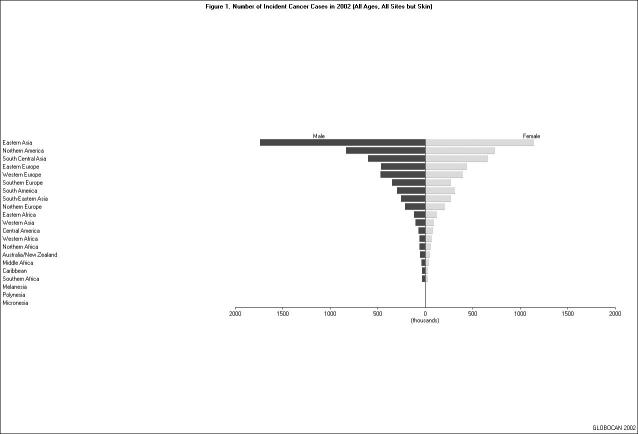
Of the 21 regions listed in the GLOBOCAN 2002 database, East Asia had the largest number of incident cancer cases (all ages, all sites except skin) in 2002 (n = 2,890,311); North America and South Central Asia were second (n = 1,570,520) and third (n = 1,261,527) on the list, respectively [Figure 1]. The pattern of cancer sites varied substantially from region to region. For example, the three most common cancer sites among individuals 15 years or older in East Asia were stomach (18.9 percent), lung (17.1 percent), and liver (14.3 percent), whereas those in North America were prostate (16.5 percent), breast (14.7 percent), and lung (14.5 percent) [Figure 2 and Figure 3].
Figure 1.
Number of Incident Cancer Cases in 2002 (all ages, all sites but skin). No bars are shown for Melanesia, Micronesia, and Polynesia because the numbers of incidence cancer cases in these three regions were very small.
Figure 2.
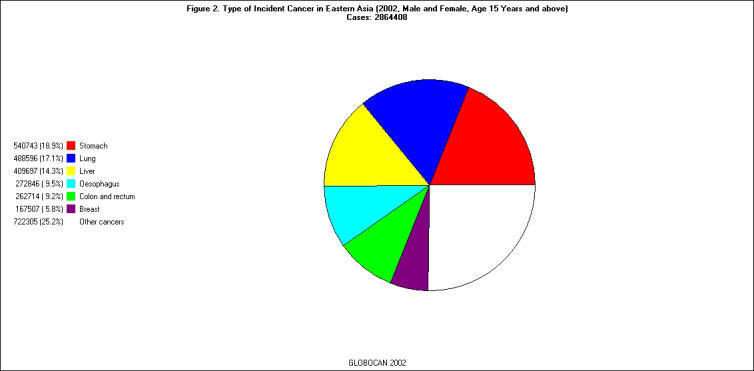
Type of Incident Cancer in Eastern Asia (male and female, age 15-65+, 2002). Cases: 2,864,408.
Figure 3.
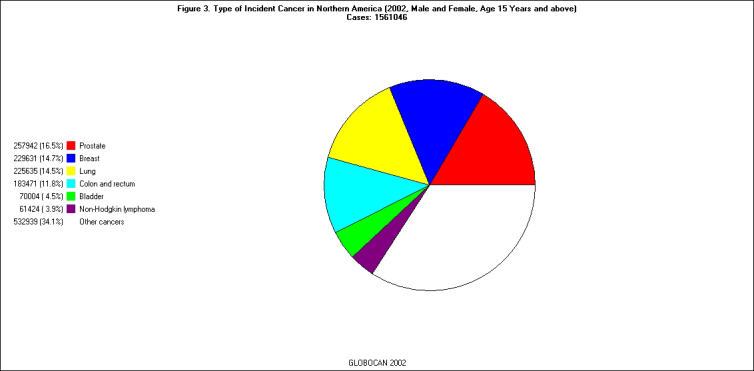
Types of Incident Cancer in North America (male and female, age 15-65+, 2002). Cases: 1,561,046.
