Abstract
In this study, we demonstrate that: (i) injection of an adenovirus (Ad) vector containing the brain-derived neurotrophic factor (BDNF) gene (Ad.BDNF) into the vitreous chamber of adult rats results in selective transgene expression by Müller cells; (ii) in vitro, Müller cells infected with Ad.BDNF secrete BDNF that enhances neuronal survival; (iii) in vivo, Ad-mediated expression of functional BDNF by Müller cells, temporarily extends the survival of axotomized retinal ganglion cells (RGCs); 16 days after axotomy, injured retinas treated with Ad.BDNF showed a 4.5-fold increase in surviving RGCs compared with control retinas; (iv) the transient expression of the BDNF transgene, which lasted ≈10 days, can be prolonged with immunosuppression for at least 30 days, and such Ad-mediated BDNF remains biologically active, (v) persistent expression of BDNF by infected Müller cells does not further enhance the survival of injured RGCs, indicating that the effect of this neurotrophin on RGC survival is limited by changes induced by the lesion within 10–16 days after optic nerve transection rather than the availability of BDNF. Thus, Ad-transduced Müller cells are a novel pathway for sustained delivery of BDNF to acutely-injured RGCs. Because these cells span the entire thickness of the retina, Ad-mediated gene delivery to Müller cells may also be useful to influence photoreceptors and other retinal neurons.
In the adult rat retina, >90% of retinal ganglion cells (RGCs) die within two weeks of axotomy (1). These central nervous system neurons, which have been shown to express the brain-derived neurotrophic factor (BDNF) signaling receptor trkB (2), can be rescued by a single intravitreal injection of this neurotrophin at the time of optic nerve (ON) transection (3). However, this survival effect is transient, only delaying the onset of RGC death by ≈3 days. Here we have investigated the possibility of extending the delivery of BDNF by using recombinant adenovirus (Ad) vectors. We found that the intravitreal injection of Ad vectors results in a selective transgene expression by Müller cells, the main glial cell type in the mammalian retina (4). Müller cells, which play a role in the metabolic maintenance and structural integrity of retinal neurons, have cytoplasmic processes in close contact with neuronal cell bodies, including RGCs and photoreceptors (5). Consequently, we investigated if: (i) Müller cells infected with an Ad vector containing the BDNF gene could produce this neurotrophin within the retina; (ii) such local production of BDNF would enhance the survival of acutely injured RGCs; and (iii) the persistent expression of Ad-mediated BDNF by transduced Müller cells could promote long-term survival of axotomized RGCs.
EXPERIMENTAL PROCEDURES
Construction and Preparation of Ad Vectors.
The mouse BDNF cDNA with the human c-myc 9E10 epitope (6) fused to K246 was cloned into an Ad5 shuttle plasmid (7) under control of the cytomegalovirus (CMV) promoter. This plasmid was cotransfected (8) into 293 cells with E1-deleted Ad5 DNA (dl309). Virus plaques generated by homologous recombination were screened by the PCR using sequence-specific primers for mouse BDNF (5′-CCGGTATCCAAAGGCCAACTG-3′) and the c-myc epitope tag (5′-TTCTTCAGAAATAAGCTTTTG-3′). The resulting replication-deficient virus (Ad.BDNF) was plaque purified, propagated in 293 cells, and concentrated on CsCl gradients by using standard procedures (9). Following addition of 10% glycerol, viral stocks were stored at −80°C. Titers of the concentrated viral stocks, determined by direct plaque assay, were in the order of 2–5 × 1010 plaque forming units/ml. Absence of wild-type Ad was verified by titration using HeLa cells and by PCR as described (10). A control Ad vector containing the CMV-LacZ reporter gene expression cassette (6) was propagated and purified in a similar fashion.
Characterization of Ad-Mediated BDNF Expression in Müller Cells in Vitro.
The rat Müller cell line HPV-16 E6/E7 (11) was maintained in DMEM (GIBCO/BRL) supplemented with 10% fetal bovine serum in a humidified incubator. Cells were plated in 48-well plates (≈3–5 × 104 cells per well) 24 hr before infection, which was performed in PBS containing 2% horse serum for 2 hr at 37°C at multiplicities of infection (plaque-forming units/cell) between 1 and 1,000. Müller cell supernatants and whole cell extracts were collected 48 hr later and used for Western blot analyses or neuronal survival bioassays. Cells were incubated with the secretion blocker Monensin (10−7 M; Sigma) for 8 hr after virus infection and processed for anti-c-myc antibody staining 48 hr later. Cells expressing the BDNF/c-myc protein were visualized using a fluorescence microscope (Polyvar, Reichert-Jung).
Protein samples were loaded onto SDS/18% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad) according to the manufacturer’s specifications. Blots were processed for anti-c-myc antibody staining and developed with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt.
