Abstract
Recent efforts to use fMRI to investigate the effects of acupuncture needle manipulation on the brain have yielded discrepant results. This study was designed to test the reliability of fMRI signal changes evoked by acupuncture stimulation. Six subjects participated in six identical scanning sessions consisting of four functional scans, one for each of the four conditions: electroacupuncture stimulation (2Hz) at GB 37, UB 60, non-acupoint (NP), and a control task of the finger-tapping. In the group analysis across all subjects and sessions, both the average ratings on a subjective acupuncture sensation scale and fMRI signal changes (increases and decreases) were similar for GB37, UB 60 and NP. Visual inspection of the activation maps from individual sessions and ICC analysis revealed that fMRI signal changes evoked by electroacupuncture stimulation were significantly more variable than those from the control finger-tapping task. The relatively large variability across different sessions within the same subject suggests multiple sessions should be used to accurately capture the activation patterns evoked by acupuncture stimulation at a particular point for a specific subject.
Keywords: fMRI, brain imaging electroacupuncture, acupuncture, test-retest, reproducibility, reliability, vision-related point
Introduction
Recently, fMRI has been used to investigate the neurobiological mechanisms of acupuncture needle manipulation (Cho et al., 1998; Wu et al., 1999; Hui et al., 2000; Manoach et al., 2001; Gareus et al., 2002; Kong et al., 2002; Siedentopf et al., 2002; Wu et al., 2002; Li et al., 2003b; Zhang et al., 2003; Fang et al., 2004; Li et al., 2004; Litscher et al., 2004; Liu et al., 2004b; Napadow et al., 2004; Yoo et al., 2004; Hui et al., 2005; Yan et al., 2005). In these studies, researchers investigated correlations between acupoint stimulation and brain activity as reflected by fMRI signal changes. BOLD signal increases in SII and insula were the most consistently observed findings and were reported regardless of acupoint or acupuncture mode (Wu et al., 1999; Hui et al., 2000; Kong et al., 2002; Wu et al., 2002; Li et al., 2003b; Zhang et al., 2003; Fang et al., 2004; Li et al., 2004; Liu et al., 2004b; Napadow et al., 2004; Yoo et al., 2004). Aside from this observation, acupuncture needle stimulation was associated with a rather variable pattern of BOLD signal changes across the studies.
These discrepant findings do not necessarily conflict with each other, as there are many sources of variability inherent in fMRI investigations that may contribute to the reported differences. These sources of variability include variations in MR scanner hardware and software, differences in MRI acquisition sequences, data post-processing methods (Smith et al., 2005) and the resting physiological state (level of arousal) of the research subject. Different modes of acupuncture, different acupuncture points and unequal duration of stimulation may also contribute to the variation in findings. In addition, because acupuncture is a therapeutic modality, it may produce different brain responses across different individuals. A systematic evaluation of the reliability of fMRI signal changes in response to acupuncture stimulation will help to clarify the origin of these variabilities and inform the design of future studies (Friston et al., 1995a; Friston et al., 1995b; Friston et al., 1999; Wei et al., 2004).
Researchers have begun to investigate test-retest reliability of fMRI BOLD signal changes evoked by paradigms ranging from simple sensory motor tasks such as finger tapping and visual simulation to more complex cognitive paradigms such as auditory oddball stimulation, working memory and learning tasks (Casey et al., 1998; Machielsen et al., 2000; McGonigle et al., 2000; Waldvogel et al., 2000; Loubinoux et al., 2001; Rutten et al., 2002; Kurland et al., 2004; Marshall et al., 2004; Wei et al., 2004; Havel et al., 2005; Wagner et al., 2005; Yoo et al., 2005; Aron et al., 2006). For instance, several studies have used the finger-tapping task (Yetkin et al., 1996; McGonigle et al., 2000; Waldvogel et al., 2000; Liu et al., 2004a; Smith et al., 2005; Yoo et al., 2005), one of the most widely used tasks in fMRI studies, to investigate the reliability of fMRI signal changes. Although the methods used across these studies are not exactly the same, they all reach the similar conclusion that a simple motor task produces relatively reliable patterns of fMRI signal increases in primary and secondary motor cortices, supplementary motor area (SMA), and cerebellum. However, substantial variability in the quantitative measurements of fMRI signal change, such as magnitude and spatial extension of activations, was found across subjects within those brain regions.
To our knowledge, no study has evaluated the test-retest reliability of fMRI signal changes evoked by acupuncture needle stimulation. In this study, we used two Traditional Chinese Medicine (TCM) acupoints (UB 60 and GB 37) to investigate the reliability and reproducibility of fMRI signal changes evoked by acupuncture stimulation. Both UB 60 and GB 37 have been previously studied with fMRI (Cho et al., 1998; Gareus et al., 2002; Li et al., 2003a). We also studied one non-acupoint (NP), to investigate the specificity of our findings. We located the NP about 1.5 cm posterior and inferior to the small head of the fibula where there is neither an acupoint nor a meridian according to the TCM theory. To assist in the interpretation of the reliability of BOLD signal change during acupuncture, we also included a finger-tapping task (Yetkin et al., 1996; McGonigle et al., 2000; Waldvogel et al., 2000; Liu et al., 2004a; Smith et al., 2005; Yoo et al., 2005).
Methods
Subjects
Eight healthy acupuncture-naïve, right-handed subjects (4 males, mean age 29±7 years, mean ± SD) participated in this study. Subjects were told that this study would investigate the reliability of fMRI in the recording of brain activity during stimulation of three acupuncture points across six sessions. The study was conducted with the understanding and written consent of each subject and approval by the Human Research Committee at Massachusetts General Hospital.
Experimental Procedures
Each subject participated in 6 identical fMRI scanning sessions. We chose variable time delays so that we could observe the effect of time between sessions on fMRI signal variability. Sessions 1 and 2 were separated by 20–30 minutes. Sessions 2 and 3 were separated by 3–6 days. After Session 3, the interval between subsequent sessions was 7–21 days.
Each fMRI session included 4 functional scans, one for each of the 4 conditions (described below), that incorporated an ON/OFF block design (Figure 1A) with 30s of baseline scanning at the beginning and the end of each scan. Four 30s blocks of stimulation (ON, electroacupuncture or finger-tapping), were separated by 3 rest periods (OFF) of 30s, 60s and 30s respectively. During scanning, the subjects were specifically instructed to keep their eyes closed and focus their attention on the sensation evoked by needle stimulation.
Figure 1.
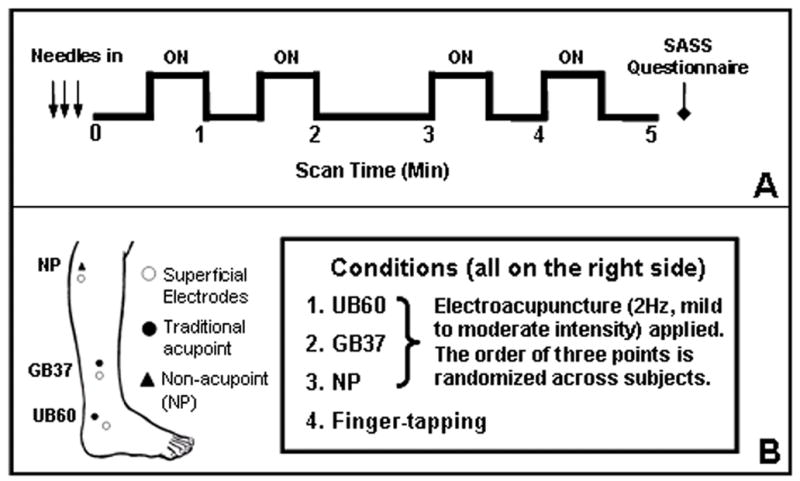
Experimental design. Figure 1A indicates the block design paradigm, each functional run incorporated an ON/OFF block design with 30s of baseline scanning at the beginning and end of each scan. Four 30s blocks of stimulation (ON) by electroacupuncture or finger-tapping were separated by delays (OFF) of 30s, 60s then 30s. Figure 1B indicates point locations and conditions. Conditions 1–3 were electroacupuncture stimulation at UB 60 GB 37, and NP. Condition 4 was a finger-tapping task in which subjects were cued by a verbal prompt to tap their right thumb to their first two fingers at a rate of about 2Hz during the “ON” periods.