For both males and females, the incidence rate of cancer increased substantially with age. For example, the annual male cancer incidence in the age group of 0 to 14 years was 6.45 per 100,000 in Western Africa, 9.07 per 100,000 in Eastern Asia, 14.10 per 100,000 in Western Europe, and 15.12 per 100,000 in North America; the rates in the same regions for those who were 65 years or older were 385.44, 1461.59, 2327.87 and 2958.14 per 100,000, respectively (Table 1). North America, Australia/New Zealand, and Europe had the highest overall incidence rates in 2002, while Northern and Western Africa had the lowest incidence rates (Tables 1-2). The geographic variation was rather substantial. For example, the age-standardized rate in North American males (398.4 per 100,000 person-years) was four times of the age-standardized rate in North African males (99 per 100,000 person-years).
The geographic disparity in cancer incidence is largely attributable to the various socioeconomic, environmental, and lifestyle factors in different regions of the world. Compared with developed countries, developing countries in general may lack the resources to ascertain incident cancer cases. For example, in developed countries, many cases of breast, prostate, colon, and cervical cancers are identified through screening (e.g. mammography, prostate-specific antigen test, colonoscopy, and Pap smear), whereas in developing countries, large-scale screening efforts are usually uncommon. Genetic factors also play a role, but the dominant effect of genetics is only observed in a relatively small percentage of the population. It is believed that the majority of cancer cases (over 90 percent) are due to the joint effect of genetic variations, environmental factors, and lifestyle choices [2]. Geographic factor per se probably has little influence on cancer risk except sunlight exposure and vitamin D metabolism, both of which have been linked to cancer risk. The major categories of cancer risk factors include tobacco use, occupational exposures, environmental contamination, infectious agents, and lifestyle factors.
Tobacco use
Knowledge about the role of tobacco smoking in the etiology of cancer has accumulated for many years [3]. In 2004, the IARC published a monograph on tobacco use and cancer, which concluded that tobacco contributed, to a greater or lesser extent, to cancer in 15 different sites, including lung, urinary tract, upper respiratory tract, pancreas, stomach, and liver.
Although the prevalence of smoking has declined in many developed countries, it is increasing in developing countries [4]. Currently, approximately 5 million people are killed annually by tobacco use; by 2030, estimates based on current trends indicate that this number will increase to 10 million, with 70 percent of deaths occurring in developing countries [5]. It is important to adopt policies such as tobacco tax increases, dissemination of information about health risks from smoking, restrictions on smoking in public places and in workplaces, comprehensive bans on advertising and promotion, and increased access to cessation therapies to reduce the incidence of cancer and other diseases related to tobacco use [5].
Occupational exposures
Occupational exposures have long been linked to the risk of cancer. A recent publication listed 28 definitive human occupational carcinogens ranging from ionizing radiation, asbestos, silica, wood dust, and arsenic to benzene [6]. Generally speaking, developed countries went through the industrialization process earlier than the developing countries, and individuals living in developed countries often had a higher chance of being exposed to various occupational exposures. However, as many developing countries go through an economic transition from primarily agricultural activities to more industrial development and manufacturing, there may be concerns about the lack of resources in monitoring occupational exposures and developing or reinforcing occupational standards, which is usually an ongoing process. For example, the current United States’ occupational standard for benzene, one of the most widely used industrial chemicals and a known human carcinogen, is 1 part per million (ppm) or 3.26 mg/m³. This is considerably lower than the occupational standard for benzene in China between 1979 and 2002, which was 40 mg/m³ (area breathing zone concentration) [7]. In 2002, the occupational standard for benzene in China was modified and significantly lowered, but the current standards (10 mg/m³ for short-term exposure limit and 6 mg/m³ for time-weighted average) are still higher than the U.S. standard [7]. Given that hematological toxicity is observed in workers with benzene exposures below the level of 1 ppm [8], the current occupational standards for benzene may still need to be reviewed and evaluated.
Environmental contamination
Exposure to environmental contamination, such as indoor air pollution and pesticides, is known to increase the risk of cancer. More than half of the world’s populations rely on dung, wood, crop waste, or coal to meet their most basic energy needs [9]. Cooking and heating with such solid fuels on open fires or stoves without chimneys lead to indoor air pollution. This indoor smoke contains a range of health-damaging pollutants, including small soot or dust particles, that are able to penetrate the lungs, increasing the risk of lung cancer and other diseases of the respiratory tract. In poorly ventilated dwellings, indoor smoke can exceed acceptable levels for small particles in outdoor air 100-fold. Exposure is particularly high among women and children, who spend the most time near the domestic hearth. The use of polluting fuels thus poses a major burden on the health of poor families in developing countries [9]. As for pesticides, the global market value is estimated at $32 billion in 2000, with the share of developing countries around $3 billion [10]. Around 30 percent of pesticides marketed in developing countries do not meet internationally accepted quality standards, and the problem is particularly widespread in sub-Saharan Africa. These poor-quality pesticides frequently contain hazardous substances and impurities that already have been banned or severely restricted elsewhere and, therefore, pose a serious threat to human health and the environment [10].