For neuronal survival bioassays, lumbar dorsal root ganglia from chicken embryos [embryonic day 8.5 (E8.5)] were prepared as described (12). Aliquots of 8,000 cells were added to 200 μl of Müller cell conditioned media and plated on poly-d-lysine and laminin-coated (17.5 μg/ml, Sigma) 18-mm diameter wells in 60-mm culture dishes. Cells were maintained in a humidified incubator at 37°C and the number of surviving neurons was determined 24 hr later by direct cell counting of the entire wells.
In Vivo Gene Delivery, Histochemical Analysis, and Quantitation of Neuronal Rescue.
All surgical procedures were performed in female Sprague–Dawley rats (180–200 g) under general anesthesia (7% chloral hydrate; 0.42 mg per g of body weight, i.p.) in accordance with the guidelines for the use of experimental animals (13). Ad stocks (5 μl) were injected intravitreally into the superior hemisphere of the retina using a posterior approach as described (3). Control eyes were injected with an equal volume of Ad.LacZ, human BDNF protein (5 μg in BSA/PBS), or Hepes-buffered saline (virus vehicle).
Serial radial sections of the entire retina were obtained for analysis of the transgene expression pattern. Cryosections (15 μm) were incubated in 10% normal goat serum in 0.2% Triton-X-100 (Sigma) in PBS for 30 min at room temperature followed by addition of anti-c-myc antibody (Oncogene Research Products, Cambridge, MA) in 2% normal goat serum in PBS for 14–18 hr at 4°C. Sections were further processed with biotinylated anti-mouse Fab fragment (Jackson ImmunoResearch), avidin-biotin-peroxidase reagent (ABC Elite Vector Labs, Burlingame, CA), followed by reaction in a solution containing 0.05% diaminobenzidine tetrahydrochloride and 0.06% hydrogen peroxide in 0.1 M phosphate buffer (pH 7.4) for 5–10 min. For 5-bromo-4-chloro-3-indolyl β-d-galactoside staining, eyes postfixed in 4% paraformaldehyde were incubated in staining solution (1 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside/5 mM K3Fe(CN)6/5 mM K4Fe(CN)6.3H2O/2 mM MgCl2, in PBS) 1 hr to overnight at 37°C, then rinsed briefly, and processed for frozen sections.
For RGC survival experiments, cells were retrogradely labeled with the fluorescent tracer Fluorogold (Fluorochrome, Englewood, CO; 2% in 0.9% NaCl containing 10% dimethyl sulfoxide) by application of the tracer to the superior colliculi 7 days prior to transection of the ON. Intravitreal administration of virus, BDNF, or vehicle solution was performed at the time of ON cut close to the eye. Rats were sacrificed by vascular perfusion with 4% paraformaldehyde and both the left (ON lesion) and right (intact control) retinas were dissected, fixed for an additional 30 min and flat-mounted vitreal side up on glass slides. The ganglion cell layer was examined under fluorescence microscopy (excitation filter, 355–425; barrier filter, LP 460) and Fluorogold-labeled neurons were counted in standard areas (14). Results were analyzed by using the sigmastat program (Jandel, San Rafael Madera, CA) by a Student’s t test (paired groups).
For immunosuppression studies, animals received daily subcutaneous injections of the immunosuppressant FK-506 (Fujisawa Pharmaceuticals, Osaka) at a dose of 1 mg/kg body weight that started 2 days prior to Ad.BDNF injection. Control eyes were injected with Ad.LacZ or vehicle. Eyes were obtained at 16 and 30 days after virus administration and processed for RGC density quantitation, c-myc immunohistochemistry, or histochemical staining using standard procedures (15).
RESULTS
Müller Cells Express the Ad-Mediated BDNF in the Intact and Injured Retina.
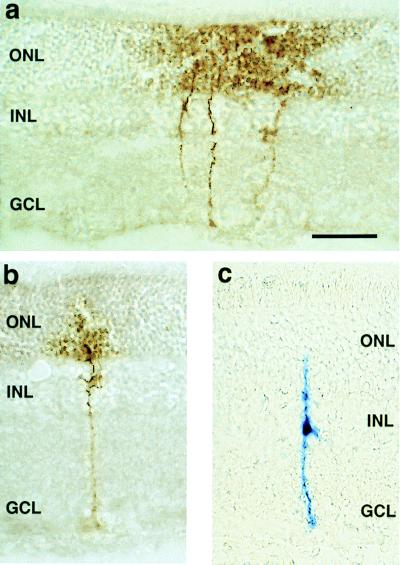
Following intravitreal injection of Ad.BDNF, c-myc staining of retinal sections demonstrated that tagged BDNF was located in Müller cells (Fig. 1 a and b). This observation was confirmed in both intact and axotomized retinas. Addition of a c-myc tag to BDNF allowed the use of a specific anti-c-myc antibody to distinguish the Ad-mediated BDNF from the endogenous BDNF, normally present in 3–5% of RGCs (16). We did not detect the tagged BDNF in RGCs or other retinal neurons. Labeling of retinal pigment epithelium was occasionally observed but only at the needle track (not shown). This was presumably due to exposure of retinal pigment epithelium to Ad particles at the time of needle insertion. Subretinal injections of Ad have been shown to result in efficient transduction of retinal pigment epithelium cells (17, 18).