There were 4 conditions in this study (Figure 1B). Condition 1 was electroacupuncture stimulation at acupoint UB 60 on the ankle; condition 2 was electroacupuncture stimulation at acupoint GB 37 on the mid-shin; condition 3 was electroacupuncture stimulation at a non-acupoint located about 1.5 cm posterior and inferior to the small head of the fibula and not on any acupoint or meridian; condition 4 was a finger-tapping task in which subjects were cued by verbal prompt to tap their right thumb to their first two fingers at a rate of about 2Hz during the “ON” periods. The order of conditions 1–3 was randomized across the subjects but kept consistent within the same subject across all 6 sessions. Half of the subjects performed condition 4 (finger-tapping task) before the other conditions and half performed it after.
All acupuncture administration was performed by the same acupuncturist using the same method of point location. To reduce any anxiety about the procedures performed during scanning, each subject was given a brief acupuncture treatment before their first scan session. For that introductory session and all subsequent scan sessions, the acupuncturist inserted needles (38-gauge stainless steel) into three points (UB 60, GB 37, and a non-acupoint) on the right leg and placed surface ground electrodes (about 1x1 cm) at specific locations inferior to each point (Figure 1B). For UB 60 the electrode was placed 2 cm anterior inferior on the same meridian at acupoint UB62; for GB 37 the electrode was placed 2 cm inferior on the same meridian at acupoint GB38; for the NP the electrode was placed about 2 cm inferior to this NP. After adjusting the needle to obtain deqi, a sensation such as numbness and fullness at the site of stimulation which is believed to be important for the efficacy of acupuncture treatment effect (Cheng, 1987; Stux, 1997), we connected the needle and electrode to an electroacupuncture device (OMS Medical Supplies IC-1107) with a wire lead modified for use in the high magnetic field environment. This modified device was approved for use by the Department of Biomedical Engineering at Massachusetts General Hospital. The specific intensity of stimulation was determined for each subject by gradually increasing and adjusting the current applied at each point until the subject reported a mild to moderate evocation of deqi. The average intensity (mean±SD) of the three conditions were 5.5±2.1 V for GB 37, 4.7±1.7 V for UB 60, and 5.7±2.0 V for NP. All electroacupuncture stimulation was applied at 2 Hz.
After the stimulation of each point, subjects were asked to quantify their sensations using the Subjective Acupuncture Sensation Scale (SASS) (Kong et al., 2005). This scale consists of eleven measures: nine descriptors of typical deqi sensations: stabbing, throbbing, tingling, burning, heaviness, fullness, numbness, soreness, and aching; one blank row for subjects to add their own word(s) if the descriptors did not completely describe the sensations they experienced; and the word anxiety for subjects to rate how anxious they felt during acupuncture treatment. Subjects indicated their ratings on a 10 cm scale with the anchor words “none,” “mild,” “moderate,” and “severe” spaced evenly along the continuum. During the introductory session, subjects were trained to use this SASS scale and to perform the finger-tapping task.
During the six fMRI sessions, the experimenter read each SASS measure to the subjects who reported their ratings orally from within the scanner after each functional run. The SASS data were analyzed using the same intraclass correlation coefficient (ICC) analysis described below for the fMRI data.
fMRI data acquisition and analysis
All brain imaging was performed with a 3-axis gradient head coil in a 3 Tesla Siemens MRI System (Erlagen, Germany) equipped for echo planar imaging. After automated scout and shimming procedures, functional MR images were acquired using gradient echo T2*-weighted sequence with TR 2000 ms, TE 40 msec and a flip angle of 90 degrees. Thirty slices (4 mm thick, 1 mm skip) oriented parallel to the AC-PC plane were collected to provide whole brain coverage. A high resolution 3D MPRAGE sequence was also collected.
During fMRI scanning, Prospective Acquisition CorrEction (PACE) was applied to adjust slice position and orientation, as well as to re-grid residual volume-to-volume motion in real-time during data acquisition for the purpose of reducing motion-induced effects (Thesen et al., 2000). In addition, we used an on-line automatic slice aligning procedure for brain MR imaging to ensure that scanning occurred with the brain registered in a similar position across different scan sessions and subjects (van der Kouwe et al., 2005).
Pre-processing and statistical analysis were performed using SPM2 software (Wellcome Department of Cognitive Neurology). Pre-processing began with motion correction. All functional runs were realigned to the first volume acquired in the scan session. We set a movement threshold of 2mm within a scan to eliminate subjects with excessive head movement. However, none of the subjects had head movements that exceeded this threshold. Thus, all data were used for this analysis. All functional runs were normalized to MNI stereotactic space and spatially smoothed with an 8mm Gaussian kernel. We specified a separate general linear model (GLM) for each session across each subject with regressors for the difference from baseline for each of the four conditions. Global signal scaling was applied. Low-frequency noise was removed with a high-pass filter applied with default values to the fMRI time series at each voxel.
In individual session analysis, a threshold of p<0.05 corrected with 10 contiguous voxels was used for finger-tapping; a less conservative threshold of p<0.0001 uncorrected with 10 contiguous voxels was used for acupuncture conditions. We chose different thresholds for finger-tapping and point stimulation because fMRI signal changes evoked by acupuncture stimulation are less robust than for finger-tapping.
To detect the activation patterns in data averaged across all sessions for each subject and data averaged across all subjects for each session, a fixed-effects group analysis was performed. For this we applied a threshold of p<0.05 corrected with 10 contiguous voxels.
Second level group analysis was performed using a random-effects model across all experimental sessions for each condition (points and tapping). We considered both subjects and sessions as components of measurement uncertainty; thus, it was appropriate to average over subjects and sessions and to perform a group analysis (6 subjects x 6 sessions). A one-way t test was used to determine group activation for each condition using a threshold of p<0.05 corrected with 10 contiguous voxels. Activations above the threshold were defined as ROIs for each condition, then β values (averaged across all the voxels above threshold within a 3mm sphere centered at peak activation) of all the ROIs for each individual subject across each individual session were extracted to further evaluate the reliability across different individuals and experimental sessions using an intraclass correlation coefficient (ICC) analysis described below.
To directly compare differences among the traditional points and NP, in another second level group analysis, a one-way ANOVA (within-subject) was performed on the electroacupuncture stimulation conditions (1, 2, and 3) across all 36 sessions. In the matrix, non-sphericity correction was performed, the replication was over subjects, and correlated repeated measures were chosen. To achieve significant activation at higher level comparisons between the three different conditions, we used a lower threshold of p <0.001 uncorrected and a cluster level p < 0.05 corrected with 10 contiguous voxels.
To investigate the influence of the time interval between consecutive scan sessions on the reliability of fMRI signal changes evoked by acupuncture stimulation, a random-effects model was fit for each ROI, using subject as a random effect and assuming a first-order autoregressive model for temporal correlation.
