Abstract
Constipation is a common gastrointestinal motility disorder that is often chronic, negatively affects patients' daily lives, and is associated with high healthcare costs. There is a considerable range of treatment modalities available for patients with constipation; however, the clinical evidence supporting their use varies widely. Nonpharmacologic modalities, such as increased exercise or fluid intake and bowel habit training, are generally recommended as first-line approaches, but data on the effectiveness of these measures are limited. The clinical benefits of various traditional pharmacologic agents (many of which are available over the counter, such as laxatives and fiber supplements) remain unclear. Although these modalities may benefit some patients with temporary constipation, their efficacy in patients for whom constipation is chronic is less well defined. Some studies suggest benefit with psyllium, polyethylene glycol, and lactulose; however, the use of other agents, such as calcium polycarbophil, methylcellulose, bran, magnesium hydroxide, and stimulant laxatives, is not supported by strong clinical evidence. More recently, newer agents have been approved for the treatment of patients with chronic constipation on the basis of comprehensive clinical investigation programs. Tegaserod, with its well-established clinical profile, and lubiprostone, the latest addition to the treatment armamentarium, represent the new generation of therapies for chronic constipation. This article reviews the efficacy and safety of traditional therapies used in the management of the multiple symptoms associated with chronic constipation and discusses recently approved and emerging therapies for this disorder.
Introduction
Constipation is a common gastrointestinal motility disorder that affects up to 28% of individuals in North America.[1] For many, this condition is chronic.[2] Epidemiologic studies consistently demonstrate an increased prevalence of constipation among certain populations, including women and nonwhites.[1,3] A distinct geographic pattern of distribution has also been described and attributed to environmental influences: Constipation is more common in rural areas and in northern and mountainous states.[4]
This article examines the clinical evidence supporting the efficacy and safety of traditional and novel therapies for the treatment of patients with chronic constipation and assesses whether the evidence supports the use of specific treatment options in these patients.
Background and Context
Available data suggest that constipation negatively affects health-related quality of life. In a Canadian survey of 1149 persons with constipation (based on Rome II diagnostic criteria and self-report),[5] poorer quality of life among participants was apparent in the physical and mental domains of the Medical Outcomes Study Short Form (SF-36) compared with Canadian normative data.[6] Data from France, using the Gastrointestinal Quality of Life Index, also demonstrated altered health-related quality of life among patients with constipation.[7] An average Gastrointestinal Quality of Life Index score of 92.3 was observed among patients with constipation (normal, >105). A study using a modified version of the Digestive Health Status Instrument measured the impact of symptoms on the daily lives of patients with various gastrointestinal disorders, including chronic constipation (n = 39). Results showed that chronic constipation-associated gastrointestinal symptoms significantly interfere with many aspects of sufferers' daily lives, including mood (44%), mobility (37%), normal work (42%), recreation (47%), and enjoyment of life (58%).[8]
Costs associated with constipation, including direct costs such as evaluation and treatment and indirect costs such as work absenteeism are high.[9,10] Recently presented data (based on the National Ambulatory Medical Care Survey database) showed that office visits for which constipation was a primary or secondary diagnosis more than doubled over a 7-year period (1997-2004), from 11.3 to 27.5 per 1000 population.[11] Mean costs of diagnostic studies for constipation have been shown to approach $3000 per patient.[12] An analysis of 3 national surveys (the 2001 National Ambulatory Medical Care Survey, the 2001 National Hospital Ambulatory Medical Care Survey, and the 2001 National Hospital Discharge Survey) estimated that the cost of ambulatory and inpatient care for constipation in the United States was $235 million in 2001.[13] Furthermore, each year, more than $800 million is spent in the United States on over-the-counter laxatives, the mainstay of therapy for constipation.[14] Results of a recently presented study (based on the Human Capital Management Services Research database, representing data from US employers) showed that over a 4-year period (2001-2005), employees with constipation were more likely than those without constipation to suffer from various comorbid gastrointestinal conditions, leading to higher costs. Constipation-associated annual mean incremental cost was $3545. Direct medical costs accounted for 76.5% of this value, prescription drug costs 11.6%, and indirect costs (ie, absenteeism and disability) 11.9%. In this study, employees with constipation missed 5 more days per year than employees without constipation.[15–17]
Although the Rome diagnostic criteria for constipation – most recently, the Rome III criteria (Table 1) – were developed to standardize the definition of constipation for enrollment in clinical trials,[5,18] no widely accepted, clinically useful definition of constipation exists. Physicians typically define constipation on the basis of stool frequency, with fewer than 3 bowel movements (BMs) per week considered abnormal.[1] However, patients typically report other symptoms, such as straining, passage of hard stool, inability to defecate at will, unproductive urges, and sensation of incomplete evacuation.[1,3]
Table 1.
| Presence of 2 or more of the following: |
|
| Additional criteria: |
|
Symptoms must have been present for the past 3 months, with symptom onset at least 6 months prior to diagnosis
As depicted in Figure 1,[19] the key steps in evaluating a patient who presents with constipation include: checking for the presence of abdominal pain as a primary symptom (if present, consider irritable bowel syndrome with constipation [IBS-C]) and assessing for the presence of red flags suggestive of organic disease (eg, unintended weight loss of more than 10 pounds, family history of colorectal cancer, anemia, hematochezia, or positive occult blood test). If present, directed testing should be undertaken. In the absence of all of these red flags, one should determine whether constipation is a result of a primary or a secondary cause (Table 2).[3,14,20–23]
Figure 1.
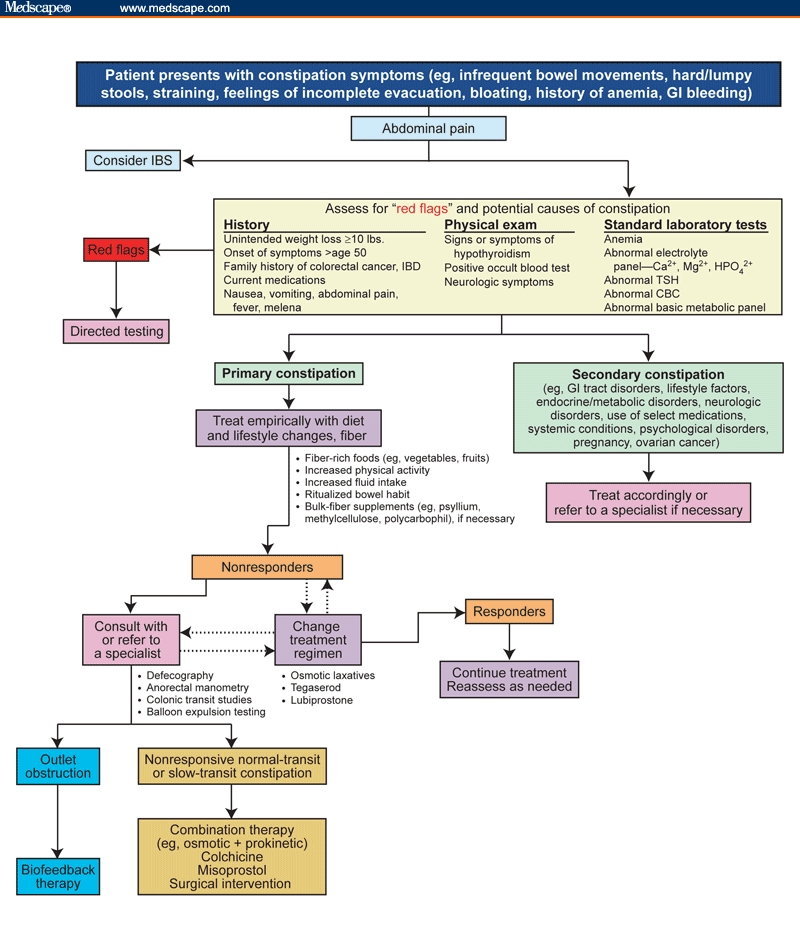
Diagnostic/treatment algorithm for constipation. CBC = complete blood count; GI = gastrointestinal; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome; TSH = thyroid-stimulating hormone. Modified from Hunt R, Lacy B. Diagnosis and management of chronic constipation in the primary care setting. Intern Med World Rep. 2004;1(suppl):3-23 with permission from Ascend Media Healthcare.