Figure 1.
Retinal radial cryosections showing typical Müller cell transgene expression at 7 days after intravitreal administration of Ad vectors (5 μl = 107 plaque-forming units/ml) in the adult rat eye. (a) A group of Müller cells and (b) a single Müller cell transduced in vivo with Ad.BDNF were visualized with an anti-c-myc antibody. (c) A single Müller cell infected with the control virus Ad.LacZ was visualized by 5-bromo-4-chloro-3-indolyl β-d-galactoside staining. Note the diffuse reaction product at the level of the ONL only in cells exposed to Ad.BDNF. INL: inner nuclear layer, GCL: ganglion cell layer. (Bar = 50 μm.)
We compared the staining pattern of single Müller cells transduced with Ad.BDNF or control Ad.LacZ (Fig. 1 b and c). C-myc immunoreactivity in Müller cells infected with Ad.BDNF was observed along the radial processes from the basal end-feet to the apical region in the outer nuclear layer (ONL). Interestingly, a diffuse immunostaining at the level of the ONL was always found in cells transduced with the Ad.BDNF vector (Fig. 1 a and b) suggesting that tagged BDNF was secreted by Müller cells. This was in contrast to the 5-bromo-4-chloro-3-indolyl β-d-galactoside staining found in cells transduced with Ad.LacZ in which the blue reaction product remained confined to the cytoplasm (Fig. 1c).
Expression of the tagged BDNF protein in Müller cells was detected as early as 1.5 days after Ad.BDNF administration. Transgene expression peaked at 6–7 days following vector injection: all retinas examined contained strongly c-myc-immunostained Müller cells. An average of 1,500 Müller cells per retina (<1% of the total number of Müller cells in the rat retina; ref. 4) were estimated to express the transgene following intravitreal administration of the Ad vectors. The highest density of immunoreactive cells was usually found in the central retina (superior hemisphere), where the vector was introduced, with progressive decreases in the density of labeled cells from the injection site. However, immunopositive cells were also detected in the peripheral retina near the ora serrata. BDNF/c-myc expression was markedly reduced at 10 days with only sparse staining detectable in a few retinas. None of the retinas examined at 14, 16, 21, and 28 days after vector administration showed positive c-myc-labeled cells. The temporal pattern of c-myc immunoreactivity in Müller cells was similar in the animals with ON transection.
Müller Cells Secrete Bioactive Ad-Mediated BDNF in Vitro.
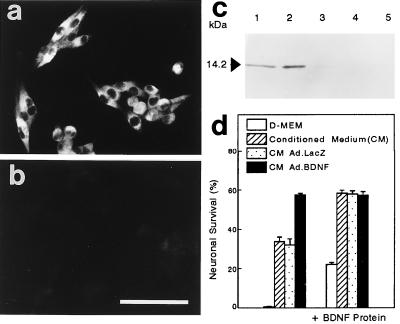
The capacity of retinal Müller cells to synthesize and secrete Ad-mediated BDNF was tested in vitro using the rat Müller cell line HPV-16 E6/E7. Cells derived from this line are highly reactive for Müller cell markers including S-100, carbonic anhydrase-C, cellular retinaldehyde binding protein, and glial fibrillary acidic protein (11). In some preparations, the cells infected with Ad.BDNF were exposed to the secretion blocker Monensin (19) to prevent loss of BDNF/c-myc protein into the culture medium and to permit localization within cells. C-myc immunoreactivity in Ad-infected Müller cells (Fig. 2a) was most intense in the perinuclear cytoplasm with little or no staining along the cellular processes. Only background staining was detected in monolayers exposed to control Ad.LacZ (Fig. 2b).
Figure 2.
Characterization of Ad-mediated BDNF expression in the rat Müller cell line HPV-16 E6/E7. Cell monolayers were infected with Ad.BDNF (a) or control Ad.LacZ (b) at a multiplicity of infection of 20 in the presence of Monensin (10−7 M) and subsequently processed for c-myc immunostaining. (Bar = 100 μm.) (c) Western blot analysis showing recombinant c-myc tagged BDNF (100 ng; lane 1), supernatant of Müller cells infected with Ad.BDNF (lane 2), supernatant of Ad.BDNF-infected Müller cells treated with Monensin (lane 3), whole cell extracts following Ad.BDNF infection (lane 4) and cell supernatant after infection with Ad.LacZ (lane 5). Five micrograms of total protein was loaded on lanes 2 to 5. (d) E8.5 chicken spinal sensory neuron survival bioassay in different conditioned media. The values are the means of triplicate determinations ± SD. All infections of Müller cell cultures for Western blots and neuronal bioassays were performed at a multiplicity of infection of 100.