ICC analysis is a statistical approach widely used to evaluate sources of variability. In this study, it was used to assess the relative contributions of subject and session to BOLD variability within a ROI. There are several types of ICC analyses that have been introduced in the literature (Fleiss, 1986). The current study presents the simple replication reliability model posed by Fleiss (Fleiss, 1986).
In this model, there are two sources of variance: between-subject (σ2bs) and within-subject (i.e., between-session, σ2ws). We employed a one-way model with session nested within subject. Thus, the total variance of a measurement in a particular individual scanning session on a subject for one particular condition is:
The ICC (which is the correlation between measurements made in different sessions on the same subject) is:
The ICC may also be interpreted as the proportion of the total variability contributed by subject differences. The between-subject and within-subject components of variance were estimated by equating mean squares to their expected values (Fleiss, 1986). This approach can lead to negative variance estimates in situations where the true variance is small. For these cases, we have set the variance component estimates and, consequently, the corresponding ICC, to zero.
Results
Subjects
Of the 8 volunteers who consented into the study, six (three female) completed all six sessions. Two subjects withdrew from the study after Session 2. Only the data from the subjects who completed all six sessions were analyzed. The average interval in days between session 2 & 3 was 4.5 ± 1.3, session 3 & 4 was 12 ± 4.5, session 4 & 5 was 13.3 ± 4.3, session 5 & 6 was 14.8 ± 9.
SASS ratings
The average stimulus intensities (mean±SD) used for GB 37 (5.5±2.1 V) and NP (5.7±2.0 V) were similar to each other, and were higher (p < 0.01 when comparing NP with UB60, p < 0.07 when comparing GB 37 with UB 60) than UB 60 (4.7±1.7 V).
Figure 2 presents the average ratings for each of the nine descriptors on the SASS across all subjects and sessions (6 by 6) for GB 37, UB 60 and NP stimulation. The ratings were similar for the three points. Average ratings of each of the nine elements of the deqi sensation for each acupoint fell between 0.0 and 4.0 on the 10.0-point scale for all three points. For each individual session, the highest reported score ranged from 4 to 7. Most of the highest scores in individual sessions were around 5 (between mild and moderate). There was no obvious trend of an increase or decrease in ratings across sessions for any subject. No subject opted to add an additional descriptor in the blank row provided. This is consistent with our previous study evaluating the typical responses to the SASS (Kong et al., 2005).
Figure 2.
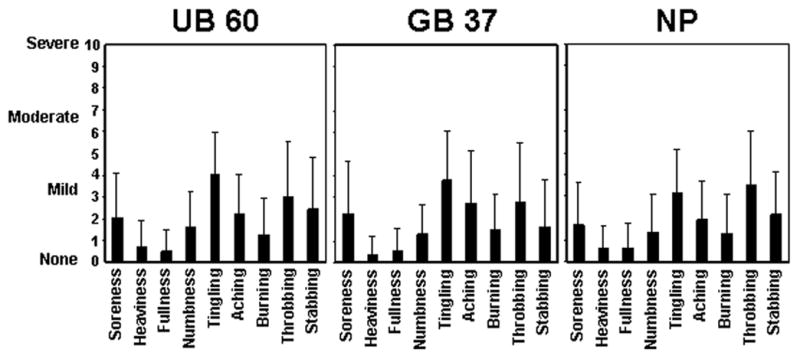
Grand average of sensations for UB 60, GB 37 and NP evoked by electroacupuncture stimulation (mean ± SD) across all subjects and all sessions. Subjects rated sensations on the 10-point SASS. In every condition, tingling and throbbing had the highest average ratings and heaviness and fullness had the lowest.
Subjects demonstrated modest reliability in their assessment of the sensations evidenced by ICCs on average SASS sensations of 0.34, 0.33, and 0.41 for GB 37, UB 60, and NP, respectively. ANOVA on ICC for SASS data showed that there was no significant difference among the GB 37, UB 60, and NP.
Anxiety Ratings
The eletroacupuncture stimulation at TCM acupoints and NP only induced very mild anxiety. The average subjective ratings of anxiety experienced during fMRI scanning for each session across all subjects ranged from 0 to 3.5 on the 10.0 scale, with an average rating of 0.7. The ratings were not significantly different across the three points with average anxiety ratings of 0.7 for stimulation at GB 37, 0.8 for stimulation at UB 60, and 0.6 for stimulation at NP. Of the total 108 fMRI functional runs (6 subjects by 6 sessions by 3 electroacupuncture conditions), 87 runs were rated 0 for anxiety.
fMRI Results
fMRI results of the finger-tapping task
The average brain activations evoked by the right handed finger-tapping task across all subjects and sessions are shown in Table 1. As expected, significant BOLD signal increases during the finger-tapping task were observed in left primary motor cortex (M1), primary somatosensory cortex (S1), thalamus and putamen, bilateral medial frontal cortex (supplementary motor area, SMA) insula/operculum, and cerebellum. Activations in the middle prefrontal gyrus were also observed.
Table 1.
Indices of activation for the brain regions with significant fMRI signal increases during finger-tapping, the associated β value and the ICCs with the corresponding p values summarizing the individual session data. Z score, number of voxels and peak coordinate are from the grand mean average (n = 36 sessions). Voxels in ROI refer to voxel numbers above the threshold within the 3mm sphere centered at peak activation of each cluster
| Condition | Area (Brodmann Area) | Z score | Voxels in cluster | Peak coordinate | Voxels in ROI | β value Mean (SD) | ICC | P value |
|---|---|---|---|---|---|---|---|---|
| Finger-tapping task | Left S1/M1 (1, 2, 3, 4) | Inf | 4469 | −34 −22 54 | 19 | 1.9 (0.46) | 0.68 | 0.00 |
| Bilateral medial frontal cortex (6) | Inf | 2 0 56 | 19 | 0.89 (0.27) | 0.51 | 0.00 | ||
| Right cerebellum | Inf | 1817 | 24 −56 −26 | 19 | 1.27 (0.33) | 0.42 | 0.00 | |
| Left insula / operculum | Inf | 1356 | −52 4 0 | 19 | 0.59 (0.23 | 0.21 | 0.05 | |
| Left putamen | −28 −8 −2 | 19 | 0.45 (0.20) | 0.00 | 0.76 | |||
| Left thalamus | Inf | 271 | −14 20 4 | 19 | 0.46 (0.19) | 0.00 | 0.82 | |
| Right cerebellum | 6.78 | 50 | 10 −68 −50 | 14 | 0.31 (0.21) | 0.25 | 0.03 | |
| Right insula / operculum | 5.75 | 75 | 58 16 0 | 18 | 0.52 (0.42) | 0.58 | 0.00 | |
| Right middle frontal gyrus (9) | 5.54 | 61 | 62 2 38 | 12 | 0.35 (0.32) | 0.76 | 0.00 | |
| Left cerebellum | 5.54 | 88 | −32 −60 −28 | 19 | 0.34 (0.29) | 0.29 | 0.01 |
Figure 3 shows sagittal maximum intensity projections (MIPs) for group and each individual session (6 subjects by 6 sessions) for the finger-tapping task. We observed a relatively consistent brain activation pattern (fMRI signal increases) across sessions and subjects. Brain activation in regions in M1, S1, SMA and contralateral cerebellum were found in every session for all subjects. We observed a more consistent activation pattern within subjects across different sessions than across different subjects.
Figure 3.
