Abstract
Background
Nocturnal hypoglycemia may be the most common type of hypoglycemia in individuals with diabetes using insulin and is particularly worrisome because it often goes undetected and may lead to unconsciousness and even death in severe cases.
Objectives
The prevalence, causes, and consequences of nocturnal hypoglycemia as well as detection and prevention strategies are reviewed, including the use of long-acting insulin analogs, which offer more physiologic and predictable time-action profiles than traditional human basal insulin.
Data Sources
A total of 307 publications (151 PubMed; 104 Adis; 52 BIOSIS) were reviewed.
Review Methods
Relevant trials were found by searching for “(detemir OR glargine) AND nocturnal AND (hypoglycemia OR hypoglycaemia) AND diabetes.” To capture trials that may not have specified “nocturnal” in the title or abstract text but still reported nocturnal hypoglycemia data, a supplemental search of PubMed using “(detemir OR glargine) AND (nocturnal OR hypoglycemia OR hypoglycaemia) AND diabetes” was undertaken.
Results
A review of these trials found that patients with type 1 and type 2 diabetes mellitus have a lower risk for nocturnal hypoglycemia when receiving long-acting insulin analogs (insulin detemir or insulin glargine), provided that glycemic control is comparable to that provided by traditional human basal insulin. Long-acting insulin analogs may be the best option to provide basal insulin coverage in patients who do not choose or require continuous subcutaneous insulin infusion.
Conclusions
Randomized clinical trials suggest that the long-acting insulin analogs are associated with a lower risk for nocturnal hypoglycemia than neutral protamine Hagedorn without sacrificing glycemic control.
Introduction
The American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) recommend that diabetes be managed with tight glycemic control (glycosylated hemoglobin [A1C] levels < 7% or ≤ 6.5%, respectively) to delay and reduce disease-related complications.[1,2] However, iatrogenic hypoglycemia is a barrier to achieving optimal glycemic goals.[3]
Nocturnal hypoglycemia occurs during sleep and is particularly dangerous because patients are unlikely to recognize symptoms or awaken during an episode. Undetected nocturnal hypoglycemia may often contribute to hypoglycemia unawareness, anxiety, loss of vitality, physical injury, poor quality of life,[4] and possibly neurocognitive deficits.[5] Major episodes can cause seizures and unconsciousness and require emergency care.[3] Progress has been made to minimize the risk for nocturnal hypoglycemia in insulin-using patients with the advent of continuous subcutaneous insulin infusion (CSII) pumps and the introduction of the long-acting [basal] analogs, insulin detemir and insulin glargine.[6] Both analogs have relatively flat and predictable time-action profiles[7,8] compared with neutral protamine Hagedorn (NPH). To answer whether these agents can help achieve tight glycemic control without incurring a higher risk for nocturnal hypoglycemia, clinical trials comparing long-acting insulin analogs with NPH insulin were identified and reviewed with specific attention to nocturnal hypoglycemia incidence in balance with good glycemic control.
Hypoglycemia
Causes and Prevalence
Therapeutic interventions used to treat individuals with diabetes facilitate cellular glucose uptake and reduce elevated blood glucose (BG) levels but cannot precisely replicate “normal” physiological insulin secretion. Insulin is the most common iatrogenic cause of hypoglycemia, 10-fold more prevalent in type 1 (T1DM) than in type 2 diabetes mellitus (T2DM).[3] Whenever BG and insulin peaks are mismatched, hypoglycemia can result. Because T2DM incidence is increasing and initiation of intensive regimens earlier in the disease is being recommended,[9–11] hypoglycemia is likely to become more prevalent. Although hypoglycemia is a recognized risk of intensive therapy,[12,13] tighter glycemic control delays development of diabetes-related complications and improves clinical outcomes.
Hypoglycemia Symptoms and Counterregulatory Processes
Symptoms and responses to hypoglycemia are idiosyncratic; therefore, its severity is difficult to characterize by BG concentration alone. Mid-normal fasting plasma glucose levels range from 88 to 96 mg/dL (4.9-5.3 mmol/L).[14] When glucose concentrations decrease and hypoglycemia goes untreated, symptoms can progress from mild to moderate and reasoning abilities and cognitive function may become impaired (Table 1 [15]). Minor hypoglycemic episodes may be self-treated, whereas severe (or major) episodes require third-party intervention.[3] Thresholds for neuroendocrine hormonal responses that counterbalance hypoglycemia (glucagon, epinephrine, and others) in tightly balanced feedback loops can vary considerably between individuals. Those who experience frequent hypoglycemic episodes may become desensitized to symptoms (hypoglycemia unawareness) and experience a dangerous cycle of self-exacerbating hypoglycemia known as hypoglycemia-associated autonomic failure (Figure).[3,16] Without the typical warning signs (ie, tremulousness, sweating, and hunger), self-treatment may not be perceived as necessary, and rapid progression to severe and potentially life-threatening hypoglycemia is possible.[3]
Table 1.