Table 2.
| Primary (Idiopathic) | Secondary |
|---|---|
| IBS-C | Inadequate diet |
| Normal-transit constipation | Dehydration |
| Slow-transit constipation | Inadequate physical activity |
Defecatory or rectal evacuation disorders:
|
Use of certain medications:
|
Older age Pregnancy Mechanical obstruction:
|
|
Endocrine/metabolic disorders:
|
|
Neurologic conditions:
|
|
Psychological conditions:
|
Primary (idiopathic) constipation generally can be classified into 3 categories: normal-transit constipation (NTC; patients in this subgroup may also fulfill criteria for IBS-C),[24] slow-transit constipation (STC), and anorectal outlet abnormalities (including dyssynergia and pelvic floor dysfunction).[3] Overlapping mechanisms often coexist (eg, STC and pelvic floor dysfunction, NTC, and IBS-C).[3,22–24] Not surprisingly, symptom overlap and comorbidity are common between chronic constipation and IBS-C as well.[25]
Patients with NTC commonly have normal physical examination findings, whereas those with STC may demonstrate mild abdominal distension or palpable stool in the sigmoid colon.[23] Abdominal tenderness is rarely seen in either group.
Anorectal outlet abnormalities should be suspected in patients who report prolonged or excessive straining, feelings of incomplete evacuation, application of perineal or vaginal pressure, or direct digital evacuation of stool (including soft stool). However, symptoms alone are insufficient to make a diagnosis.[22] Reduced perineal descent noted on rectal examination supports a diagnosis of pelvic floor dysfunction.[20] Physiologic studies (colonic-transit tests, anorectal manometry, balloon expulsion tests, and defecography; Table 3 [14,22,26]) can be helpful in further evaluating constipation.[3] The use of these tests is frequently delayed until dietary and lifestyle changes and trials of fiber and laxatives have produced no improvement.[3]
Table 3.
| Test | Use | Method | Purpose |
|---|---|---|---|
| Anorectal manometry | Assesses the anal sphincter, pelvic floor, and associated nerves | Special pressure-sensitive catheter is inserted into the anus to measure resting pressure and squeeze pressure of the sphincter | Principal purpose in chronic constipation is to exclude adult-onset or short-segment Hirschsprung's disease (congenital megacolon) |
| Balloon-expulsion test | Demonstrates rectal evacuation | Either a silicone-filled stool-like device or a 4-cm long balloon filled with 50 mL warm water is placed in the rectum, and the patient is asked to expel the device | Healthy volunteers can expel the balloon within 1 minute; if the patient is unable to expel the device within 3 minutes, dyssynergic defecation should be suspected |
| Colonic-transit study | Measures rate at which fecal residue moves through colon | Serial abdominal radiographs are obtained after the patient swallows a capsule filled with radiopaque markers | In persons with normal transit time, most markers should pass by day 5; in patients with slow colonic transit, markers will be scattered throughout the colon; in patients with pelvic outlet obstruction, > 20% of markers will be delayed in the rectum |
| Defecography | Provides information on anatomic and functional changes of the anorectum | Approximately 150 mL barium is placed in the patient's rectum, and the patient is asked to squeeze, cough, and bear down | Test may reveal poor activation of levator muscles, prolonged retention of contrast material, or inability to expel the barium in patients with dyssynergic defecation |
| MRI defecography | Provides a global view of pelvic viscera and musculature | Rectum is filled with a semi-solid substance that is labeled with a contrast marker. Patient sits on a commode between 2 magnetic rings. A series of MRI images are collected during pelvic floor contraction and defecation. | This technique allows for analysis of anorectal angle, opening of anal canal, pelvic floor descent during defecation, and functioning of the puborectal muscle. Clear visibility of rectal wall can reveal intussusceptions and rectoceles. Visibility of structures surrounding the rectoanal can reveal enteroceles |
Ideally, therapies for patients with chronic constipation should restore normal bowel function and relieve constipation-related symptoms (eg, straining, bloating, and feelings of incomplete evacuation). Laxatives or promotility agents may be used in patients with STC and some patients with NTC. Most patients with pelvic floor dyssynergia are refractory to pharmacologic therapy and benefit from referral to a specialist, who may use neuromuscular training with biofeedback, alone or in combination with pharmacologic therapy, to help patients relearn how to coordinate their pelvic floor and anal sphincter muscles.[3,14]
Nonpharmacologic Interventions
In patients with no known secondary causes of constipation, conservative nonpharmacologic treatment measures generally are recommended as first-line therapy (Figure 1). These strategies typically include regular exercise, increased fluid intake, and bowel habit training.[14] However, these measures are effective in only a subset of patients. Clinical trial data supporting the effectiveness of such approaches are limited and, in general, do not support their benefit in providing relief of symptoms, particularly when evaluated in patients with chronic constipation.[3] Ritualizing bowel habits may also help some patients. This recommendation is made on the basis of observations that most patients without constipation have a regular pattern of defecation and that certain activities (eg, waking and eating) stimulate colonic activity.[14] Despite its rational appeal, the concept of ritualizing bowel habits has never been tested prospectively in patients with constipation.[27]
Other nonpharmacologic therapies include biofeedback therapy, behavior therapy, and electric stimulation; however, these therapies are generally reserved for patients with outlet obstruction and are typically performed at highly specialized centers.[28–32] Biofeedback therapy involves retraining the pelvic floor and anal sphincter muscles with a small balloon or electronic probe. This type of therapy may be helpful in patients with symptoms or physical examination findings that suggest pelvic floor dysfunction, or for those in whom conservative therapies have failed and who have diagnostic test results indicative of this disorder.[3,33] However, in clinical trials, positive response rates for biofeedback varied greatly,[33] and many of these studies had a poor experimental design or were too small to provide clinically meaningful data. For example, one such trial, a prospective, randomized, comparative study of 4 biofeedback techniques, found a statistically significant increase in unassisted BMs and a reduction in cathartic use with electromyographic biofeedback training alone or with intrarectal balloon training and home training.[32] Although this trial followed a relatively well-designed protocol and is one of the better studies in the field of nonpharmacologic treatment of constipation, it enrolled only 36 patients, which makes it difficult to determine whether the findings are applicable to the wider population with chronic constipation and pelvic floor dyssynergia. More recently, a randomized controlled trial of 54 patients with pelvic floor dyssynergia demonstrated that biofeedback therapy is significantly more effective than laxative use in this patient population. At 6 months, 80% of patients receiving biofeedback vs 22% of those receiving laxatives reported symptom improvement (P < .001).[34] These data further support the benefit of biofeedback as an efficacious therapy for patients with pelvic floor dysfunction. Nevertheless, additional well-designed trials are needed to confirm the effectiveness of biofeedback training in patients with defecatory disorders.[35]
Electric stimulation therapy, which uses brief waves of electrical stimulation to strengthen the muscles in the lower pelvis, has also been associated with some success in small studies as a potential treatment for constipation.[28-30,35]
Surgery (subtotal colectomy with ileorectal anastomosis) should be reserved for patients with STC (or those with both STC and pelvic floor dysfunction) whose symptoms are unresponsive to other measures; surgery is inappropriate for patients with pelvic floor dysfunction alone, or those with NTC attributed to IBS-C.[36,37] Additionally, extensive evaluation at a tertiary center should be undertaken before consideration of surgery.