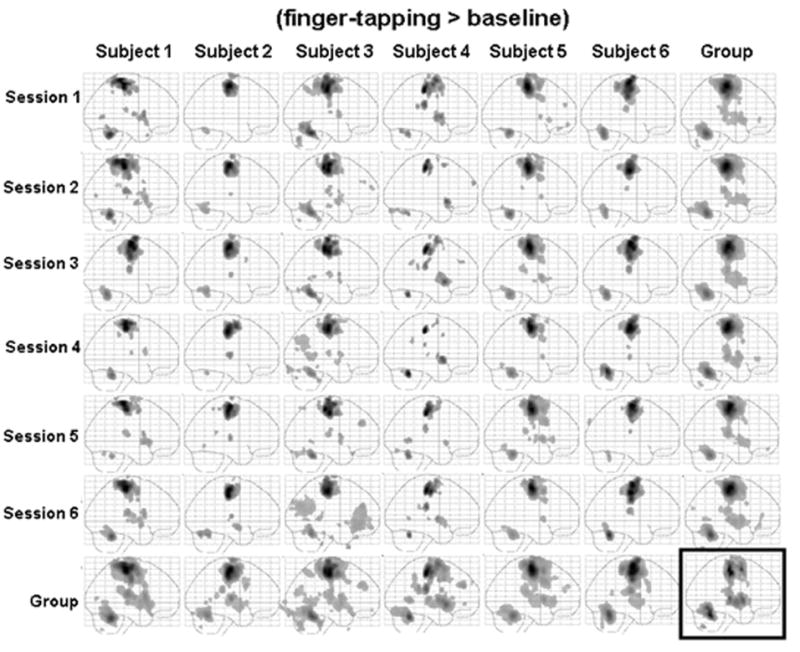
Sagittal maximum intensity projections (MIPs) for the finger-tapping task in both individual session and group analysis. The subject average for all 36 sessions is shown in the box at the bottom right. The group averages across all six sessions for each subject are shown along the bottom row. The group averages across the 6 subjects in each session are shown in the far right column.
fMRI results of the electroacupuncture stimulation
The average brain activations evoked by electroacupuncture stimulation at two TCM acupoints and a NP across all subjects and sessions are presented in Figure 4. The figures show a similar activation pattern among the two Traditional acupoints and NP. Detailed results are shown in Table 2. As expected, BOLD signal increases in bilateral frontal operculum and insula, and left S2 were observed during the stimulation of all points. Increases in right S2 and left S1 were observed during the stimulation of GB 37 and NP, but not during UB 60 stimulation. In addition, positive activations were also observed in left medial frontal cortex and right inferior parietal lobule for GB 37; right medial frontal cortex and bilateral cerebellum for UB 60; and left medial frontal cortex and cerebellum for NP.
Figure 4.
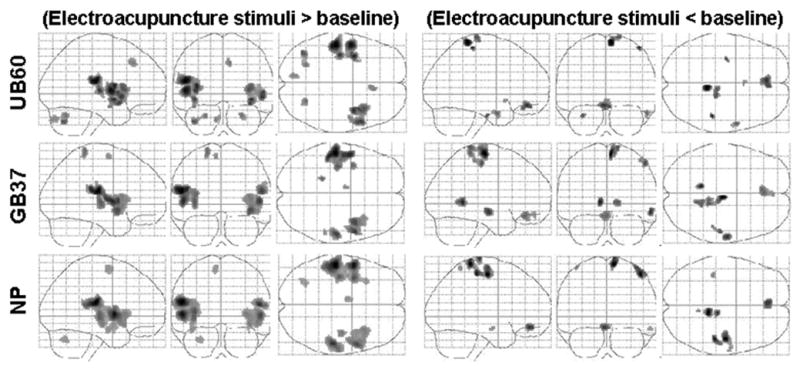
Group results of fMRI signal increases and decreases evoked by three electroacupuncture stimulation conditions across all 36 sessions, p value is set at p<0.05 corrected with 10 contiguous voxels.
Table 2.
Indices of activation for the brain regions with significant fMRI signal changes (↑indicates signal increase, ↓indicates signal decrease) evoked by electroacupuncture stimulation on TCM acupuncture points (GB 37 and UB 60) and NP, and the ICCs with the corresponding p values. Regions common to electroacupuncture at all 3 points are indicated by italics. The indices are the same as in Table 1.
| Condition | Area (Brodmann Area) | Z score | Voxels in cluster | Peak coordinate | Voxels in ROI | β value Mean (SD) | ICC | P value | |
|---|---|---|---|---|---|---|---|---|---|
| GB 37 | ↑ | Left postcentral gyrus S2 (40) | 7.46 | 992 | −54 −20 18 | 19 | 0.48 (0.24) | 0.19 | 0.06 |
| Left frontal operculum cortex (44, 45) | 6.84 | −52 0 4 | 19 | 0.36 (0.22) | 0.15 | 0.10 | |||
| Right frontal operculum cortex/insula (45) | 6.45 | 813 | 52 6 4 | 19 | 0.33 (0.21) | 0.27 | 0.02 | ||
| Right insula | 6.27 | 40 6 −8 | 19 | 0.32 (0.22) | 0.00 | 0.70 | |||
| Right postcentral gyrus, S2 (2, 40) | 6.43 | 196 | 56 −20 18 | 19 | 0.37 (0.25) | 0.34 | 0.01 | ||
| Left postcentral gyrus, S1 (2) | 5.79 | 55 | −18 −40 74 | 16 | 0.25 (0.20) | 0.42 | 0.00 | ||
| Left insula | 5.76 | 69 | −36 4 6 | 19 | 0.29 (0.25) | 0.23 | 0.04 | ||
| Left medial frontal cortex (6) | 5.26 | 27 | −6 −6 64 | 14 | 0.28 (0.26) | 0.22 | 0.04 | ||
| Right inferior parietal cortex (4) | 5.23 | 21 | 54 −36 22 | 11 | 0.27 (0.25) | 0.18 | 0.07 | ||
| ↓ | Right superior frontal gyrus(6) | 6.54 | 266 | 6 −20 70 | 19 | −0.25 (0.18) | 0.40 | 0.00 | |
| Right paracentral lobule (4, 2) | 5.58 | 14 −38 76 | 19 | −0.35 (0.30) | 0.54 | 0.00 | |||
| Left lingual gyrus (19) | 6.01 | 63 | −8 −50 2 | 18 | −0.42 (0.32) | 0.03 | 0.33 | ||
| Right middle temporal gyrus (20) | 5.86 | 75 | 60 −14 −12 | 19 | −0.23 (0.18) | 0.17 | 0.07 | ||
| Right postcentral gyrus (3,1,2) | 5.66 | 61 | 52 −22 56 | 17 | −0.25 (0.21) | 0.40 | 0.00 | ||
| Right lingual gyrus (19) | 5.52 | 88 | 12 −54 2 | 18 | −0.35 (0.29) | 0.11 | 0.16 | ||
| Left superior parietal lobule | 5.51 | 23 | −22 −82 48 | 10 | −0.36 (0.32) | 0.15 | 0.10 | ||
| Bilateral medial orbital prefrontal cortex (11) | 5.45 | 149 | 0 36 −16 | 19 | −0.63 (0.53) | 0.06 | 0.26 | ||
| Right superior frontal gyrus (6) | 5.38 | 22 | 26 −8 66 | 13 | −0.18 (0.16) | 0.00 | 0.91 | ||
| UB 60 | ↑ | Left post-central gyrus S2 (6, 40) | 7.17 | 1164 | −52 −22 18 | 19 | 0.49 (0.27) | 0.04 | 0.30 |
| Left operculum cortex (6, 44) | 6.89 | −50 −2 6 | 19 | 0.37 (0.22) | 0.00 | 0.43 | |||
| Left insula | 6.57 | −36 2 10 | 19 | 0.26 (0.17) | 0.09 | 0.20 | |||
| Right insula | 6.47 | 705 | 38 0 −8 | 19 | 0.25 (0.17) | 0.04 | 0.30 | ||
| Right frontal operculum cortex (47 ) | 6.31 | 52 10 −4 | 19 | 0.44 (0.31) | 0.26 | 0.02 | |||
| Left cerebellum | 5.91 | 106 | −38 −66 −38 | 18 | 0.17 (0.13) | 0.29 | 0.01 | ||
| Right cerebellum | 5.58 | 50 | 26 −64 −30 | 19 | 0.16 (0.14) | 0.00 | 0.42 | ||
| Right medial frontal gyrus (8) | 5.48 | 55 | 10 28 42 | 14 | 0.19 (0.16) | 0.00 | 0.44 | ||
| Left cerebellum | 5.35 | 51 | −10 −80 −34 | 15 | 0.16 (0.14) | 0.12 | 0.14 | ||