Fig. 2c shows an immunoblot in which the conditioned media (CM) or whole cell protein extracts of Müller cells infected with Ad vectors were visualized by anti-c-myc staining. In agreement with the predicted relative molecular mass of 14.2 kDa for recombinant BDNF/c-myc (Fig. 2c, lane 1), a single protein corresponding to the fully processed BDNF/c-myc monomer was detected in the supernatant of Müller cells exposed to Ad.BDNF (Fig. 2c, lane 2). The absence of BDNF/c-myc protein was confirmed in all negative controls: (i) supernatant of cells transduced with Ad.BDNF in the presence of Monensin (Fig. 2c, lane 3); (ii) whole cell protein extracts of Müller cells infected with Ad.BDNF (Fig. 2c, lane 4); and (iii) CM of Müller cells infected with Ad.LacZ (Fig. 2c, lane 5). The lack of detectable amounts of pre-pro BDNF/c-myc in whole cell extracts suggests that Ad-mediated BDNF is rapidly processed and secreted by these cells.
The biological activity of BDNF secreted by Ad-transduced Müller cells was assayed by using spinal sensory neurons from chicken embryos (E8.5). Quantitation of neuronal survival in the presence of this medium indicated that the biological activity of Ad-mediated BDNF was strikingly similar to that observed when recombinant BDNF was administered at concentrations that supported maximal neuronal survival (10 ng/ml) (Fig. 2d). This result also indicates that the biological activity of the Ad-mediated BDNF was not compromised by addition of the c-myc tag. CM from Müller cells exposed to Ad.LacZ only produced background survival activity similar to that normally found in the CM of uninfected Müller cells. Interestingly, Müller cell cultures normally express a significant amount of endogenous neurotrophic activity. The nature of this activity is presently unknown but it could be attributed to the production of trophic factors other than BDNF. For example, it has been suggested that Müller cells up-regulate expression of basic fibroblast growth factor (bFGF) and ciliary neurotrophic factor (CNTF) after retinal injury (20). Addition of recombinant BDNF at saturating amounts (10 ng/ml) to the CM of Müller cells transduced with Ad.BDNF did not result in further increases in neuronal survival. However, addition of BDNF protein significantly increased the survival effect of medium alone, CM alone or CM of cells infected with Ad.LacZ, indicating that BDNF was the factor responsible for this survival effect in vitro (Fig. 2d). These results also suggest that the BDNF introduced via the Ad vector was present at levels capable of saturating the trkB receptors present in the BDNF-responsive population of sensory neurons (21).
Expression of Ad-Mediated BDNF by Müller Cells Transiently Enhances the Survival of Injured RGCs in Vivo.
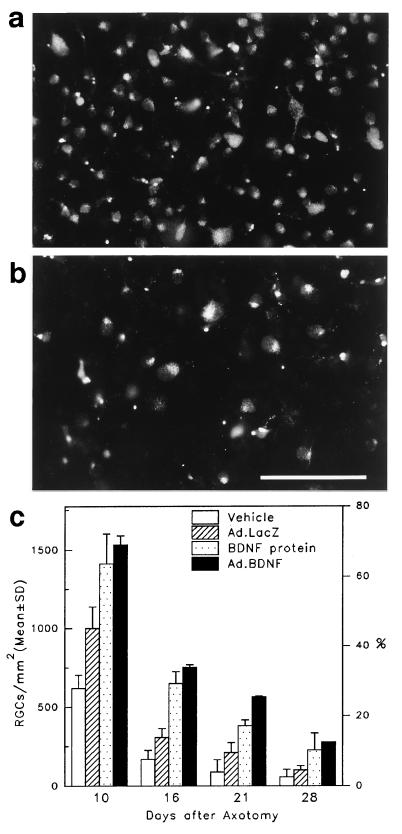
To test if gene transfer of BDNF into Müller glia was sufficient to rescue injured RGCs in vivo, an intravitreal injection of Ad.BDNF was carried out immediately after ON transection. Retinas were examined histologically at 10, 16, 21, and 28 days after ON transection and neuronal densities determined, using retrograde labeling with the tracer Fluorogold to identify surviving RGCs. Examination of retinas that received Ad.BDNF showed significantly more Fluorogold-labeled RGCs than retinas from animals that received Ad.LacZ or vehicle at all times examined (Fig. 3). At 10 days after axotomy, neuronal counts indicated that 65% (1,530 ± 189 RGCs/mm2; mean ± SD) of the total number of RGCs survived in retinas exposed to Ad.BDNF compared with controls: 42% with Ad.LacZ (999 ± 84 RGCs/mm2) and 26% with vehicle (619 ± 128 RGCs/mm2). Surviving RGCs were evenly distributed within each standard area examined, suggesting a widespread effect of Ad-mediated BDNF. At 10 days, a higher RGC survival was observed in eyes injected with Ad.LacZ when compared with vehicle-injected eyes. This may have been due to the Ad-triggered immune response characterized by infiltration of inflammatory cells capable of providing trophic support (22). This Ad.LacZ related effect was not statistically significant at later times. Although the total number of surviving RGCs decreased with time after axotomy, the values for the retinas that received Ad.BDNF remained significantly greater than for the Ad.LacZ or vehicle injected eyes (Fig. 3). At all times examined, the effect of Ad.BDNF was as potent as that observed following a single intravitreal administration of recombinant BDNF, indicating that in vivo synthesis of BDNF by Ad-infected Müller cells effectively delayed the death of axotomized RGCs.