Finally, in a study that included more patients with IBS than constipation, cognitive behavior therapy (an integrated treatment approach using cognitive restructuring and behavioral modification techniques) showed some benefit in women with moderate to severe constipation.[31] However, the role of cognitive therapy in patients with chronic constipation remains unclear.
Traditional Pharmacologic Agents
Fiber
Increasing fiber intake is commonly recommended for the initial treatment of constipation,[3,22] and this can be accomplished by recommending that patients eat high-fiber foods (fruits, vegetables) or taking fiber/bulk supplements (bran, psyllium, methylcellulose, or polycarbophil). However, patients must be counseled that they may need to continue such therapy for 2-3 months before they experience any measurable symptom relief. Despite the widespread use of fiber supplementation, this approach is effective in only a subset of patients, and clinical trial evidence supporting the use of increased fiber intake is limited.
A meta-analysis of 20 clinical studies (conducted from 1974 to 1988) examining the impact of fiber on BM frequency showed that although bran increased stool weight and decreased transit time in patients with chronic constipation, patients still had lower stool output and slower transit than persons without constipation, regardless of whether they had consumed bran.[38] Moreover, the increase in BM frequency with fiber supplements or bulk laxatives typically was 1 to 1.4 BMs per week, similar to that observed with nonbulk laxatives, an increase of 1.5 BMs per week.[39] For many patients, fiber exacerbates bloating and distension, leading to poor compliance (estimated to be as low as 50%).[3,40] Finally, large quantities of fiber (up to 25 g/day) are required for efficacy, but this may be inconvenient and unpalatable.[3,14,40]
Two recently published evidence-based systematic reviews[2,41] regarding treatment options for patients with chronic constipation revealed a lack of high-quality data demonstrating the efficacy of many commonly used bulking agents in this population. On the basis of the parameters shown in Table 4,[2] the American College of Gastroenterology Chronic Constipation Task Force (ACG-CCTF) found that psyllium increases stool frequency in patients with chronic constipation; however, this was the only bulking agent for which sufficient data were available to make an evidence-based recommendation (grade B; Table 5).[2] They also ascertained that available data on the efficacy of calcium polycarbophil, methylcellulose, and bran did not demonstrate statistically significant improvements in stool frequency or consistency (grade B). The ACG-CCTF concluded that available data were insufficient to make recommendations regarding the efficacy of these agents in the treatment of chronic constipation. On the basis of the parameters established by the US Preventive Services Task Force for levels of evidence and grading recommendations, Ramkumar and Rao[41] found fair evidence supporting the efficacy and safety of calcium polycarbophil (grade B) and poor evidence supporting the use of psyllium, methylcellulose, and bran (grade C) in the treatment of chronic constipation.
Table 4.
ACG Grading Recommendations and Levels of Evidence[2]
| Grading Recommendations | Levels of Evidence |
|---|---|
| A Two or more level 1 trials without conflicting evidence from other level 1 trials | 1 RCTs with P < .05, adequate sample size, appropriate methodology |
| B Single level 1 trial or 2 or more level 1 trials with conflicting evidence from other level 1 trials or 2 or more level 2 trials | 2 RCTs with P > .05 or inadequate sample size and/or inappropriate methodology |
| C Based on evidence from levels 3 to 5 | 3 Non-RCTs with contemporaneous controls 4 Non-RCTs with historical controls 5 Case series |
RCT = randomized controlled trial
Table 5.
| Agent | Efficacy | ACG Grade Recommendation |
|---|---|---|
| Fiber | ||
| Psyllium | Increases stool frequency in patients with chronic constipation | Grade B |
| Calcium polycarbophil Methylcellulose Bran | Data are insufficient to make a recommendation about the efficacy of these agents in patients with chronic constipation | Grade B |
| Laxatives | ||
| Polyethylene glycol | Effective at improving stool frequency and stool consistency in patients with chronic constipation | Grade A |
| Lactulose | Effective at improving stool frequency and stool consistency in patients with chronic constipation | Grade A |
| Magnesium hydroxide | Data are insufficient to make a recommendation about the efficacy of these agents in patients with chronic constipation | Grade B |
| Stimulant laxatives | Data are insufficient to make a recommendation about the efficacy of these agents in patients with chronic constipation | Grade B |
| Novel agents | ||
| Tegaserod | Effective at improving the frequency of CSBMs, straining, stool frequency, and stool consistency in patients with chronic constipation | Grade A |
CSBM = complete spontaneous bowel movement
Note: Lubiprostone was not FDA approved at the time that these recommendations were published. This agent is therefore not included in this table.
Laxatives
Osmotic laxatives (poorly absorbed/nonabsorbed sugars, saline laxatives, and polyethylene glycol [PEG]) cause intestinal water secretion and may be recommended if fiber therapy fails.[3] Many osmotic laxatives need a few days to become effective, and they may result in electrolyte and volume overload in patients with renal insufficiency or cardiac dysfunction.[3] They also may cause abdominal cramping, bloating, and flatulence.[40] Clinical trials suggest that PEG-based laxatives increase stool frequency, improve consistency,[42,43] and reduce the time to first BM.[42,44] However, the duration of most of these studies was relatively short, ranging from 72 hours to 4 weeks.[42–47] Therefore, the implications of long-term therapy with PEG-based laxatives remain unclear. Only one placebo-controlled study has examined long-term (24 weeks) safety, tolerability, and efficacy with a PEG electrolyte balance solution.[48] However, it enrolled 70 patients, and only those who responded positively to treatment and tolerated the drug during the first 4 weeks of treatment completed the remaining 20 weeks. Furthermore, a significant number of subjects dropped out before completing the study. Thus, the long-term clinical benefits of this agent in patients who show limited or delayed response to therapy remain unclear.