| ↓ | Right paracentral lobule (2, 4) | 5.91 | 82 | 6 −40 70 | 19 | −0.41 (0.32) | 0.40 | 0.00 | |
| Right postcentral gyrus (1,2) | 5.54 | 20 | 46 −32 64 | 13 | −0.23 (0.19) | 0.00 | 0.69 | ||
| Bilateral medial orbital prefrontal cortex (11) | 5.49 | 153 | −2 42 −16 | 19 | −0.57 (0.48) | 0.11 | 0.16 | ||
| Right medial frontal gyrus (6, 4) | 5.29 | 70 | 10 −26 76 | 17 | −0.30 (0.26) | 0.36 | 0.00 | ||
| Left uncus / parahippocampus (20, 36) | 5.28 | 10 | −32 −12 −28 | 7 | −0.16 (0.14) | 0.06 | 0.26 | ||
| Right middle temporal gyrus (21) | 5.21 | 41 | 56 0 −26 | 17 | −0.22 (0.21) | 0.08 | 0.20 | ||
| Bilateral hypothalamus | 4.99 | 11 | 2 10 −8 | 6 | −0.38 (0.37) | 0.12 | 0.14 | ||
| NP | ↑ | Left postcentral gyrus, S2 (40, 43) | Inf | 2307 | −52 −22 18 | 19 | 0.58 (0.25) | 0.14 | 0.11 |
| Left frontal operculum cortex (45, 47) | 7.74 | −52 2 2 | 19 | 0.54 (0.25) | 0.07 | 0.23 | |||
| Left insula | 6.59 | −36 2 6 | 19 | 0.35(0.23) | 0.07 | 0.24 | |||
| Right postcentral gyrus, S2 (40, 43) | 7.12 | 340 | 52 −26 16 | 19 | 0.42 (0.24) | 0.00 | 0.94 | ||
| Right frontal operculum cortex (45, 47) | 6.79 | 1079 | 54 6 2 | 19 | 0.49 (0.30) | 0.02 | 0.36 | ||
| Right insula | 5.81 | 34 16 4 | 19 | 0.36 (0.29) | 0.25 | 0.03 | |||
| Left medial frontal cortex (6) | 5.34 | 70 | −8 −6 64 | 19 | 0.37 (0.34) | 0.34 | 0.01 | ||
| Left cerebellum | 5.22 | 58 | −30 −66 −30 | 15 | 0.23 (0.22) | 0.19 | 0.06 | ||
| Left post-central gyrus, S1 (2) | 5.02 | 13 | −20 −38 72 | 11 | 0.27 (0.26) | 0.05 | 0.29 | ||
| ↓ | Right paracentral lobule (4, 2) | 6.32 | 146 | 10 −38 72 | 19 | −0.31 (0.22) | 0.41 | 0.00 | |
| Right precentral gyrus (4, 6) | 6.27 | 251 | 46 −16 58 | 19 | −0.22 (0.16) | 0.06 | 0.26 | ||
| Right postcentral gyrus (3,2) | 6.02 | 46 −30 60 | 18 | −0.28 (0.22) | 0.01 | 0.40 | |||
| Bilateral medial orbital prefrontal cortex (11) | 5.71 | 111 | −4 44 −14 | 19 | −0.43 (0.35) | 0.10 | 0.18 | ||
| Left postcentral gyrus (3, 1, 2) | 5.11 | 13 | −42 −32 66 | 9 | −0.24 (0.23) | 0.06 | 0.26 | ||
| Right precuneus (7) | 5.02 | 12 | 8 −52 66 | 11 | −0.34 (0.34) | 0.24 | 0.03 | ||
| Right middle temporal gyrus (21) | 4.95 | 19 | 58 −8 −16 | 13 | −0.25 (0.25) | 0.15 | 0.10 | ||
Significant BOLD signal decreases were observed in bilateral medial orbital prefrontal cortex, right paracentral lobule, and right postcentral gyrus and middle temporal gyrus during electroacupuncture stimulation at all three points. In addition, decreases were also found in bilateral lingual gyrus and superior parietal lobule for GB 37; bilateral hypothalamus, left uncus / parahippocampus for UB 60, right precentral gyrus, precuneus and left postcentral gyrus for NP.
The ANOVA directly comparing electro-acupuncture conditions showed only minor fMRI signal differences evoked by stimulation at GB 37, UB 60 and NP. Stimulation at UB 60 produced relatively more fMRI signal increases than GB 37 in left inferior parietal lobule (−46 −44 56). Stimulation at NP was associated with relatively more fMRI signal increases in left insula/operculum (−36 −30 12) and SMA (−4 −28 62) than GB 37, and more signal increases in SMA (−6 −14 58) than UB 60.
Figures 5 and Figure 6 show sagittal maximum intensity projections (MIPs) for group and each individual session (6 subjects by 6 sessions) for eletroacupuncture stimulation at UB 60 and NP, respectively. In contrast to the relatively consistent brain activation pattern evoked by the finger-tapping task, the brain activation patterns evoked by electroacupuncture showed more variability. Figure 5 indicates the fMRI signal changes evoked by electroacupuncture stimulation at UB 60. We observed a relatively consistent positive activation pattern in the contralateral postcentral gyrus (S2) and frontal operculum / insula in most sessions for all subjects. The negative activation pattern appeared to be more variable than the positive activation pattern. Similar results were observed for the fMRI signal changes evoked by electroacupuncture stimulation at GB 37. fMRI signal changes evoked by stimulation at NP are shown in Figure 6. The result is very similar to that evoked by stimulation on the two TCM acupoints, UB 60 and GB 37. There was no obvious correlation among fMRI signal changes evoked by electroacupuncture stimulation at GB 37, UB 60 and NP, and that evoked by finger tapping task. It is worth noting that the average data within the same subject across different sessions or within the same session and across different subjects was more reliable than the individual session data (Figures 5–6).
Figure 5.