Infectious agents
In 2002, an estimated total of 1.9 million cancer cases, or 17.8 percent of the global cancer burden, were attributed to various infections [11]. Several infectious agents are considered to be causes of cancer [11]. For example, Helicobacter pylori infection is known to increase the risk of stomach cancer [12], whereas infection with hepatitis B and C viruses can lead to liver cancer [13]. The relationship between socioeconomic status and the acquisition of Helicobacter pylori infection has been confirmed in a number of studies; the prevalence of infection varies from 8.9 percent to 72.8 percent among children from developed and developing countries, respectively; the re-infection rate is also significantly higher in the latter [14]. Similarly, the prevalence of infection with hepatitis B and C viruses is higher in developing countries than in developed countries [15]. The higher incidences of stomach and liver cancers in developing countries are largely due to the higher prevalence of related infections. Other infections considered to be important in cancer include human papilloma virus, Epstein-Barr virus, and human immunodeficiency virus [11].
Diet and physical activity
Excessive weight and obesity, resulting from excess calorie intake and physical inactivity, have become a serious health issue in many developed countries. In the United States, for example, it is estimated that nearly a third of the adult population is obese and two-thirds are overweight [16]. Obesity and excessive weight have been linked to many types of cancers, including those of the colon, breast, and prostate [17]. The higher incidences of colon, breast, and prostate cancers in developed countries are attributed in part to a lifestyle of high-calorie diet and physical inactivity. This lifestyle results in positive energy imbalance that further leads to insulin resistance (or metabolic syndrome) characterized by hyperinsulinemia, dyslipidemia, hypertension, and glucose intolerance [18]. Insulin resistance has been linked to a number of health problems including cancer, type 2 diabetes, and cardiovascular diseases [19]. These lifestyle-related health issues have begun to spread to certain developing countries where there has been steady economic growth. This spread also results in changes in regional cancer patterns. For example, dramatic increases in the incidences of breast, prostate, and colon cancers have been observed in the major cities of China [20].
Mortality of Cancer by Geographic Regions
In 2002, Asia had the largest number of cancer deaths in the world, a total of 3,355,928 deaths, including 1,983,473 males and 1,372,455 females, followed by Europe (1,701,472), and North America (631,971). The mortality of cancer also increased dramatically with age for both males and females (Table 3-4). For males, the highest age-standardized mortality rate was observed in Eastern Europe (197.2 per 100,000 person-years) and the lowest in Western Africa (73.5 per 100,000 person-years). The geographic variation in cancer mortality was not as substantial as the geographic variation in cancer incidence. More developed regions such as North America had relatively low cancer mortality despite having high cancer incidence. On the other hand, some less developed regions had relatively high cancer mortality despite having low cancer incidence. This disparity is clearly shown in Figure 4 — the gap between incidence and mortality in males was larger in more developed countries than in less developed countries. There was a similar pattern in females (data not shown). The disparity could be due to the different profiles of cancer in more or less developed countries. For example, prostate cancer is common in more developed countries, and it is usually associated with a fairly good prognosis and does not have a severe impact on survival. The disparity also could be due to the resources available for the screening and treatment of cancer in more or less developed countries. For example, the incidence rates of female breast cancer were nearly three-fold higher in more-developed than less-developed countries; whereas, mortality rates were less than two-fold higher in more-developed than less-developed countries. Consequently, the mortality/incidence rate ratio varied widely, from a low of 0.19 in North America to a high of 0.69 in Africa [21]. The prognosis of certain types of malignancies, such as cervical cancer and colon cancer, can be remarkably improved by identifying cases early through effective screening. Less developed countries may lack resources to carry out large scale screenings. It is also possible that various completeness in cancer surveillance and vital records in more or less developed countries contributed to the apparent disparity.
Figure 4.