Given their overall safety, osmotic laxatives represent an alternative therapy for patients who do not respond to lifestyle changes or fiber supplements. The primary limitations of osmotic laxatives are the lack of effectiveness in alleviating global symptoms of constipation and the associated adverse effects (abdominal cramping, bloating, and flatulence). Data indicate that PEG and lactulose are effective in increasing stool frequency and consistency. The efficacy and safety of these agents in the treatment of patients with chronic constipation are supported by good-quality evidence (grade A), but the efficacy and safety of magnesium hydroxide are not supported by sufficient data (grade B; Table 5).[2] Ramkumar and Rao[41] concluded that good evidence supports the efficacy of PEG (grade A), but that only moderate evidence supports the efficacy of lactulose (grade B) and that there is little evidence to support the efficacy of magnesium hydroxide (grade C) in the treatment of patients with chronic constipation. It should be noted that all formulations of PEG (eg, PMF-100, PEG 3350, PEG/electrolyte solutions, and high-molecular-weight PEG [PEG 4000]) are included in this review but that only PEG 3350 (MiraLax; Braintree Laboratories; Braintree, Massachusetts) is FDA approved for the treatment of patients with occasional constipation.[49] A recently published systematic review of FDA-approved prescription medications for constipation concluded that the majority of clinical trials with lactulose were methodologically flawed (eg, small sample sizes, short duration, lack of prospectively defined measures). Overall they found lactulose to be more effective in relieving constipation than placebo but less effective than PEG or bulk laxatives plus senna.[50]
Stimulant laxatives (diphenylmethane and anthraquinone derivatives) produce rhythmic muscle contractions in the intestines and may be recommended if osmotic laxatives fail.[40] These agents increase intestinal motility and secretion and work within hours of ingestion, but they may be associated with severe abdominal cramps.[3] Continuous daily use may result in hyponatremia, hypokalemia, and dehydration.[40] Although concerns remain regarding the long-term safety of these agents (eg, their potential to cause rebound constipation, damage to the intestinal smooth muscle or enteric nervous system, and colorectal cancer), the prevailing opinion is that they are likely safe.[51] Nevertheless, they should be used judiciously, preferably on a short-term basis when fiber or osmotic laxatives provide an insufficient response or when patients cannot tolerate other agents. Clinical data regarding stimulant laxatives are generally weak, as noted by the ACG-CCTF[2] (grade B) (Table 5) and the Ramkumar and Rao[41] (grade C) systematic reviews. Although these approved therapies may be beneficial in some patients with chronic constipation, data supporting their long-term use are inadequate. Compliance with treatment is often an issue, as most therapies are associated with limited efficacy (ie, they do not relieve associated symptoms such as bloating and discomfort) and can have disturbing adverse effects.
Novel Agents
In the past, cisapride, a first-generation promotility agent, which increases intestinal motor activity, was used clinically for the treatment of chronic constipation, but its efficacy was not established.[22] Results of one study showed that cisapride reduced the need for laxatives and normalized stool consistency, but that it did not have an effect on other symptoms or on bowel motility parameters in patients with chronic idiopathic constipation.[52] Cisapride was withdrawn from the US market in July 2000 because of safety concerns (cardiac arrhythmias).[22] Studies with prucalopride, a 5-HT4 agonist, have demonstrated good clinical efficacy,[53] but development was suspended because of safety issues.[35] Other drugs that increase intestinal motor activity (cholinergic agents, metoclopramide) are generally ineffective, poorly tolerated, or inappropriate for most patients.[40]
Recent advances in the understanding of gastrointestinal physiology have paved the way for innovative new approaches to the treatment of patients with chronic constipation. The advent of tegaserod, a gastrointestinal motility agent, and lubiprostone, which enhances gastrointestinal secretion, has extended the therapeutic options available to patients with chronic constipation.
Tegaserod
Research advances have identified the neurotransmitter serotonin (5-hydroxytryptamine [5-HT]), found primarily (95%) in the gastrointestinal tract, as an important mediator of gut function.[54] Serotonin facilitates communication between the enteric nervous system and its effector systems (muscles, secretory endothelium, endocrine cells, and vasculature) and between the enteric nervous system and the central nervous system.[54] Serotonin acts by binding to its receptors, of which type 3 (5-HT3) and type 4 (5-HT4) have been shown to be particularly important in the regulation of gut function.[54]
Tegaserod, a selective 5-HT4 receptor agonist, is significantly more effective than placebo in providing relief from the symptoms of chronic constipation, including decreased BM frequency, straining, hard or lumpy stool, abdominal discomfort/pain, and bloating/distension.[55–57] In 2 large, double-blind, randomized, placebo-controlled trials involving adult patients with chronic constipation (Europe, South Africa, and Australia: N = 1264[57]; North and South America: N = 1348[55]), patients were treated with either placebo or tegaserod 6 mg or 2 mg twice daily for 12 weeks. In these studies, chronic constipation was defined as an average of fewer than 3 complete spontaneous bowel movements (CSBMs; defined as bowel movements that were unassisted by laxative or enema within the previous 24 hours and that completely relieved the feeling of stool in the distal bowel) per week with straining, incomplete evacuation, and hard or very hard stool associated with at least one fourth of all bowel movements. The primary efficacy variable was a responder rate where a responder was defined as an individual with an increase of at least 1 CSBM per week over weeks 1-4. This variable was chosen instead of spontaneous bowel movements (SBMs; bowel movements that occur without the use of laxatives or enemas) because it is thought to be a more clinically meaningful end point than merely counting bowel movements. Additional efficacy variables included the responder rate during the entire 12 weeks of active treatment, change in individual symptom scores, laxative intake, and median time to first CSBM. Results showed that tegaserod administration was associated with a statistically significant increase in the number of CSBMs (for weeks 1-4 and weeks 1-12) and reduced constipation symptoms (abdominal discomfort/pain, bloating/distension, straining, and stool consistency [P < .05 for all symptoms compared with placebo]).[55,57] Figure 2 depicts the response rate for the primary efficacy variable in the North and South American trial.[55] Improvements in BM frequency and most other symptoms were evident within the first week.[55,57] Within the first 24 hours of treatment with 2 mg or 6 mg tegaserod twice daily, 54% and 62% of patients, respectively, had their first SBM, compared with 41% of patients who received placebo (in the trial conducted in Europe, South Africa, and Australia).[57]
Figure 2.