Approximate Blood Glucose Concentrations for Counterregulatory Hormonal and Physiologic, Somatic, and Cognitive (Neuroglycopenic) Responses in Persons Without Diabetes
| Response | Glucose mg/dL (mmol/L) |
|---|---|
| Inhibition of endogenous insulin secretion | 83 (4.6) |
| Counterregulatory hormone release (glucagon, epinephrine) | 68 (3.8) |
| Onset of autonomic and neuroglycopenic symptoms | 58–50 (3.2–2.8) |
| Neurophysiologic dysfunction, evoked responses | 54–43 (3.0–2.4) |
| Widespread electroencephalographic changes | 54 (3.0) |
| Cognitive dysfunction, inability to perform complex tasks | 50 (2.8) |
| Severe neuroglycopenia, reduced conscious level, seizures, coma | 27 (< 1.5) |
Data from Zammitt and Frier[15]
Nocturnal Hypoglycemia
Cyclic diurnal variations in BG occur normally, making it difficult to precisely define nocturnal hypoglycemia.[6,17] An episode of abnormally low BG (typically ≤ 63 mg/dL [approx. 3.5 mmol/L]) occurring at nighttime during sleep is a useful working definition.[17] Nocturnal hypoglycemia is common, especially in patients with T1DM.[6] During the Diabetes Control and Complications Trial (DCCT), 43% of all hypoglycemic episodes and 55% of severe episodes reported occurred during sleep.[18] Incidence rates vary from 12% to 56%[6]; however, because 49% to 100%[6] of episodes occur without symptoms, the actual incidence may be much higher. Patients with T2DM treated with long-acting sulfonylureas, insulin,[19] or insulin combined with oral antidiabetes drugs (OADs) are also susceptible.[20,21] Ingestion of alcohol in the evening may increase the risk for nocturnal hypoglycemia.[6]
Clinical features of nocturnal hypoglycemia include vivid dreams or nightmares, poor sleep quality or restlessness during sleep, morning headache, chronic fatigue, mood changes, increased muscle tone, night sweats, convulsions, and enuresis in children.[6,22,23] Patients with diabetes may have a reduced tendency to be awakened by hypoglycemia, mediated by reduced plasma epinephrine, cortisol, and pancreatic polypeptide responses.[24] Nocturnal hypoglycemia episodes average 86 minutes in duration,[25] but hypoglycemia unawareness and frequent severe daytime hypoglycemia may lead to prolonged nighttime hypoglycemic episodes.[26]
In addition to the reduced insulin and counterregulatory responses in T1DM and T2DM patients, diminished physiologic defenses during sleep, behavioral factors, and limitations of therapy used for diabetes management contribute to nocturnal hypoglycemia (Table 2).[6,17,23,27–32]
Table 2.
Risk Factors for Nocturnal Hypoglycemia
| Pharmacologic | Behavioral | Physiologic |
|---|---|---|
| Slow clearance of prandial insulin or OAD | Missed meal | ↓Pancreatic counterregulation involving insulin, glucagon, and pancreatic polypeptide |
| Peak insulin effects of basal insulin at night | Type of meal | ↓ Sympathetic counterregulation: ↓ epinephrine ↓ norepinephrine |
| Variability of insulin time-action profile: ∼ absorption | Missed SMBG | ↓ Adrenal counterregulation: ↓ cortisol |
| ∼ time to peak | Unplanned exercise | Age |
| ∼ duration | Alcohol | Infection |
| Poor judgment | Other comorbidity | |
| Risk factors that can be both behavioral and physiologic: Hypoglycemia unawareness | ||
| Stress | ||
| Sleep (decreased cognition) | ||
OAD = oral antidiabetic drug; SMBG = self-monitoring of blood glucose.