Figure 3.
Flatmounted retinas showing Fluorogold labeled RGCs at 10 days after ON transection and intravitreal injection of Ad.BDNF (a) or vehicle (b). (Bar = 100 μm.) (c) Quantitative analysis of RGC survival in vivo at 10, 16, 21, and 28 days after axotomy and intravitreal administration of 5 μl of Ad.BDNF, recombinant BDNF, Ad.LacZ, or vehicle (n = 3–8 rats per group). At all times examined, significantly greater numbers of RGCs survived in the retinas treated with Ad.BDNF (solid bars) than in the retinas exposed to Ad.LacZ (hatched bars), or vehicle (open bars) (Student’s t test, P < 0.001). RGC densities were similar for the groups of retinas treated with Ad.BDNF (solid bars) or recombinant BDNF (stippled bars) but decreased in all groups at longer times after axotomy.
Immunosuppression Prolongs BDNF Transgene Expression in Müller Cells but Does Not Extend RGC Survival.
Histological examination of retinas exposed to Ad vectors demonstrated an early infiltration of macrophages and mononuclear cells primarily at the site of injection (data not shown). This cellular immune response was apparent within the first week of recombinant Ad injection, but was considerably decreased 2 weeks later. Daily injections of the immunosuppressant FK-506 effectively eliminated the cellular immune response triggered by Ad vectors (not shown). There were no signs of inflammation in retinas of immunosuppressed rats examined at 7, 10, and 16 days after injection of recombinant Ad.
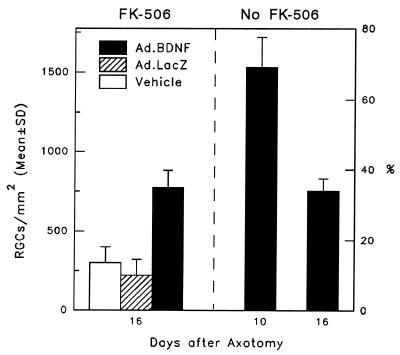
It has been shown previously that the immune response elicited by Ad vectors abolishes long-term expression of the transgene (23, 24). Maximal gene expression peaks during the first week of injection and rapidly declines to basal levels within the next few weeks. In the present experiments, immunosuppression lead to a sustained and strong expression of BDNF/c-myc in Müller cells for the entire period studied (30 days) in both axotomized and intact retinas (data not shown). This was in contrast to the shorter expression, which gradually disappeared at 10–14 days after Ad.BDNF inoculation, observed in immunocompetent animals. In immunosuppressed rats studied from 16 days to 1 month after Ad.BDNF administration, the number of Müller cells expressing the transgene as well as their c-myc staining pattern was similar to that observed at the time of peak transgene expression in immunocompetent rats (1 week). The effect of prolonged BDNF transgene expression on RGC survival was determined in FK-506-treated rats at 16 days after ON transection. The 16-day time was selected for several reasons: (i) transgene expression was no longer detectable in any of the immunocompetent rats; (ii) in these rats, the cellular immune response was still apparent; and (iii) the survival effect of BDNF or Ad-mediated BDNF was 4.5-fold greater than the effect of vehicle injection. One concern regarding the use of FK-506 in these experiments was its possible effect on RGC survival. It has been shown previously that FK-506 promotes neurite outgrowth (25), but its role on neuronal survival is less clear. As evidenced from our experiments, the densities of surviving RGCs in control eyes in the presence of FK-506 indicated that this drug per se did not have a significant effect on RGC survival (Fig. 4).
Figure 4.
Comparison of RGC survival in immunosuppressed and immunocompetent rats 16 days after axotomy and intravitreal administration of Ad.BDNF, Ad.LacZ, or vehicle (n = 3–6 animals per group). The bar representing RGC densities in immunocompetent rats 10 days after axotomy and intravitreal Ad.BDNF injection is included as a reference. At day 16, RGC survival in the retinas treated with Ad.BDNF (solid bar) was greater than in the retinas exposed to Ad.LacZ (hatched bar) or vehicle (open bar) (Student’s t test, P < 0.001), but RGC densities in the immunosuppressed and immunocompetent rats were not significantly different (P > 0.05).