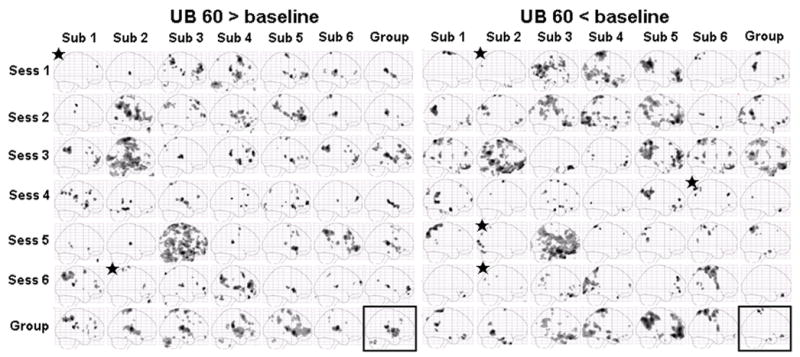
Sagittal maximum intensity projections (MIPs) for electroacupuncture stimulation at UB 60 in both individual session and group analysis. The subject average for all 36 sessions is shown in the box at the bottom right. The group averages across all six sessions for each subject are shown along the bottom row. The group averages across the 6 subjects in each session are shown in the far right column. Signal increases are shown on the left and signal decreases on the right. Star indicates no activation was observed at the original threshold of p<0.0001 uncorrected with 10 contiguous voxels, a lowered threshold of p<0.001 uncorrected with 10 contiguous voxels was used in this case.
Figure 6.
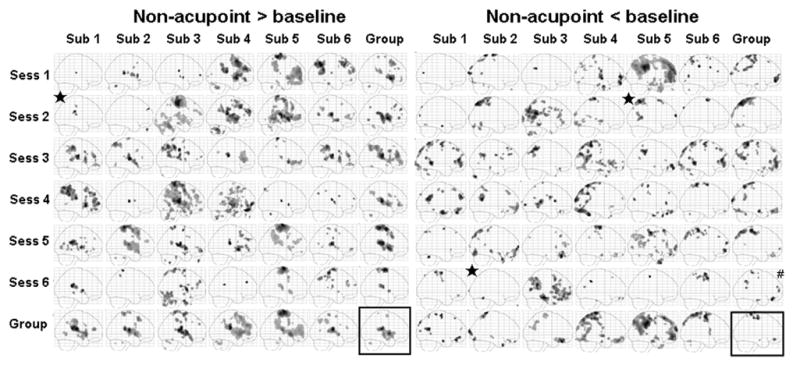
Sagittal maximum intensity projections (MIPs) for electroacupuncture stimulation at NP in both individual session and group analysis. The subject average for all 36 sessions is shown in the box at the bottom right. The group averages across all six sessions for each subject are shown along the bottom row. The group averages across the 6 subjects in each session are shown in the far right column. Signal increases are shown on the left and signal decreases on the right. Star indicates no activation was observed at the original threshold of p<0.001 uncorrected with 10 contiguous voxels, a lowered threshold of p<0.001 uncorrected with 10 contiguous voxels was used in this case.
The intersession interval was varied to allow us to evaluate effects of time interval between consecutive scan sessions on repeatability. A random-effects analysis for temporal correlation was performed for each ROI to investigate the influence of time interval on the reliability of fMRI signal changes. The results showed that there is no statistically significant effect of intersession interval on the magnitude of BOLD signal change in any of the ROIs for any of the conditions.
The result of ROI analysis
The β values of common regions evoked by electroacupuncture stimulation at both acupoints and NP (bilateral operculum, left S2, insula, bilateral MOF and right paracentral lobule ) of all 36 sessions (6 subjects by 6 sessions) are shown in Figure 7. The direction of BOLD signal change (increase VS decrease) in most of the individual sessions is consistent with the results of the group dataset with few exceptions. However, the amplitude of these signal changes showed a large variability.
Figure 7.
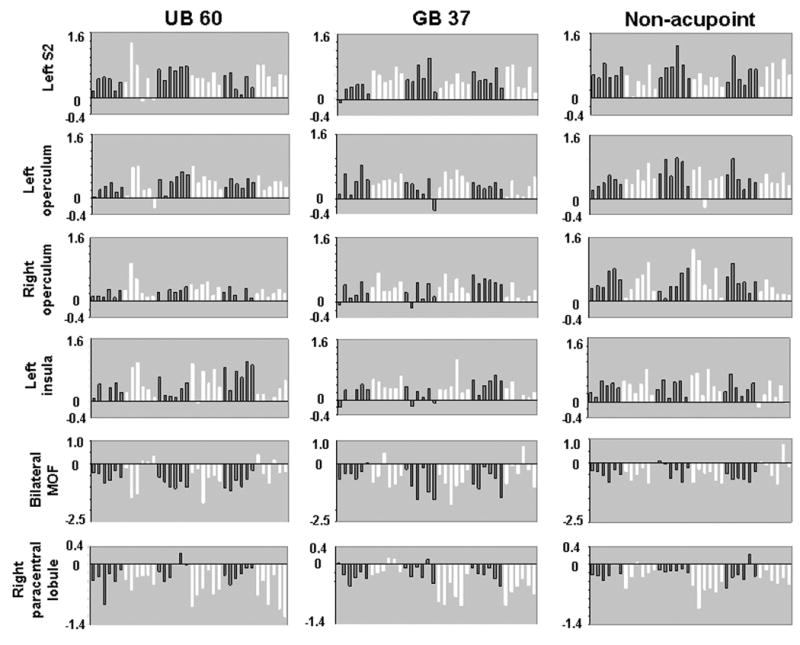
β values of some representative common regions evoked by electroacupuncture stimulation. Common regions of activation including left S2, bilateral operculum, left insula, bilateral MOF and right paracentral lobule are presented for all 36 sessions (6 subjects by 6 sessions). Each bar in each graph represents the data from one experimental session in order of session 1 to session 6. Alternating colors separate the data of one subject from the next subject.
To quantify the reliability of the fMRI signal changes, an ICC analysis was performed. The results of the analysis are shown in the last 2 columns of Table 1 and Table 2. The data indicate that the ICC of the finger-tapping ROIs were significantly greater than that of electroacupuncture ROIs (p < 0.04, Kruskal-Wallis test), suggesting finger-tapping task can produce more reliable fMRI signal changes than that evoked by electroacupuncture stimulation. There was no significant difference in reliability among the ROIs among the GB 37, UB 60 and NP (p < 0.13, Kruskal-Wallis test). Although visual observation suggested that the patterns of activation are more variable for the BOLD signal increases than for the BOLD signal decreases, the ICCs of the signal increases and decreases were not significantly different for the three electroacupuncture conditions.
Discussion
In this study, we investigated the fMRI signal changes evoked by finger-tapping and electroacupuncture stimulation. Group analysis showed that finger-tapping of the right hand evoked the expected activation in left M1, S1, thalamus and putamen, bilateral insula/operculum, SMA and cerebellum. Visual observation showed that the finger-tapping task evoked a reliable brain activation pattern across individual sessions for all subjects, consistent with previous reports (Yetkin et al., 1996; McGonigle et al., 2000; Waldvogel et al., 2000; Liu et al., 2004a; Smith et al., 2005; Yoo et al., 2005).
Electroacupuncture stimulation at UB 60, GB 37 and NP each evoked similar patterns of fMRI signal increases in bilateral operculum, left S2 and right insula, and fMRI signal decreases in bilateral medial orbital prefrontal cortex and paracentral lobule in group data. This result is similar to previous studies of the brain activation evoked by electroacupuncture stimulation (Kong et al., 2002; Napadow et al., 2004) that used comparable methods of acupuncture stimulation. The brain activation patterns evoked by electroacupuncture for each individual session were much less reliable than those evoked by the finger-tapping task. ICC analysis confirmed that finger-tapping produced higher reliability across sessions than that of the electroacupuncture stimulation.