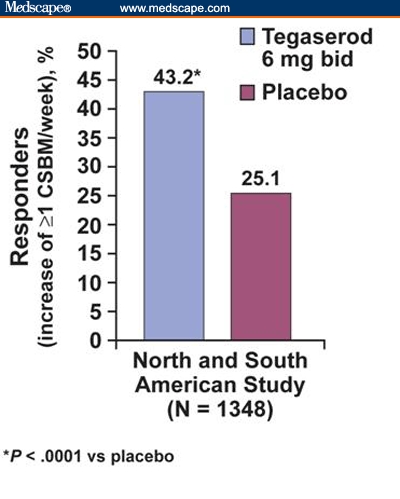
Tegaserod (6 mg BID) responders for increased complete spontaneous bowel movements (CSBMs). Percentage of patients experiencing an increase of 1 or more CSBMs per week during weeks 1-4 (primary efficacy variable).[55] BID = twice daily
Tegaserod was safe and well tolerated in this population.[55–57] Pooled analyses of the 2 trials revealed that the most commonly reported adverse events included headache, nasopharyngitis, and abdominal pain, all of which were more common in the placebo group than in the tegaserod groups. Mild to moderate diarrhea was reported more frequently among subjects randomly assigned to tegaserod than among those receiving placebo.[58] The frequency of diarrhea was 6.6%, 4.2%, and 3.0% for tegaserod 6 mg twice daily, tegaserod 2 mg twice daily, and placebo, respectively (P = .0005; tegaserod 6 mg twice daily vs placebo). However, the diarrhea was generally of short duration (less than 2 days), typically resolved with continued therapy, and rarely resulted in the interruption of therapy.[58] Overall, 4.3% of patients discontinued the study due to adverse effects; abdominal pain and diarrhea were the most common reason for discontinuation in patients receiving tegaserod 2 mg twice daily and 6 mg twice daily, respectively. However, the discontinuation rate attributed to diarrhea was less than 1% among those treated with tegaserod. Furthermore, clinically relevant laboratory and electrocardiogram parameters were comparable among tegaserod and placebo groups.[58] On the basis of the design, conduct, and results of these 2 studies, the ACG-CCTF concluded that tegaserod is effective at improving the frequency of CSBMs, straining, and stool consistency in patients with chronic constipation (grade A; Table 5).[2] The systematic review on FDA-approved prescription agents for constipation concluded that these 2 clinical trials with tegaserod were of high quality and successfully demonstrated the positive benefits of tegaserod in relieving the multiple symptoms of patients with chronic constipation.[50]
Additional studies examining the long-term (13 months) safety and efficacy of tegaserod demonstrated that the clinical efficacy of tegaserod was maintained among patients who initially responded to tegaserod. This long-term study also confirmed that it was safe and well tolerated.[59,60] Tegaserod, dosed at 6 mg twice daily, was approved by the FDA in 2004 as a treatment for men and women younger than 65 years with chronic idiopathic constipation.[61]
Table.
FDA Public Health Advisory
| Ed. Note: As this manuscript was being prepared for publication, on March 30, 2007, the US FDA issued the following statement. |
|---|
| FDA Public Health Advisory Tegaserod maleate (marketed as Zelnorm) |
| FDA is issuing this public health advisory to inform patients and health care professionals that the sponsor of Zelnorm (tegaserod maleate), Novartis Pharmaceuticals Corporation, has agreed to stop selling Zelnorm. Zelnorm is being taken off the market because a new safety analysis has found a higher chance of heart attack, stroke, and worsening heart chest pain that can become a heart attack in patients treated with Zelnorm compared to those treated with a sugar pill they thought was Zelnorm. |
FDA announces the following, effective immediately:
|
| Zelnorm is a prescription medication approved for short term treatment of women with irritable bowel syndrome with constipation and for patients younger than 65 years with chronic constipation. In late February and early March 2007, Novartis Pharmaceuticals gave FDA the results of new analyses of 29 clinical studies of Zelnorm for treatment of a variety of gastrointestinal tract conditions; the data from all the studies were combined to assess the chance of side effects on the heart and blood vessels. In each study, patients were assigned at random to either Zelnorm or a sugar pill they thought was Zelnorm. These 29 studies included 11,614 patients treated with Zelnorm and 7,031 treated with a sugar pill. The average age of patients in these studies was 43 years and most patients–88%–were women. |
| The number of patients who suffered a heart attack, stroke or severe heart chest pain that can turn into a heart attack was small. However, patients treated with Zelnorm had a higher chance of having any of these serious and life-threatening side effects than did those who were treated with a sugar pill. Thirteen patients treated with Zelnorm (0.1%) had serious and life-threatening cardiovascular side effects; among these, four patients had a heart attack (one died), six had a type of severe heart chest pain which can quickly turn into a heart attack, and three had a stroke. Among the patients taking the sugar pill, only one (or 0.01%) had symptoms suggesting the beginning of a stroke that went away without complication. |
| There may be patients for whom no other treatment options are available and in whom the benefits of Zelnorm treatment outweigh the chance of serious side effects. FDA will work with Novartis to allow access to Zelnorm for those patients through a special program. |
| FDA has also indicated to Novartis a willingness to consider limited re-introduction of Zelnorm at a later date if a population of patients can be identified in whom the benefits of the drug outweigh the risks. However, before FDA makes a decision about limited re-introduction, any proposed plan would be discussed at a public advisory committee meeting. |
Lubiprostone
Lubiprostone, the newest agent to receive FDA approval for the treatment of adult patients with chronic idiopathic constipation,[62] is a gastrointestinal system-targeted bicyclic functional fatty acid that acts as a selective chloride channel (ClC-2) activator in the apical membrane of the gastrointestinal epithelium to increase intestinal water secretion.[63,64] This enhanced secretion of chloride leads to an increase in intraluminal fluid in the gut, which facilitates transit in the intestine and thereby eases stool passage.
The efficacy of lubiprostone in the treatment of patients with chronic constipation was demonstrated in several trials, including 2 identical placebo-controlled trials enrolling 479 (237 and 242) patients.[65–67] Both studies enrolled patients with chronic idiopathic constipation, defined as fewer than 3 SBMs per week for at least 6 months before randomization, plus hard or very hard stool, incomplete evacuation, and straining with defecation, each for at least 25% of BMs.[65–67] Patients were randomly assigned to receive lubiprostone (dosed at 24 micrograms twice daily) or placebo for 4 weeks. The primary end point was SBM frequency after the initiation of treatment. In the first of the two phase 3 studies, lubiprostone was significantly more effective than placebo in increasing SBM frequency, decreasing straining, and improving stool consistency (over a 4-week period; P < .05). Additionally, 61% of patients receiving lubiprostone vs 31% of those receiving placebo, experienced an SBM within 24 hours of first dose (P≤ .0001).[65,66] In the other phase 3 study enrolling 242 patients with constipation, participants were likewise randomized to receive either lubiprostone 24 mcg or placebo twice daily for 4 weeks. Results (for the intent-to-treat analysis) again demonstrated significant increases in SBM frequency in patients receiving lubiprostone vs placebo, with weekly SBM frequencies ranging from 5.1 to 5.7 in the lubiprostone group vs 2.8 to 3.5 in the placebo group (P < .002). In addition, significantly more patients in the lubiprostone group experienced SBMs within 24 hours of initiating therapy compared with placebo recipients (57% vs 37% for lubiprostone and placebo, respectively; P = .0024).[67] In a randomized withdrawal study (consisting of 4 weeks of active treatment and a 3-week randomized withdrawal period), consistent efficacy was observed, whereas discontinuation of lubiprostone was not associated with any rebound effect.[68] That is, although SBM frequency progressively declined in patients receiving placebo but not in those receiving lubiprostone, bowel frequency among those randomized to placebo remained higher than at baseline (P = .0020).[68] The systematic review on FDA-approved prescription agents for constipation noted a statistically significant benefit of lubiprostone compared with placebo but concluded that because clinical trials with this agent have only been published in abstract form to date, clinical applicability of findings remains to be determined.[50]