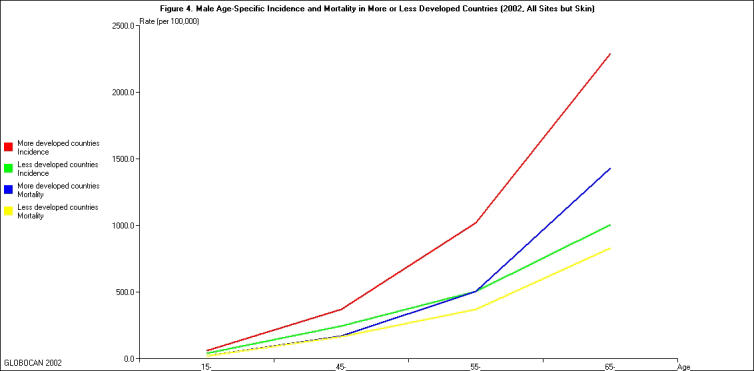
Male age-specific incidence and mortality in more or less developed countries, 2002. (All sites but skin: male, 2002)
Looking into the Future
Cancer is primarily a disease of old age. If all other factors remain the same, the demographic change (population growth and an increasingly higher percentage of older individuals in the world population) will lead to a global increase of cancer incidence. The GLOBOCAN 2002 database provides a means to project cancer incidence in the future. In 2002, there were an estimated total of 5,801,839 male individuals diagnosed with cancer (all sites except skin). If the age-specific incidence rates remain the same, population growth and aging of the world population would result in a projected total of 6,993,778 in 2010, a 20.5 percent increase within eight years (Table 4). The picture is similar for females. The total number of females diagnosed with cancer (all sites except skin) is projected to change from 5,060,657 in 2002 to 6,037,753 in 2010, which represents a 19.3 percent increase (detailed data not shown). This underscores the importance to improve our understanding of the risk factors of cancer, design and implement practical prevention strategies, and develop better and more effective treatment options.
The population compositions are dramatically different between more and less developed countries. Less developed countries have a smaller percentage of older individuals [Figure 5]. As cancer incidence increases with age, less developed countries have a larger population base and potentially more room for population aging. The foreseeable increase in the global burden of cancer likely will be more profound in less developed countries. In a recently published report, Cancer Control Opportunities in the Developing World [22], by the Institute of Medicine of the National Academy of Sciences, it is recognized that cancer is a significant disease burden in low- and middle-income countries, and the burden will become increasingly heavy for these countries not only because these nations are more populous, which give rise to more cases, but there are also more aggressive cancers and lower cure rates. The report also indicates that cancer causes and outcomes are very different between more and less developed countries. For example, one in four cancer cases in developing countries are related to infectious agents compared to less than one in 10 cases in the developed nations. These disparities suggest different cancer prevention and control strategies for more and less developed countries in directing resources into the field of cancer research with regard to etiology, prevention, treatment, and health policy to reduce the burden of cancer globally.
Figure 5.
Population Pyramid for More (top) and Less (bottom) Developed Countries, 2002.
Table 1. Age-specific incidence rate of all cancers (except skin) among males in 2002*.