In this study, we found that electroacupuncture stimulation at TCM acupoints and a NP evoked similar fMRI activation patterns in group data sets. However, this does not mean that there is no difference between TCM acupoints and non-acupoints. Neuroimaging can only capture one aspect of the effect of acupuncture stimulation, changes in the central nervous system, by detecting the correlation between fMRI signal changes and short time periods of acupuncture needle stimulation. In addition, acupuncture’s effect in healthy subjects may differ from that which may occur in patients. Finally, the immune system, endocrine system or other biomechanical signaling pathways may all be involved in mediating the salubrious effects of acupuncture (Gollub et al., 1999; Langevin et al., 2004; Langevin et al., 2006). Given the importance of acupoint specificity in Traditional Chinese Medicine system, further study is needed regarding this issue.
In our study, considerable efforts were made to control for and minimize common sources of variabilities owing to technical factors, physiological instability, and acupuncture procedures. The result from the finger-tapping task showed a consistent activation pattern in the brain across all sessions and subjects. This suggests that the technical limitations due to imaging are unlikely to be the main sources of the variability in the activation patterns seen in the electroacupuncture conditions.
To minimize variability due to acupuncture administration, such as frequency of stimulation, we used electroacupuncture instead of manual acupuncture. In addition, a single licensed acupuncturist followed consistent procedures to locate GB 37, UB 60 and NP throughout the entire study. Rather than use a fixed stimulus intensity, which may produce different sensations across different subjects (or even within the same subject), we adjusted the intensity of stimulation for each subject in each session until the subject reported a similar rating for the deqi sensation (mild to moderate sensation, indicated by having subjects rate the highest score about a 5 on one of the SASS scales) for each session.
It is worth noting that while it is almost impossible to evoke exactly the same sensation in two separate sessions even in the same subjects, the overall range of sensations evoked by electroacupuncture stimulation was quite narrow in this study. The ICC analysis on the SASS sensations showed that there are no significant differences among the GB 37, UB 60 and NP. Visual inspection of the individual scan data did not reveal an obvious trend between the magnitude of average and peak SASS ratings and fMRI signal activation patterns. Thus, subtle differences in SASS ratings are also unlikely to explain the large variability in fMRI signal changes across individual sessions.
One potential source of variability that is very hard to control is individual physiological status, such as arousal level and attention. To minimize variability due to possible changes in physiology throughout the day, the scan times for each individual were kept within 3 hours of each other. However, the circumstances of daily life may cause an individual’s baseline level of activation and capacity for attention to vary from day to day. The theoretical framework upon which acupuncture treatment was developed includes the belief that acupuncture stimulation can act in ‘two-directions’ to regulate and balance the body’s systems (Yang and Cao, 1987); this concept may be critical to understanding the effects of acupuncture stimulation on the brain. Extending this theoretical framework to the blood flow changes we measure with brain imaging tools such as fMRI suggests that the same acupuncture stimulation could result in fMRI signal increase or decrease in a given brain region depending on a subject’s baseline state.
This effect may also be related to the well-known finding that attention and arousal can significantly modulate brain response to sensory stimulation including touch and pain (Peyron et al., 1999; Johansen-Berg and Lloyd, 2000; Petrovic et al., 2000; Tracey et al., 2002; Porro et al., 2004). Thus, a subject’s level of distraction away from the sensation may influence the amount of activation in some areas of the brain. In this study, to reduce the confounding effects of other stimuli, all subjects were required to close their eyes and focus their attention on the sensation evoked by the acupuncture stimulation. All subjects expected and did indeed perform a SASS rating task after each five-minute scan. Theoretically, their attention to the stimulus should be similar across different sessions; however, unlike the finger-tapping task that requires the active involvement of the subjects, electroacupuncture stimulation is passively received, making it harder to control subjects’ attention during the experiment.. We speculate that variability in baseline brain status, attention and arousal may partly account for the variability in brain responses observed across different sessions and subjects. Further studies should examine this point more closely.
In this study, we found that ICCs evoked by electroacupuncture stimulation are significantly less than that of finger-tapping. This may reflect the relatively low contrast to noise ratio of acupuncture stimulation evoked fMRI signals (Hui et al., 2000), so that consistent activation of a small magnitude just at the threshold for detection may appear less reliable. Despite the variability across individual sessions, it is still possible to make useful inferences about the brain response to acupuncture stimulation from group averaged data as shown in previous acupuncture brain imaging studies. As our results demonstrated, the activation patterns in the group average data from each experimental session are more similar to each other (Figures 5–6). This supports the view that fMRI is a useful tool for characterizing average behavior. However, the relatively large variability across different sessions within the same subject suggests multiple sessions should be used to accurately capture the activation patterns evoked by acupuncture stimulation at a particular point for a specific subject. Our results provide useful information for the design of future studies and explain some of the inconsistencies between different studies.
Finally, it is worth noting that this large variability across different individual sessions is not something unique to acupuncture stimulation (Huettel et al., 2004). Previous studies have reported relatively large variabilities across different sessions in the same subject for tasks such as finger tapping, visual stimulation of flickering black and white checkerboard images (McGonigle et al., 2000; Waldvogel et al., 2000) and verbal working memory (Wei et al., 2004). Given the fact that during acupuncture, subjects only passively feel the sensations evoked by acupuncture stimulation, we believe the degree of variability observed in our study is consistent with other similarly controlled experimental paradigms.
In summary, we observed a similar pattern of group average fMRI signal changes (increases and decreases) during electroacupuncture stimulation at Traditional Chinese acupuncture points (GB 37 and UB 60) and a NP. Visual inspection of the activation maps from individual sessions and ICC analysis reveals that in contrast to the relatively reliable activation evoked by finger-tapping task, activations evoked by electro-acupuncture are much more variable. The relatively large variability across different sessions within the same subject suggests multiple sessions should be used to accurately capture the activation patterns evoked by acupuncture stimulation at a particular point for a specific subject.