Gastrointestinal adverse events were the most often encountered tolerability issues in patients receiving lubiprostone in clinical trials. Nausea and diarrhea were the most frequent adverse events, but patients also reported abdominal distension, abdominal pain, and flatulence. Almost one third of patients receiving lubiprostone at the recommended dose (24 mcg twice daily) experienced nausea; of these, 9% discontinued therapy due to nausea. The severity of nausea was reduced when lubiprostone was administered with food, which is the recommended practice and was the practice employed during clinical trials.[62] Of the nongastrointestinal adverse effects, headache was the most frequently reported event. Because of concerns regarding fetal loss observed in a preclinical animal study, it is advised that women of childbearing age have a pregnancy test before lubiprostone administration and that lubiprostone be used during pregnancy only when the potential benefit justifies the potential risk to the fetus (pregnancy category C).[62]
Investigational Agents
Research is also focusing on newer investigational agents that take novel mechanistic approaches to the treatment of patients with chronic constipation. Sodium phosphate in tablet formulation has been marketed as a prescription osmotic purgative since 2001 and is indicated for colonic cleansing before colonoscopy. Its efficacy in the treatment of adult patients with functional constipation or IBS with constipation was demonstrated in a 4-week, open-label, dose-ranging pilot study (N = 43).[69] Results showed that sodium phosphate tablets (2-4 tablets: 3-6 g sodium phosphate) produced prompt relief of constipation symptoms (on the basis of the change from baseline in weekly number of BMs), usually within the first week of treatment. In another open-label study of 11 patients with chronic constipation, 8 (73%) demonstrated improvement in their constipation symptoms with either sodium phosphate tablets alone or in combination with other laxatives.[70] Although sodium phosphate is generally well tolerated, it should be used with caution in patients who have congestive heart failure, impaired renal function, or comorbidities or who are taking medications associated with increased risk for electrolyte disturbances.[71]
Neurotrophin-3 is a neurotrophic factor that stimulates the development, growth, and function of the nervous system. An initial randomized controlled trial of neurotrophin administered subcutaneously enrolled 107 patients.[72] Results demonstrated significant increases in the frequency of CSBMs and improvements in other secondary measures of constipation among the patients who received 9 mg neurotrophin-3 subcutaneously 3 times per week compared with placebo.
The opiate antagonists methylnaltrexone and alvimopan are under investigation for the treatment of opiate-induced constipation and postoperative ileus, but, unlike other opiate antagonists, they do not have an impact on the central effects of analgesia.[73,74] Their utility in treating nonopioid-induced constipation remains to be determined. Recently presented data demonstrated a lack of efficacy of alvimopan compared with placebo in the treatment of patients with chronic idiopathic constipation in an 8-week, double-blind trial.[75]
Linaclotide, a novel, poorly absorbed guanylate cyclase agonist, is also under development for the treatment of chronic constipation.[76] Recently presented data from a double-blind, randomized, placebo-controlled study in patients with chronic constipation demonstrated that linaclotide was more effective than placebo in increasing bowel movement frequency and improving stool consistency. This study enrolled only 42 patients, however, and results did not report symptom scores or P values.[77]
The mixed 5-HT4 receptor agonist/5-HT3 receptor antagonist renzapride relieves symptoms of constipation by improving stool consistency and increasing colonic transit and has been tested only in patients with IBS and constipation.[78] Other 5-HT4 agonists, such as norcisapride and mosapride,[79] cannabinoid receptor antagonists (SR141716A),[79] neurotrophic factors,[80] and probiotic agents,[81,82] are also under investigation. Additional work is needed to determine what role, if any, these agents may play in the treatment of patients with chronic constipation.
Conclusion
Constipation is a common and often chronic disorder, with multiple symptoms for which few treatment strategies have proven effective. The goal of treatment for patients with chronic constipation is global relief of constipation symptoms and normalization of gastrointestinal motility.
High-quality clinical evidence is unavailable to support the efficacy of nonpharmacologic treatment in patients with chronic constipation. In addition, although traditional pharmacologic therapies are used widely and benefit some patients with chronic constipation, evidence of their effectiveness is limited in this patient population, and many patients with chronic constipation are not satisfied with these treatments.[83] Conversely, the efficacy of tegaserod (which is FDA approved for the treatment of chronic idiopathic constipation in men and women younger than 65 years[61]) is supported by high-quality evidence. Similarly, lubiprostone, a selective chloride channel activator, has also demonstrated efficacy in high-quality trials and recently received FDA approval for the treatment of adult patients with chronic idiopathic constipation.[62] Full disclosures of the clinical trial data are anticipated shortly.
It is important for clinicians to choose treatment options for constipation that are most efficacious for the individual patient. Although some patients respond to traditional treatment approaches, such as fiber and laxatives, others do not. If patients have chronic symptoms refractory to traditional treatments, agents such as tegaserod and lubiprostone may be effective. Information on the efficacy and safety of investigational agents in development for the treatment of chronic constipation is forthcoming. As research into the pathophysiology of gastrointestinal motility disorders continues and new classes of therapeutic agents are developed, the current armamentarium of treatment options will increase, offering additional choices for patients with chronic constipation.
Acknowledgments
The author thanks Sophia Shumyatsky, PharmD; Maribeth Bogush, PhD; and Cathy Winter, PhD, for their editorial assistance with this manuscript.
Funding Information
Funding support was provided to the author by Novartis Pharmaceuticals.