Implications of Nocturnal Hypoglycemia
Nocturnal hypoglycemia is more worrisome than daytime hypoglycemia because sympathoadrenal responses to hypoglycemia, subjective symptoms that provide warning, and cognitive function are suppressed during sleep. Stimuli for self-treatment are absent, thus, waking during an episode to ingest a snack is unlikely.[6] Mild or moderate hypoglycemic episodes that progress without intervention have the potential to result in more serious sequelae. Coma, seizures, serious injuries such as fractures, joint dislocations, cardiac arrhythmia, or death, although rare, have been reported.[6] Although disturbed sleep secondary to nocturnal hypoglycemia also affects vitality and mood,[6] its impact on neuropsychologic performance is less clear.[17]
Experimentally induced asymptomatic nocturnal hypoglycemia augments hypoglycemia unawareness, decreasing the threshold of BG that produces neuroglycopenic symptoms, counterregulatory hormone responses, and cognitive impairment during subsequent hypoglycemic episodes.[33] Although rare, permanent brain damage has been suspected to result from severe recurrent hypoglycemia[34]; however, a recent meta-analysis concluded otherwise.[35] Young children are particularly susceptible to severe and prolonged episodes of nocturnal hypoglycemia.[36]
Monitoring Nocturnal Hypoglycemia
Bedtime BG levels may predict nocturnal hypoglycemia,[23] thus, bedtime self-monitoring of BG (SMBG) is the simplest way for patients to predict and take measures to prevent nocturnal hypoglycemia. Noninvasive intermittent monitoring devices equipped with audible alarms that detect BG by iontophoresis are available. Questionable accuracy[22] and the lag time between glucose diffusion from blood to interstitial fluid limits the real-time utility of these devices.[23] Newer electronic devices are being developed to address these limitations. In high-risk patients, such as young children or individuals with frequent daytime hypoglycemia or hypoglycemia unawareness,[37] continuous glucose monitoring systems (CGMS) that transmit real-time BG information directly to the patient may be useful. A randomized clinical trial using a patient-inserted implantable CGMS found that hypo- and hyperglycemic excursions could be reduced 21% and 23%, respectively.[38] CGMS devices are being designed for use in “closed-loop” systems with CSII pumps and insulin adjustment algorithms.[39]
Treatment of Nocturnal Hypoglycemia
The key to treatment is early recognition. In an awake individual, mild-to-moderate nocturnal hypoglycemia is easily corrected by ingesting a snack containing approximately 5 g of fast-acting carbohydrate (eg, glucose-containing drink, cookies, sandwich, or glucose tablets), which should increase BG by approximately 15 mg/dL.[23] When treating an episode, foods high in fat should be avoided because they delay glucose absorption.
Glucagon, a counterregulatory hormone that stimulates hepatic gluconeogenesis and glycogenolysis, will raise BG levels rapidly if glycogen stores have not been depleted. Subcutaneous glucagon administration is recommended if the hypoglycemic episode is suspected to be severe (ie, if the patient is asleep and cannot be aroused).[40] Convenient glucagon kits with subcutaneous injection supplies are available. Nausea and vomiting are common side effects of glucagon, thus, prone positioning shortly after restoration of consciousness should be avoided because of the risk for aspiration. If response to glucagon is not rapid, emergency services should be called to administer intravenous glucose.
Prevention of Nocturnal Hypoglycemia
Patient behavior may influence whether nocturnal hypoglycemia will occur. Providing education about risks and risk-reduction strategies is critical in preventing nocturnal hypoglycemia. Patients should be encouraged to plan meals and exercise, adhere to dosing guidelines for diabetes therapy, moderate their alcohol intake, carefully and consistently perform SMBG, and be made aware that a simple change in routine (eg, change in time zone, holidays, vacation) may increase their risk for nocturnal hypoglycemia.
When NPH is used as the basal insulin, a bedtime snack may be necessary. A snack that is slowly absorbed and provides a steady supply of nighttime glycemic support may be more appropriate than fast-acting carbohydrates, whose glycemic effects tend to wane too early. Adjunctive agents that delay starch hydrolysis (alpha-glucosidase inhibitors) and slow intestinal glucose absorption also lower nocturnal hypoglycemia incidence when taken with the evening meal,[41] and may be appropriate in high-risk adults.
Therapeutic Insulin
Irrespective of time of day or sleep-wake status, insulin in excess of the physiologic requirement is a major iatrogenic cause of hypoglycemia.[3] When insulin replacement fails to mimic normal physiologic patterns of insulin secretion, causing a mismatch between nighttime insulin requirements and the peak action of the basal insulin replacement, nocturnal hypoglycemia results.[17,42,43]
Conventional Basal Insulin Formulations
Intermediate-acting NPH, Lente, or long-acting Ultralente insulins have traditionally been used to replace basal insulin. Even within the same patient, these formulations have highly variable and unpredictable absorption rates, onsets of action, and times to peak activity,[44] leading to questions about their therapeutic utility.[16,45] In fact, human Lente and Ultralente insulin are no longer available in the United States.[46]
Variations in crystal size and inadequate suspension of NPH insulin prior to subcutaneous injection interfere with precise dosing and contribute to substantial within-patient variability.[44,47] NPH action occurs within 1-2 hours of administration and its duration of action is 16-24 hours.[48] NPH activity has a distinct peak, and even when measured in healthy volunteers under controlled glucose-clamp conditions (0.3 U/kg), the peak occurred after 4.9 hours with a standard deviation of 3.1 hours.[49] Because the sleep interval represents the longest time between meals and SMBG, variability in peak activity of NPH increases nocturnal hypoglycemia risk. Inherent within-patient variability and unpredictability render NPH less than ideal for basal replacement.