Acknowledgments
Funding and support for this study came from: NIH (NCCAM) R21 R21AT00949 to Randy Gollub, PO1-AT002048 to Bruce Rosen / Randy Gollub, KO1AT003883 to Jian Kong. M01-RR-01066 for Mallinckrodt General Clinical Research Center Biomedical Imaging Core, P41RR14075 for Center for Functional Neuroimaging Technologies from NCRR and the MIND Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;29:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, O’Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, Truwitt CL, Turski PA. Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage. 1998;8:249–261. doi: 10.1006/nimg.1998.0360. [DOI] [PubMed] [Google Scholar]
- Cheng XN. Chinese acupuncture and moxibustion. Beijing: Foreign Language Press; 1987. [Google Scholar]
- Cho ZH, Chung SC, Jones JP, Park JB, Park HJ, Lee HJ, Wong EK, Min BI. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci U S A. 1998;95:2670–2673. doi: 10.1073/pnas.95.5.2670. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fang JL, Krings T, Weidemann J, Meister IG, Thron A. Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology. 2004;46:359–362. doi: 10.1007/s00234-003-1125-7. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. The design and analysis of clinical experiments. New York: A Wiley-Interscience Publication; 1986. [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995a;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995b;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gareus IK, Lacour M, Schulte AC, Hennig J. Is there a BOLD response of the visual cortex on stimulation of the vision-related acupoint GB 37? J Magn Reson Imaging. 2002;15:227–232. doi: 10.1002/jmri.10059. [DOI] [PubMed] [Google Scholar]
- Gollub R, Hui K, Stefano G. Acupuncture: pain management coupled to immune stimulation. Acta Pharmacol Sina. 1999;20:769–777. [PubMed] [Google Scholar]
- Havel P, Braun B, Rau S, Tonn JC, Fesl G, Bruckmann H, Ilmberger J. Reproducibility of activation in four motor paradigms An fMRI study. J Neurol. 2005 doi: 10.1007/s00415-005-0028-4. [DOI] [PubMed] [Google Scholar]
- Huettel AA, Song AW, M G. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates, Inc; 2004. [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27:479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Lloyd DM. The physiology and psychology of selective attention to touch. Front Biosci. 2000;5:D894–904. doi: 10.2741/A558. [DOI] [PubMed] [Google Scholar]
- Kong J, Fufa DT, Gerber AJ, Rosman IS, Vangel MG, Gracely RH, Gollub RL. Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. J Pain. 2005;6:55–64. doi: 10.1016/j.jpain.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Kong J, Ma L, Gollub RL, Wei J, Yang X, Li D, Weng X, Jia F, Wang C, Li F, Li R, Zhuang D. A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J Altern Complement Med. 2002;8:411–419. doi: 10.1089/107555302760253603. [DOI] [PubMed] [Google Scholar]
- Kurland J, Naeser MA, Baker EH, Doron K, Martin PI, Seekins HE, Bogdan A, Renshaw P, Yurgelun-Todd D. Test-retest reliability of fMRI during nonverbal semantic decisions in moderate-severe nonfluent aphasia patients. Behav Neurol. 2004;15:87–97. doi: 10.1155/2004/974094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin HM, Bouffard NA, Badger GJ, Churchill DL, Howe AK. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207:767–774. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Konofagou EE, Badger GJ, Churchill DL, Fox JR, Ophir J, Garra BS. Tissue displacements during acupuncture using ultrasound elastography techniques. Ultrasound Med Biol. 2004;30:1173–1183. doi: 10.1016/j.ultrasmedbio.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Li G, Cheung RT, Ma QY, Yang ES. Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. Neuroreport. 2003a;14:669–673. doi: 10.1097/00001756-200304150-00002. [DOI] [PubMed] [Google Scholar]
- Li G, Huang L, Cheung RT, Liu SR, Ma QY, Yang ES. Cortical activations upon stimulation of the sensorimotor-implicated acupoints. Magn Reson Imaging. 2004;22:639–644. doi: 10.1016/j.mri.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Li G, Liu HL, Cheung RT, Hung YC, Wong KK, Shen GG, Ma QY, Yang ES. An fMRI study comparing brain activation between word generation and electrical stimulation of language-implicated acupoints. Hum Brain Mapp. 2003b;18:233–238. doi: 10.1002/hbm.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litscher G, Rachbauer D, Ropele S, Wang L, Schikora D, Fazekas F, Ebner F. Acupuncture using laser needles modulates brain function: first evidence from functional transcranial Doppler sonography and functional magnetic resonance imaging. Lasers Med Sci. 2004;19:6–11. doi: 10.1007/s10103-004-0291-0. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Zhang L, Brown RW, Yue GH. Reproducibility of fMRI at 1.5 T in a strictly controlled motor task. Magn Reson Med. 2004a;52:751–760. doi: 10.1002/mrm.20211. [DOI] [PubMed] [Google Scholar]
- Liu WC, Feldman SC, Cook DB, Hung DL, Xu T, Kalnin AJ, Komisaruk BR. fMRI study of acupuncture-induced periaqueductal gray activity in humans. Neuroreport. 2004b;15:1937–1940. doi: 10.1097/00001756-200408260-00021. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test--retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001;21:592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Witter MP. FMRI of visual encoding: reproducibility of activation. Hum Brain Mapp. 2000;9:156–164. doi: 10.1002/(SICI)1097-0193(200003)9:3<156::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, Kennedy DN, Gollub RL. Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry. 2001;158:955–958. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]
- Marshall I, Simonotto E, Deary IJ, Maclullich A, Ebmeier KP, Rose EJ, Wardlaw JM, Goddard N, Chappell FM. Repeatability of motor and working-memory tasks in healthy older volunteers: assessment at functional MR imaging. Radiology. 2004;233:868–877. doi: 10.1148/radiol.2333031782. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP. Variability in fMRI: an examination of intersession differences. Neuroimage. 2000;11:708–734. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2004;24:193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire M, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122:1765–1779. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Porro CA, Lui F, Facchin P, Maieron M, Baraldi P. Percept-related activity in the human somatosensory system: functional magnetic resonance imaging studies. Magn Reson Imaging. 2004;22:1539–1548. doi: 10.1016/j.mri.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, van Veelen CW. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421–437. doi: 10.1006/brln.2001.2600. [DOI] [PubMed] [Google Scholar]
- Siedentopf CM, Golaszewski SM, Mottaghy FM, Ruff CC, Felber S, Schlager A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci Lett. 2002;327:53–56. doi: 10.1016/s0304-3940(02)00383-x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Ramnani N, Woolrich MW, Bannister PR, Jenkinson M, Matthews PM, McGonigle DJ. Variability in fMRI: a re-examination of inter-session differences. Hum Brain Mapp. 2005;24:248–257. doi: 10.1002/hbm.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stux G. Technique of Acupuncture. In: Stux G, Pomeranz B, editors. Basics of Acupuncture. Berlin: Springer-Verlag; 1997. pp. 202–213. [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Wagner K, Frings L, Quiske A, Unterrainer J, Schwarzwald R, Spreer J, Halsband U, Schulze-Bonhage A. The reliability of fMRI activations in the medial temporal lobes in a verbal episodic memory task. Neuroimage. 2005;28:122–131. doi: 10.1016/j.neuroimage.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Immisch I, Pfeiffer C, Hallett M. The variability of serial fMRI data: correlation between a visual and a motor task. Neuroreport. 2000;11:3843–3847. doi: 10.1097/00001756-200011270-00048. [DOI] [PubMed] [Google Scholar]
- Wei X, Yoo SS, Dickey CC, Zou KH, Guttmann CR, Panych LP. Functional MRI of auditory verbal working memory: long-term reproducibility analysis. Neuroimage. 2004;21:1000–1008. doi: 10.1016/j.neuroimage.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Wu M-T, Hsieh J-C, Xiong J, Yang P-C, Pan H-B, Chen Y-CI, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain- preliminary experience. Radiology. 1999;212:133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- Wu MT, Sheen JM, Chuang KH, Yang P, Chin SL, Tsai CY, Chen CJ, Liao JR, Lai PH, Chu KA, Pan HB, Yang CF. Neuronal specificity of acupuncture response: a fMRI study with electroacupuncture. Neuroimage. 2002;16:1028–1037. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- Yan B, Li K, Xu J, Wang W, Li K, Liu H, Shan B, Tang X. Acupoint-specific fMRI patterns in human brain. Neurosci Lett. 2005;383:236–240. doi: 10.1016/j.neulet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Yang JS, Cao YM. Aupuncture point. Shanghai: Shanghai science and technology press; 1987. [Google Scholar]
- Yetkin FZ, McAuliffe TL, Cox R, Haughton VM. Test-retest precision of functional MR in sensory and motor task activation. AJNR Am J Neuroradiol. 1996;17:95–98. [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Teh EK, Blinder RA, Jolesz FA. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. Neuroimage. 2004;22:932–940. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Wei X, Dickey CC, Guttmann CR, Panych LP. Long-term reproducibility analysis of fMRI using hand motor task. Int J Neurosci. 2005;115:55–77. doi: 10.1080/00207450490512650. [DOI] [PubMed] [Google Scholar]
- Zhang WT, Jin Z, Cui GH, Zhang KL, Zhang L, Zeng YW, Luo F, Chen AC, Han JS. Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Res. 2003;982:168–178. doi: 10.1016/s0006-8993(03)02983-4. [DOI] [PubMed] [Google Scholar]