| 0-14 | 15-44 | 45-54 | 55-64 | 65+ | All ages | ASR** | |
| Eastern Africa | 16.04 | 76.94 | 251.11 | 443.9 | 821.06 | 91 | 158.7 |
| Middle Africa | 10.13 | 60.1 | 238.13 | 443.01 | 731.93 | 78.1 | 141.9 |
| Northern Africa | 11.09 | 27.56 | 138.95 | 344.95 | 583.31 | 65.8 | 99 |
| Southern Africa | 9.45 | 52.44 | 324.88 | 755.78 | 1315.28 | 127.4 | 213.7 |
| Western Africa | 6.49 | 37.69 | 193.74 | 293.41 | 385.44 | 51.9 | 90 |
| Caribbean | 12.43 | 31.28 | 189.29 | 532.17 | 1624.15 | 177.3 | 194.4 |
| Central America | 14.81 | 33.86 | 136.57 | 386.3 | 1157.13 | 100.3 | 146.1 |
| South America | 13.47 | 40.81 | 235.81 | 662.67 | 1653.23 | 168.5 | 216.4 |
| North America | 15.12 | 79.56 | 424.55 | 1321.14 | 2958.14 | 529.7 | 398.4 |
| Eastern Asia | 9.07 | 53.53 | 346.87 | 663.89 | 1461.59 | 227.2 | 219.4 |
| Southeastern Asia | 10.47 | 30.31 | 194.44 | 429.39 | 833.46 | 94.3 | 130.4 |
| South Central Asia | 7.24 | 26.95 | 166.82 | 379.62 | 612.97 | 76.3 | 105.5 |
| Western Asia | 12.11 | 33.3 | 185.69 | 522.08 | 989.35 | 104.4 | 149.5 |
| Eastern Europe | 12.23 | 50.37 | 372.94 | 973.17 | 1619.75 | 328.3 | 257.7 |
| Northern Europe | 13.67 | 51.88 | 259.85 | 802.67 | 2340.02 | 456.6 | 283.1 |
| Southern Europe | 14.22 | 61.91 | 378.34 | 941.65 | 2163.41 | 493.2 | 299.4 |
| Western Europe | 14.1 | 71.67 | 402.15 | 1050.75 | 2327.87 | 525.6 | 326.4 |
| Australia/New Zealand | 15.91 | 77.61 | 344.04 | 1024.79 | 2736.44 | 483.9 | 349.7 |
| Melanesia | 9.04 | 36.86 | 213.33 | 489.47 | 912 | 80.6 | 145.1 |
| Micronesia | 11.04 | 36.41 | 170 | 404.58 | 1159.27 | 107.1 | 151.3 |
| Polynesia | 5.47 | 33.56 | 266.33 | 676.44 | 996.07 | 116.7 | 169.3 |
*Rates are per 100,000 person-years. **Age-standardized rates using the world standard.
Table 2. Age-specific incidence rate of all cancers (except skin) among females in 2002*.
| 0-14 | 15-44 | 45-54 | 55-64 | 65+ | All ages | ASR** | |
| Eastern Africa | 10.76 | 82.75 | 339.77 | 511.03 | 564.26 | 97.7 | 156.7 |
| Middle Africa | 7.55 | 64.93 | 274.96 | 391.14 | 424.09 | 76.1 | 121.5 |
| Northern Africa | 8.32 | 42.25 | 183.95 | 271.61 | 320.83 | 66.7 | 85.2 |
| Southern Africa | 7.57 | 69.92 | 353.05 | 537.91 | 698.69 | 126 | 163.2 |
| Western Africa | 5.25 | 58.06 | 272.49 | 341.17 | 293.34 | 66.8 | 104.4 |
| Caribbean | 10.81 | 68 | 301.37 | 448.1 | 903.83 | 166.6 | 164.9 |
| Central America | 11.84 | 54.4 | 303.63 | 438.82 | 824.11 | 119.7 | 153.3 |
| South America | 11.72 | 69.57 | 355.88 | 562.68 | 1055.34 | 176.5 | 191.6 |
| North America | 13.06 | 116.97 | 502.96 | 950.27 | 1705.66 | 454.8 | 305.1 |
| Eastern Asia | 5.98 | 52.56 | 267.63 | 399.11 | 728.11 | 157.2 | 136.8 |
| Southeastern Asia | 8.44 | 51.27 | 284.71 | 367.38 | 507 | 101.4 | 120.9 |
| South Central Asia | 4.64 | 47.4 | 265.69 | 384.08 | 404.08 | 88.9 | 110.1 |
| Western Asia | 9.81 | 48.44 | 252.93 | 398.74 | 602.27 | 99.3 | 125.7 |
| Eastern Europe | 9.85 | 76.09 | 347.65 | 554.81 | 809.86 | 277.2 | 175.1 |
| Northern Europe | 11.18 | 91.32 | 438.43 | 778.15 | 1414.85 | 439.1 | 252.3 |
| Southern Europe | 12.48 | 89.11 | 398.3 | 596.42 | 1062.4 | 362.5 | 208.1 |
| Western Europe | 11.84 | 105.2 | 460.26 | 731.53 | 1236.72 | 429.3 | 244.6 |
| Australia/New Zealand | 13.75 | 116.05 | 479.5 | 851.99 | 1503.38 | 405.5 | 280.3 |
| Melanesia | 6.47 | 70.43 | 376.98 | 590.38 | 628.68 | 108.1 | 165 |
| Micronesia | 7.61 | 58.21 | 304.28 | 401.98 | 725.41 | 110.2 | 143.8 |
| Polynesia | 6.99 | 92.78 | 432.81 | 373.09 | 568.88 | 131.3 | 159.3 |
*Rates are per 100,000 person-years. **Age-standardized rates using the world standard.