Footnotes
Readers are encouraged to respond to the author at johnfj@uic.edu or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Brandt LJ, Schoenfeld P, Prather CM, Quigley EMM, Schiller LR, Talley NJ. Evidenced-based position statement on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl):S1–S21. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- 3.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 4.Johanson JF. Geographic distribution of constipation in the United States. Am J Gastroenterol. 1998;93:188–191. doi: 10.1111/j.1572-0241.1998.00188.x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(suppl II):II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 7.Damon H, Dumas P, Mion F. Impact of anal incontinence and chronic constipation on quality of life. Gastroenterol Clin Biol. 2004;28:16–20. doi: 10.1016/s0399-8320(04)94835-x. [DOI] [PubMed] [Google Scholar]
- 8.Legoretta A, Clark S, Marehbian J, et al. Symptoms associated with chronic gastrointestinal disorders interfere with daily living as assessed by patient reported outcomes in the primary care setting. Am J Gastroenterol. 2006;101:S490. [Abstract 1267] [Google Scholar]
- 9.Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. PharmacoEconomics. 2005;23:461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kleinman NL, Brook RA, Melkonian AK, et al. Assessment of work absences associated with constipation. Am J Gastroenterol. 2006;101:S409. [Abstract 1040] [Google Scholar]
- 11.Shah ND, Locke GR, Meek PD, et al. Ambulatory care for constipation in the United States, 1997-2004. Am J Gastroenterology. 2006;101:S220. doi: 10.1111/j.1572-0241.2008.01910.x. [Abstract 522] [DOI] [PubMed] [Google Scholar]
- 12.Rantis PC, Jr, Vernava AM, III, Daniel GL, Longo WE. Chronic constipation–is the work-up worth the cost? Dis Colon Rectum. 1997;40:280–286. doi: 10.1007/BF02050416. [DOI] [PubMed] [Google Scholar]
- 13.Martin BC, Barghout V, Cerulli A. Direct medical costs of constipation in the United States. Managed Care Interface. 2006;19:43–39. [PubMed] [Google Scholar]
- 14.Rao SS. Constipation: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:659–683. doi: 10.1016/s0889-8553(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 15.Brook RA, Kleinman NL, Melkonian AK, et al. The prevalence and costs to treat GI comorbidities in persons with constipation. Am J Gastroenterol. 2006;101:S407. [Abstract 1037] [Google Scholar]
- 16.Brook RA, Kleinman NL, Melkonian AK. Cost of illness for constipation: medical, pharmacy, and work absence costs in employees with or without constipation. Am J Gastroenterol. 2006;101:S408. [Abstract 1038] [Google Scholar]
- 17.Kleinman NL, Brook RA, Melkonian AK, et al. Assessment of work absences associated with constipation. Am J Gastroenterol. 2006;101:S409. [Abstract 1040] [Google Scholar]
- 18.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Hunt R, Lacy B. Diagnosis and management of chronic constipation in the primary care setting. Intern Med World Rep. 2004;1(suppl):3–23. [Google Scholar]
- 20.National Digestive Diseases Information Clearinghouse. Constipation. Bethesda, Md: National Institute for Diabetes and Digestive and Kidney Diseases, National Institutes of Health; [Google Scholar]
- 21.Arce DA, Ermocilla CA, Costa H. Evaluation of constipation. Am Fam Physician. 2002;65:2283–2290. [PubMed] [Google Scholar]
- 22.Locke GR, III, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology. 2000;119:1766–1778. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- 23.Velio P, Bassotti G. Chronic idiopathic constipation: pathophysiology and treatment. J Clin Gastroenterol. 1996;22:190–196. doi: 10.1097/00004836-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Cash BD, Chey WD. Review article: The role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment Pharmacol Ther. 2005;22(11-12):1047–1060. doi: 10.1111/j.1365-2036.2005.02696.x. [DOI] [PubMed] [Google Scholar]
- 25.Crowell MD, Schettler VA, Harris L, et al. Symptom overlap and irritable bowel syndrome (IBS-C) Comorbidity in women with chronic constipation. Gastroenterology. 2006;130 Abstract M1191. [Google Scholar]
- 26.Schwizer W, et al. Noninvasive investigation of gastrointestinal functions with magnetic resonance imaging: towards an “ideal” investigation of gastrointestinal function. Gut. 2003;52:iv34. doi: 10.1136/gut.52.suppl_4.iv34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris LA. Prevalence and ramifications of chronic constipation. Manag Care Interface. 2005:23–30. [PubMed] [Google Scholar]
- 28.Shafik A, Shafik AA, el-Sibai O, Ahmed I. Colonic pacing in patients with constipation due to colonic inertia. Med Sci Monitor. 2003;9:CR243–CR248. [PubMed] [Google Scholar]
- 29.Kenefick NJ, Vaizey CJ, Cohen CRG, Nicholls RJ, Kamm MA. Double-blind placebo-controlled crossover study of sacral nerve stimulation for idiopathic constipation. Br J Surg. 2002;89:1570–1571. doi: 10.1046/j.1365-2168.2002.02278.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang HS, Myung SJ, Yang SK, et al. Effect of electrical stimulation in constipated patients with impaired rectal sensation. Int J Colorectal Dis. 2003;18:433–438. doi: 10.1007/s00384-003-0483-2. [DOI] [PubMed] [Google Scholar]
- 31.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 32.Heymen S, Wexner SD, Vickers D, Nogueras JJ, Weiss EG, Pikarsky AJ. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Dis Colon Rectum. 1999;42:1388–1393. doi: 10.1007/BF02235034. [DOI] [PubMed] [Google Scholar]
- 33.Heymen S, Jones KR, Scarlett Y, Whitehead WE. Biofeedback treatment of constipation: a critical review. Dis Colon Rectum. 2003;46:1208–1217. doi: 10.1007/s10350-004-6717-8. [DOI] [PubMed] [Google Scholar]
- 34.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Schiller LR. New and emerging treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4(suppl 2):S43–S51. [PubMed] [Google Scholar]
- 36.Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg. 1991;214:403–411. doi: 10.1097/00000658-199110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyam DC, Pemberton JH, Ilstrup DM. Long term results of surgery for chronic constipation. Dis Colon Rectum. 1997;40:273–279. doi: 10.1007/BF02050415. [DOI] [PubMed] [Google Scholar]
- 38.Mueller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. BMJ (Clin Res Ed) 1988;296:615–617. doi: 10.1136/bmj.296.6622.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petticrew M, Rodgers M, Booth A. Effectiveness of laxatives in adults. Qual Health Care. 2001;10:268–273. doi: 10.1136/qhc.0100268... [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borum ML. Constipation: evaluation and management. Primary Care. 2001;28:577–590. doi: 10.1016/s0095-4543(05)70054-3. [DOI] [PubMed] [Google Scholar]
- 41.Ramkumar D, Rao SSC. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 42.Chaussade S, Minic M. Comparison of efficacy and safety of two doses of two different polyethylene glycol-based laxatives in the treatment of constipation. Aliment Pharmacol Ther. 2003;17:165–172. doi: 10.1046/j.1365-2036.2003.01390.x. [DOI] [PubMed] [Google Scholar]
- 43.DiPalma JA, DeRidder PH, Orlando RC, Kolts BE, Cleveland MVB. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95:446–450. doi: 10.1111/j.1572-0241.2000.01765.x. [DOI] [PubMed] [Google Scholar]
- 44.Di Palma JA, Smith JR, Cleveland MVB. Overnight efficacy of polyethylene glycol laxative. Am J Gastroenterol. 2002;97:1776–1779. doi: 10.1111/j.1572-0241.2002.05840.x. [DOI] [PubMed] [Google Scholar]
- 45.Bouhnik Y, Neut C, Raskine L, et al. Prospective, randomized, parallel-group trial to evaluate the effects of lactulose and polyethylene glycol-4000 on colonic flora in chronic idiopathic constipation. Aliment Pharmacol Ther. 2004;19:889–899. doi: 10.1111/j.1365-2036.2004.01918.x. [DOI] [PubMed] [Google Scholar]