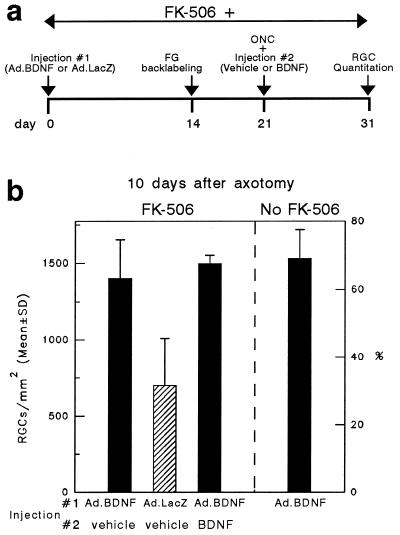
Quantitation of RGC densities indicated that there was an Ad.BDNF-mediated survival effect in animals that received daily doses of FK-506 (773 ± 109 RGCs/mm2). However, this effect was not significantly different from that observed in immunocompetent rats at 16 days after administration of this vector (753 ± 75 RGCs/mm2) (Fig. 4). To determine if a loss of BDNF activity was responsible for the failure of prolonged transgene expression to enhance RGC survival, the experiment outlined in Fig. 5a was performed in immunosuppressed animals. Briefly, intravitreal injections of Ad.BDNF or Ad.LacZ (control) were done at day 0 followed by Fluorogold back-labeling of RGCs at day 14. On day 21, the ON was cut immediately followed by injection of vehicle or BDNF protein. RGC survival was then measured on day 31 (10 days after axotomy). Neuronal survival was significantly higher in retinas exposed to Ad.BDNF (1,402 ± 254 RGCs/mm2; 63%) when compared with those exposed to control Ad.LacZ (742 ± 359 RGCs/mm2; 33.4%) (Fig. 5b), demonstrating that the Ad-mediated BDNF expressed at later times under immunosuppression is still bioactive. In addition, the extent of this survival effect was comparable to that observed when Ad.BDNF was injected at day 0 followed by BDNF injection at the time of ON cut.
Figure 5.
(a) Outline of the experimental protocol to test long-term biological activity of Ad-mediated BDNF under constant immunosuppression. FG, Fluorogold; ONC, optic nerve cut. (b) Quantitation of surviving RGCs at 31 days after intravitreal injection of Ad.BDNF or Ad.LacZ (10 days after axotomy) (n = 3–6 animals per groups). More RGCs survived in the retinas treated with Ad.BDNF than in those exposed to Ad.LacZ (Student’s t test, P < 0.02). A second injection of BDNF protein on day 21 did not result in a further increase in RGC survival. The bar representing RGC survival at 10 days after axotomy in immune competent rats is included as a reference.
DISCUSSION
Functional Expression of Bioactive BDNF by Ad-Infected Müller Cells.
In this study, retinal Müller cells were transduced by intravitreal injection of an Ad vector containing the BDNF gene. One possible explanation for the selective infection of Müller cells is that the basal end-feet of these glial cells provide a large surface for adsorption of viral particles from the vitreous chamber. Alternative explanations include the possibility that receptors mediating Ad internalization (26) are preferentially expressed by Müller cells or that the CMV promoter driving BDNF expression is selectively active or stronger in these glial cells than in neurons. For example, CMV-directed gene expression has been shown to be markedly down-regulated in postmitotic neurons (27). Interestingly, certain DNA-binding dyes accumulate specifically in Müller cells after intravitreal injection and are not taken up by other retinal cells (28), a finding that points toward some specificity in the entry of molecules into these cells.
Our results strongly suggest that infected Müller cells secrete Ad-mediated BDNF that is biologically active both in vitro and in vivo. In our experiments using the cell line HPV-16 E6/E7, which retains the Müller cell phenotype in vitro (11), we found that after infection with Ad.BDNF these cells can process and secrete BDNF that is bioactive. The characteristic diffuse staining pattern surrounding Ad.BDNF-infected Müller cells in the ONL, which was never observed after Ad.LacZ infection, favors the idea of secretion of BDNF by the transduced Müller cells. Interestingly, the use of secretion blockers was not necessary to visualize the transgene product in Müller cells in the retina, suggesting some differences in the way these cells transport and secrete the BDNF/c-myc protein in vivo and in vitro. The reason for this is not known, but it is possible that extrinsic factors such as cell-cell interactions or extracellular matrix components play a role in regulating the rate of secretion of BDNF/c-myc in the in vivo situation. Most importantly, expression of Ad-mediated BDNF by Müller cells in vivo prolonged the survival of axotomized RGCs over the entire retina. This survival effect may involve secretion of Ad-mediated BDNF by Müller cells into the surroundings of neurons within the retina and also into the vitreous chamber with further diffusion to the RGC layer.
Other studies have focused on the transfer of reporter or therapeutic genes directly into retinal neurons (29, 30) or photoreceptors (31, 32). For example, RGCs have been targeted by retrograde transport following application of an Ad.LacZ vector to the superior colliculus (29) or by intravitreal injection of an adeno-associated virus vector (30). The approach we describe here takes advantage of the typical Müller glia cytoarchitecture with processes closely apposed to different classes of retinal neurons. Our study also differs from other indirect but more invasive approaches to deliver trophic factors to the lesioned adult central nervous system in which primary fibroblasts (33) or central nervous system-derived neural stem cells (34) were genetically engineered to secrete nerve growth factor and then transplanted into the site of injury. Given the characteristic morphology of Müller glial cells, which span the entire retina, this strategy might be extended to rescue photoreceptor cells and other retinal neurons.
Persistent Expression of Ad-Mediated BDNF with Immunosuppression and its Effect on Injured RGCs.