Table 3. Age-specific mortality rate of all cancers (except skin) among males in 2002*.
| 0-14 | 15-44 | 45-54 | 55-64 | 65+ | All ages | ASR** | |
| Eastern Africa | 10.66 | 59.17 | 204.22 | 380.4 | 736.85 | 73.9 | 133.2 |
| Middle Africa | 7.11 | 49.73 | 198.74 | 375.94 | 646.45 | 65.6 | 120.8 |
| Northern Africa | 7.28 | 19.81 | 111.83 | 293.42 | 522.28 | 53.9 | 83.1 |
| Southern Africa | 6.13 | 38.82 | 240.95 | 584.94 | 951.5 | 94.5 | 158.5 |
| Western Africa | 4.14 | 28.02 | 152.52 | 244.21 | 340.43 | 41.3 | 73.5 |
| Caribbean | 5.98 | 16.74 | 120.01 | 343.39 | 1229.53 | 123.8 | 135.8 |
| Central America | 6.06 | 14.61 | 75.72 | 230.21 | 859.4 | 63 | 95.1 |
| South America | 6.35 | 18.4 | 131.69 | 369.27 | 1112.8 | 101.1 | 131.8 |
| North America | 2.65 | 16.3 | 128.69 | 417.08 | 1394.91 | 210.2 | 153 |
| Eastern Asia | 5.05 | 31.07 | 229.81 | 471.03 | 1198.95 | 167 | 161.8 |
| Southeastern Asia | 6.87 | 19.89 | 147.18 | 339.23 | 692.48 | 73 | 102.5 |
| South Central Asia | 4.3 | 15.79 | 113.69 | 283.14 | 495.86 | 55.2 | 78 |
| Western Asia | 7.27 | 18.86 | 122.48 | 379.68 | 778.81 | 74.4 | 108.7 |
| Eastern Europe | 5.53 | 28.64 | 256.3 | 750.41 | 1356.35 | 253.5 | 197.2 |
| Northern Europe | 3.33 | 14.52 | 131.68 | 413.01 | 1517.66 | 269.9 | 161 |
| Southern Europe | 3.47 | 20.38 | 175.51 | 495.03 | 1447.52 | 294.4 | 170.1 |
| Western Europe | 3.09 | 19.58 | 177.63 | 483.88 | 1518.44 | 294.6 | 173.9 |
| Australia/New Zealand | 3.7 | 15.37 | 110.58 | 378.27 | 1412.69 | 213.9 | 149.1 |
| Melanesia | 5.7 | 24.69 | 152.6 | 358.93 | 667.37 | 57.3 | 104.6 |
| Micronesia | 7.97 | 23.48 | 128.46 | 323.52 | 884.31 | 80.4 | 114.5 |
| Polynesia | 3.45 | 20.46 | 203.46 | 529.58 | 738.05 | 86.1 | 126.3 |
*Rates are per 100,000 person-years. **Age-standardized rates using the world standard.
Table 4. Age-specific mortality rate of all cancers (except skin) among females in 2002*.