- 46.Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999;44:226–230. doi: 10.1136/gut.44.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cleveland MV, Flavin DP, Ruben RA, Epstein RM, Clark GE. New polyethylene glycol laxative for treatment of constipation in adults: a randomized, double-blind, placebo-controlled study. South Med J. 2001;94:478–481. [PubMed] [Google Scholar]
- 48.Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46:522–526. doi: 10.1136/gut.46.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MiraLax polyethylene glycol 3550, NF powder for solution [prescribing information] Braintree, Mass: Braintree Laboratories, Inc.; June 2001. [Google Scholar]
- 50.Cash BD, Lacy BE. Systematic Review: FDA-Approved prescription medications for adults with constipation. Gastroenterol Hepatol. 2006;2:736–749. [PMC free article] [PubMed] [Google Scholar]
- 51.Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232–242. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 52.Altabas K, Bilic A, Jurcic D, et al. The efficacy of cisapride vs. placebo and diet in patients with chronic constipation. Coll Antropol. 2003;27:197–204. [PubMed] [Google Scholar]
- 53.Johanson JF, Miner PB, Parkman HP, et al. Prucalopride (PRU) improves bowel movement (BM) frequency and symptoms (SX) in patient (PTS) with chronic constipation (CC): results of two double-blind, placebo-controlled trials [abstract] Gastroenterology. 2000;118:A175. [Google Scholar]
- 54.Crowell MD. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am J Manag Care. 2001;7(suppl):S252–S260. [PubMed] [Google Scholar]
- 55.Johanson JF, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: a randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol. 2004;2:796–805. doi: 10.1016/s1542-3565(04)00356-8. [DOI] [PubMed] [Google Scholar]
- 56.Johanson JF. Review article: tegaserod for chronic constipation. Aliment Pharmacol Ther. 2004;20(suppl 7):20–24. doi: 10.1111/j.1365-2036.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 57.Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study [published correction appears in Am J Gastroenterol. 2005;100:735] Am J Gastroenterol. 2005;100:362–372. doi: 10.1111/j.1572-0241.2005.40749.x. [DOI] [PubMed] [Google Scholar]
- 58.Quigley EMM, Wald A, Fidelholtz J, Boivin M, Pecher E, Earnest D. Safety and tolerability of tegaserod in patients with chronic constipation: pooled data from two phase III studies. Clin Gastroenterol Hepatol. 2006;4:605–613. doi: 10.1016/j.cgh.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 59.Chang L, Cohard-Radice M, Dunger-Baldauf C, Kralstein J, Shetzline MA. Tegaserod is effective for long-term use in patients with chronic constipation [abstract] Gastroenterology. 2006;130(suppl 2):A-599. Abstract T2039. [Google Scholar]
- 60.Mueller-Lissner S, Kamm M, Haeck P, et al. Long-term safety and tolerability of tegaserod in chronic constipation (CC) [abstract] Gastroenterology. 2004;126(suppl 2):A-642. Abstract W1468. [Google Scholar]
- 61.Zelnorm [R] (tegaserod maleate) tablets [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corporation; August 2004. [Google Scholar]
- 62.Amitiza [R] (lubiprostone) soft gelatin capsules [prescribing information] Bethesda, Md: Sucampo Pharmaceuticals, Inc.; 2006. [Google Scholar]
- 63.Orr KK. Lubiprostone: a novel chloride channel activator for the treatment of constipation. Formulary. 2006;41:118–120. 122, 128–129. [Google Scholar]
- 64.Winpenney JP. Lubiprostone. IDrugs. 2005;8:416–422. [PubMed] [Google Scholar]
- 65.Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Phase III study of lubiprostone, a chloride channel-2 (CIC-2) activator for the treatment of constipation: safety and primary efficacy [abstract] Am J Gastroenterol. 2005;100(suppl):S328–S329. [Google Scholar]
- 66.Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Initial and sustained effects of lubiprostone, a chloride channel-2 (CIC-2) activator for the treatment of constipation: data from a 4-week phase III study [abstract] Am J Gastroenterol. 2005;100(suppl):S324–S325. [Google Scholar]
- 67.Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Phase III efficacy and safety of RU-0211, a novel chloride channel activator, for the treatment of constipation [abstract] Gastroenterology. 2003;124:A104. [Google Scholar]
- 68.Johanson JF, Gargano MA, Holland PC, Patchen ML, Ueno R. Phase III, randomized withdrawal study of RU-0211, a novel chloride channel activator for the treatment of constipation [abstract] Gastroenterology. 2004;126(suppl 2):A100. [Google Scholar]
- 69.Medoff J, Katz S, Malik P, et al. Open-label, dose-ranging pilot study of 4 weeks of low-dose therapy with sodium phosphate tablets in chronically constipated adults. Clin Ther. 2004;26:1479–1491. doi: 10.1016/j.clinthera.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Aronchick CA. Efficacy of sodium phosphate (NaP) tablets in the longterm treatment of refractory chronic constipation. Am J Gastroenterol. 2006;101:S494. [Google Scholar]
- 71.Visicol [R] tablets [prescribing information] Morrisville, NC: Salix Pharmaceuticals, Inc.; March 2006. [Google Scholar]
- 72.Parkman HP, Rao SS, Reynolds JC, et al. Neurotrophin-3 improves functional constipation. Am J Gastroenterol. 2003;98:1338–1347. doi: 10.1111/j.1572-0241.2003.t01-1-07477.x. [DOI] [PubMed] [Google Scholar]
- 73.Camilleri M. Alvimopan, a selective peripherally acting mu-opioid antagonist. Neurogastroenterol Motil. 2005;17:157–165. doi: 10.1111/j.1365-2982.2005.00640.x. [DOI] [PubMed] [Google Scholar]
- 74.Yuan CS, Wei G, Foss JF, O'Connor M, Karrison T, Osinski J. Effects of subcutaneous methylnaltrexone on morphine-induced peripherally mediated side effects: a double-blind randomized placebo-controlled trial. J Pharmacol Exp Ther. 2002;300:118–123. doi: 10.1124/jpet.300.1.118. [DOI] [PubMed] [Google Scholar]
- 75.Kelleher D, Johanson J, Pobiner B, Carter E, Dukes G. Alvimopan, a peripherally acting mu-opioid receptor (PAM-OR) antagonist – a study in patients with chronic idiopathic constipation (CIC) not taking opioid medications. Am J Gastroenterol. 2006;101:S480. [Abstract 1239] [Google Scholar]
- 76.Kurtz C, Fitch D, Busby RW, Fretzen A, Geis GS, Currie MG. Effects of multidose administration of MD-1100 on safety, tolerability, exposure, and pharmacodynamics in healthy subjects [abstract] Gastroenterology. 2006;130:A-26. [Google Scholar]
- 77.Lacy BE. Evaluation and treatment of IBS and chronic constipation. Medscape Gastroenterology coverage of American College of Gastroenterology 2006 Annual Scientific Meeting and Postgraduate Course; http://www.medscape.com/viewarticle/547772 Accessed February 12, 2007. [Google Scholar]
- 78.Camilleri M, McKinzie S, Fox J, et al. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004;2:895–904. doi: 10.1016/s1542-3565(04)00391-x. [DOI] [PubMed] [Google Scholar]
- 79.Cremonini F, Talley NJ. Treatments targeting putative mechanisms in irritable bowel syndrome. Nat Clin Practice Gastroenterol Hepatol. 2005;2:82–88. doi: 10.1038/ncpgasthep0096. [DOI] [PubMed] [Google Scholar]
- 80.Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 81.Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2003;17:655–659. doi: 10.1155/2003/654907. [DOI] [PubMed] [Google Scholar]
- 82.Ouwehand AC, Lagstrom H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab. 2002;46:159–162. doi: 10.1159/000063075. [DOI] [PubMed] [Google Scholar]
- 83.Schiller LR, Dennis E, Toth G. An Internet-based survey of the prevalence and symptom spectrum of chronic constipation [abstract] Am J Gastroenterol. 2004;99:S234. [Google Scholar]