The expression of the BDNF transgene in Müller cells declined rapidly and was barely detectable by anti-c-myc immunoreactivity 10 days after gene transfer. A similar decrease in foreign gene expression was observed previously in other Ad-transduced tissues (35–37) and has been attributed to the immune response elicited by the vector, which is directed toward the infected cells (23). Consistent with this idea, the transient BDNF transgene expression in Müller cells, which lasted ≈10 days, was prolonged for >30 days by immunosuppression using FK-506. This suggests that the Ad-triggered immune response rather than decreased CMV promoter activity limits transgene expression within the period studied. The results from the experiment outlined in Fig. 5a demonstrated that under these conditions: (i) the Ad-mediated BDNF remained bioactive; and (ii) prolonged exposure to BDNF did not alter the capacity of RGCs to respond to this factor. In spite of these conditions, persistent availability of the bioactive BDNF did not promote long-term survival of axotomized RGCs. These results are in agreement with our previous observations in similarly injured rat retinas where multiple intravitreal injections of BDNF (3) or sustained administration of NT-4 using osmotic minipumps did not prolong RGC survival (38). Regardless of the delivery system, the RGC survival effect of BDNF is most pronounced within the first week after axotomy and rapidly declines thereafter. It has been shown that exposure of central nervous system neurons to BDNF induces down-regulation of full-length trkB receptor expression at the mRNA and protein level both in vitro and in vivo (39, 40). However, our data suggest that in spite of constant exposure to biologically active BDNF, RGCs can express sufficient levels of trkB receptors to mediate a BDNF-elicited survival response, at least during the first 10 days after axotomy.
Alternatively, changes triggered by injury rather than by prolonged exposure to BDNF might limit the response of RGCs to this neurotrophin. Thus, the loss of RGCs appears to be closely related to cellular events that take place within 10–16 days after axotomy. One possibility is that axotomy is the trigger of a down-regulation of trkB receptor levels. In addition, results from our laboratory using quantitative in situ hybridization (P. Kittlerova and A.J.A., unpublished observations) indicate a down-regulation of the full-length trkB receptor mRNA levels by 3 days after axotomy that could lead to a desensitization of RGCs to BDNF. Interestingly, ON cut has also been shown to induce an up-regulation of the truncated trkB receptor levels in astrocytes in the ON stump at approximately one week after injury (T. Jelsma, G.M.B., and A.J.A., unpublished data). This raises the possibility of a limited availability of BDNF to injured RGCs due to competitive binding of this ligand by nonneuronal cells. While axotomized RGCs appear to respond only transiently to BDNF, different neuronal populations or pathologies other than axotomy might not lead to such resilience. Future studies will need to focus on the elucidation of the molecular mechanisms responsible for the decline in the effectiveness of this neurotrophin in enhancing neuronal survival after axotomy.
Acknowledgments
We thank Drs. Y.-A. Barde (Max-Planck Institute for Neurobiochemistry, Martinsried, Germany) for providing the BDNF/c-myc construct, Regeneron Pharmaceuticals (Tarrytown, NY) for providing the human BDNF protein, R. S. Roque (University of North Texas Health Science Center, Fort Worth) for providing the rat Müller cell line HPV-16 E6/E7, M. Ferns (Centre for Research in Neuroscience, McGill University, Montreal), and S. Hardy (Cell Genesys, Foster City, CA) for providing the Ad shuttle plasmid. The assistance of S. Singel, R. Gill, J. Trecarten, M. Attiwell, and W. Wilcox is gratefully acknowledged. This research was supported by postdoctoral awards to A.D.P. from the Canadian Neuroscience Network and L.J.A. from the Deutscher Akademischer Austauschdienst and the Rick Hansen Foundation; and by grants to A.J.A. from the Medical Research Council and the Canadian Neuroscience Network.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Ad, adenovirus; BDNF, brain-derived neurotrophic factor; RGC, retinal ganglion cell; ON, optic nerve; CM, conditioned media; CMV, cytomegalovirus; ONL, outer nuclear layer; En, embryonic day n.