| 0-14 | 15-44 | 45-54 | 55-64 | 65+ | All ages | ASR** | |
| Eastern Africa | 7.15 | 60.04 | 256.42 | 408.34 | 482.72 | 75 | 122.7 |
| Middle Africa | 5.04 | 52.21 | 213.85 | 313.89 | 375.8 | 61.5 | 99 |
| Northern Africa | 5.27 | 29.24 | 135.05 | 208.92 | 276.45 | 50.2 | 65.1 |
| Southern Africa | 4.67 | 40.94 | 219.9 | 364.99 | 483.42 | 81.1 | 106.3 |
| Western Africa | 3.3 | 41.77 | 198.17 | 264.1 | 253.65 | 50.2 | 79.7 |
| Caribbean | 5.01 | 26.66 | 140.65 | 269.81 | 690.16 | 100.3 | 98.4 |
| Central America | 5.22 | 20.72 | 133.72 | 253.04 | 630.68 | 67.9 | 89.6 |
| South America | 5.19 | 23.87 | 148.37 | 285.8 | 731.22 | 93.4 | 102.2 |
| North America | 2.32 | 19.4 | 124.07 | 321.35 | 910.18 | 185.8 | 112.1 |
| Eastern Asia | 3.74 | 20.79 | 134.74 | 250.45 | 590.09 | 100.8 | 86.3 |
| Southeastern Asia | 5.47 | 24.98 | 156.44 | 244.17 | 385.43 | 62.5 | 76.2 |
| South Central Asia | 2.79 | 22.32 | 151.7 | 265.77 | 307.54 | 55.2 | 69.9 |
| Western Asia | 5.82 | 21.36 | 123.79 | 238.9 | 432.93 | 57.7 | 74 |
| Eastern Europe | 4.56 | 28.97 | 158.84 | 326.95 | 633.93 | 175.7 | 101.9 |
| Northern Europe | 2.6 | 20.44 | 138.34 | 327.26 | 958.56 | 236.9 | 118.1 |
| Southern Europe | 3.04 | 19.93 | 119.1 | 244.68 | 714.06 | 189.03 | 92.2 |
| Western Europe | 2.21 | 20.7 | 127.62 | 274.95 | 864.06 | 224.9 | 106.1 |
| Australia/New Zealand | 2.67 | 19.18 | 116.09 | 283.8 | 841.32 | 167.1 | 103.4 |
| Melanesia | 3.84 | 37.06 | 233.53 | 400.91 | 424.78 | 66.5 | 104.6 |
| Micronesia | 4.12 | 29.33 | 175.51 | 261.52 | 492.51 | 66.8 | 88.6 |
| Polynesia | 4.81 | 48.83 | 268.4 | 247.04 | 368.27 | 79.2 | 97.6 |
*Rates are per 100,000 person-years. **Age-standardized rates using the world standard.
Abbreviations
- IARC
International Agency of Research on Cancer
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Parkin DM. International variation. Oncogene. 2004;23(38):6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92(3):426–429. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization; Tobacco smoke and involuntary smoking: Views and expert opinions of an IARC working group on the evaluation of carcinogenic risks to humans; 2002 11-18 June; Lyon. Lyon, France: IARC; 2004. [PMC free article] [PubMed] [Google Scholar]
- Jha P, Chaloupka FJ, Corrao M, Jacob B. Reducing the burden of smoking world-wide: effectiveness of interventions and their coverage. Drug Alcohol Rev. 2006;25(6):597–609. doi: 10.1080/09595230600944511. [DOI] [PubMed] [Google Scholar]
- Siemiatycki J, Richardson L, Straif K, et al. Listing occupational carcinogens. Environ Health Perspect. 2004;112(15):1447–1459. doi: 10.1289/ehp.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Wong O, Yang L, Li T, Su Z. The development and regulation of occupational exposure limits in China. Regul Toxicol Pharmacol. 2006;46(2):107–113. doi: 10.1016/j.yrtph.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Lan Q, Zhang L, Li G, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306(5702):1774–1776. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO.int: Media centre fact sheets [Internet] Geneva, Switzerland: World Health Organization; [updated 2005 June; cited 2007 April 2]. Available from: http://www.who.int/mediacentre/factsheets/fs292/en/ [Google Scholar]
- WHO.int: Press release: FAO/WHO: Amount of poor-quality presticides sold in developing countries alarmingly high [Internet] Geneva, Switzerland: World Health Organization; [updated 2001 February 1; cited 2007 April 2]. Available from: http://www.who.int/inf-pr-2001/en/pr2001-04.html. [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: Summary of a clinical research workshop. Hepatology. 2007;45(4):1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- Magalhaes Queiroz DM, Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2006;11(Suppl 1):1–5. doi: 10.1111/j.1478-405X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- Stuver SO, Boschi-Pinto C, Trichopoulos D. Infection with hepatitis B and C viruses, social class and cancer. IARC Sci Publ. 1997;138:319–324. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29(1):109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991-2005. Br J Cancer. 2004;90(11):2157–2166. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- Sloan FA, Gelband H, editors. Institute of Medicine (U.S.) Committee on Cancer Control in Low- and Middle-Income Countries. Cancer control opportunities in low- and middle-income countries. Washington DC: National Academies Press; 2007. [PubMed] [Google Scholar]