References
- 1.Berkelaar M, Clarke D B, Wang Y-C, Bray G M, Aguayo A J. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jelsma T N, Hyman Friedman H, Berkelaar M, Bray G M, Aguayo A J. J Neurobiol. 1993;24:1207–1214. doi: 10.1002/neu.480240907. [DOI] [PubMed] [Google Scholar]
- 3.Mansour-Robaey S, Clarke D B, Wang Y-C, Bray G M, Aguayo A J. Proc Natl Acad Sci USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao T I, Grosche J, Friedrich K J, Biedermann B, Francke M, Pannicke T, Reichelt W, Wulst M, Mühle C, Pritz-Hohmeier S, et al. J Neurocytol. 1997;26:439–454. doi: 10.1023/a:1018525222826. [DOI] [PubMed] [Google Scholar]
- 5.Newman E, Reichenbach A. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 6.Munro S, Pelham H R. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 7.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham F L, van der Erb A J. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 10.Lochmüller H, Jani A, Huard J, Prescott S, Simoneau M, Massie B, Karpati G, Acsadi G. Hum Gene Ther. 1994;5:1485–1491. doi: 10.1089/hum.1994.5.12-1485. [DOI] [PubMed] [Google Scholar]
- 11.Roque R S, Agarwal N, Wordinger R J, Brun A M, Xue Y, Huang L C, Nguyen L P, Shay J W. Exp Eye Res. 1997;64:519–527. doi: 10.1006/exer.1996.0230. [DOI] [PubMed] [Google Scholar]
- 12.Aigner L, Caroni P. J Cell Biol. 1993;123:417–429. doi: 10.1083/jcb.123.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olfert E D, Cross B M, McWilliams A A. The Guide to the Care and Use of Experimental Animals, Canadian Council on Animal Care. ON, Canada: Ottawa; 1993. [Google Scholar]
- 14.Villegas-Pérez M P, Vidal-Sanz M, Rasminsky M, Bray G M, Aguayo A J. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- 15.Bancroft J D. In: Theory and Practice of Histological Techniques. Bancroft J D, Stevens A, editors. New York: Churchill Livingstone; 1990. pp. 81–92. [Google Scholar]
- 16.Perez M T R, Caminos E. Neurosci Lett. 1995;183:96–99. doi: 10.1016/0304-3940(94)11123-z. [DOI] [PubMed] [Google Scholar]
- 17.Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Invest Ophthalmol Visual Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 18.Li T, Adamian M, Roof D J, Berson E L, Dryja T P, Roessler B J, Davidson B L. Invest Ophthalmol Visual Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- 19.Mollenhauer H H, Morre D J, Rowe L D. Biochim Biophys Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen R, Song Y, Cheng T, Matthes M T, Yasumura D, LaVail M M, Steinberg R H. J Neurosci. 1995;15:7377–7385. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay R M, Thoenen H, Barde Y-A. Dev Biol. 1985;112:319–328. doi: 10.1016/0012-1606(85)90402-6. [DOI] [PubMed] [Google Scholar]
- 22.Richardson P M, Lu X. J Neurol. 1994;242:57–60. doi: 10.1007/BF00939244. [DOI] [PubMed] [Google Scholar]
- 23.Wood M J, Charlton H M, Wood K J, Kajiwara K, Byrnes A P. Trends Neurosci. 1996;19:497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- 24.Lochmüller H, Petrof B J, Pari G, Larochelle N, Dodelet V, Wang Q, Allen C, Prescott S, Massie B, Nalbantoglu J, et al. Gene Ther. 1996;3:706–716. [PubMed] [Google Scholar]
- 25.Steiner J P, Connolly M A, Valentine H L, Hamilton G S, Dawson T M, Hester L, Snyder S H. Nat Med. 1997;3:421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- 26.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 27.Wiederkehr A, Caroni P. Exp Cell Res. 1995;219:664–670. doi: 10.1006/excr.1995.1277. [DOI] [PubMed] [Google Scholar]
- 28.Jeon C J, Masland R H. J Histochem Cytochem. 1993;41:1651–1658. doi: 10.1177/41.11.8409373. [DOI] [PubMed] [Google Scholar]
- 29.Cayouette M, Gravel C. Invest Opthalmol Visual Sci. 1996;37:2022–2028. [PubMed] [Google Scholar]
- 30.Ali R R, Reichel M B, Thrasher A J, Levinsky R J, Kinnon C, Kanuga N, Hunt D M, Bhattacharya S S. Hum Mol Genet. 1996;5:591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- 31.Flannery J, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennet J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire A M. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 33.Tuszynski M H, Roberts J, Senut M C, U, H S, Gage F H. Gene Ther. 1996;3:305–314. [PubMed] [Google Scholar]
- 34.Martinez-Serrano A, Fischer W, Söderstrom S, Ebendal T, Björklund A. Proc Natl Acad Sci USA. 1996;93:6355–6360. doi: 10.1073/pnas.93.13.6355. . 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quantin B, Perricaudet L D, Tajbakhsh S, Mandel J L. Proc Natl Acad Sci USA. 1992;89:2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Gall La Salle G, Robert J J, Berrard S, Ridoux V, Stratford-Perricaudet L D, Perricaudet M, Mallet J. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 37.Engelhardt J F, Simon R H, Yang Y, Zepeda M, Weber-Pendleton S, Doranz B, Grossman M, Wilson J M. Hum Gene Ther. 1993;4:759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- 38.Clarke, D. B., Bray, G. M. & Aguayo, A. J. (1998) Vis. Res., in press. [DOI] [PubMed]
- 39.Knüsel B, Okazaki T, Yoshida T, Mori N, Gao H, Hefti F, Kaplan D R. Neuroscience. 1997;78:851–862. doi: 10.1016/s0306-4522(96)00616-1. [DOI] [PubMed] [Google Scholar]
- 40.Frank L, Wiegand S J, Siuciak J A, Lindsay R M, Rudge J S. Exp Neurol. 1997;145:62–70. doi: 10.1006/exnr.1997.6440. [DOI] [PubMed] [Google Scholar]