Long-acting Insulin Analogs for Preventing Nocturnal Hypoglycemia
Insulin regimens are now available that provide equivalent or better levels of glycemic control and reduce nocturnal hypoglycemia risk. CSII is an excellent option for motivated patients. CSII technology has improved considerably since the 1970s,[50] and when used with rapid-acting insulin analogs, provides consistent and near-physiologic basal and prandial insulin coverage, lowering A1C levels with fewer severe nocturnal hypoglycemia episodes.[51] CSII also provides lifestyle flexibility, improved quality of life, and independence.[52] However, this therapy is not widely used in patients with T2DM, and for patients who choose not to use CSII or whose insulin needs do not require it, the long-acting insulin analogs offer a suitable alternative.
Long-acting insulin analogs were specifically developed to solve the issues of variable peaks in activity and unpredictable durations of action observed with traditional human insulin formulations. Insulin detemir and insulin glargine are the 2 currently available long-acting analogs. These agents possess the characteristics of an ideal basal insulin in that they nearly mimic basal physiologic insulin secretion, are slowly absorbed and distributed, and therefore have relatively flat time-action profiles and durations of action up to 24 hours.[7,53] In the case of insulin glargine, formation of slowly dissolving microprecipitates at the subcutaneous injection site accounts for its delayed absorption and 24-hour duration of action.[7] Insulin detemir remains soluble after subcutaneous injection yet also has a duration of action up to 24 hours.[54] Self-aggregation into hexamers and dihexamers and subsequent dissociation into dimers and monomers that bind reversibly to albumin account for the prolonged and flat time-action profile of insulin detemir relative to NPH insulin.[53] In contrast to the inherent variability and distinct peak of NPH insulin, the reproducible and relatively flat time-action profiles of these analogs reduce the likelihood of hyper- and hypoglycemic excursions.[7,53,55,56] Both long-acting analogs are suitable for once-daily dosing[7,53] and may be administered at dinner or bedtime. Twice-daily insulin detemir is also an option for patients if predinner BG levels are not at target.[53]
To address whether these newer agents are able to reduce nocturnal hypoglycemia, a comprehensive search of the literature for randomized clinical trials was performed. Trials including commercially available formulations of insulin detemir or insulin glargine were compared with NPH. Pertinent English-language clinical trials published between 2000 and 2006 were identified by searching PubMed, Adis, and the BIOSIS literature databases using the following search strategy: “(detemir OR glargine) AND nocturnal AND (hypoglycemia OR hypoglycaemia) AND diabetes.” To capture trials that may not have specified “nocturnal” in the title or abstract text but still reported nocturnal hypoglycemia data, a supplemental search of PubMed using “(detemir OR glargine) AND (nocturnal OR hypoglycemia OR hypoglycaemia) AND diabetes” was performed. Reviews from 2004 to 2006 were included and scanned to identify missing trials. A total of 307 publications (151 PubMed; 104 Adis; 52 BIOSIS) were evaluated and exclusions were made as follows: duplication (or abstracts since published in full), reviews older than 2004, letters, commentaries, case reports, simulations, cost or economic evaluations, subgroup analyses, patient-information summaries, trials involving CSII, nonparallel comparisons (eg, to a prestudy period), trials of fewer than 16 weeks' duration, comparisons to analog-containing premixes, studies conducted with non-commercially available analog formulations, or trials conducted with insulin preparations that are no longer commercially available.
Trials in patients with T1DM used NPH insulin as the primary “basal” comparator (Table 3).[57–72] Prandial insulin replacement, schedules of insulin administration, and insulin dosing varied from study to study. Most trials were not blinded because NPH is a cloudy suspension, whereas insulin detemir and insulin glargine are clear solutions. Because more patients with T2DM are now receiving insulin therapy, with or without OADs, relevant trials in insulin-treated patients with T2DM are also summarized (Table 4).[73–81]
Table 3.
Nocturnal Hypoglycemia and Glycemic Control in Clinical Trials Comparing Insulin Analogs vs Human Insulin (T1DM)
| Reference | Length | Treatments (Regimen) [N] | Nocturnal Hypoglycemia (P value)a | % A1Cb (P value) | |
|---|---|---|---|---|---|
| Fulcher[57] | 30 wks | Glargine (HS) + lispro (PP) [62] | 1.77c;d (.04) | 0.22c;e (.02) | −1.04 (<.01) −0.51 |
| NPH (HS) + lispro (PP) [63] | 2.30c;d | 0.37c;e | |||
| Home[58] | 28 wks | Glargine (HS) + RHI (PP) [292] | 61%f | +0.21 | |
| NPH (HS or AM+HS) + RHI (PP) [293] | 61%f | +0.10 | |||
| Raskin[59] | 16 wks | Glargine (HS) + lispro (PP) [310] | 69%f | 12.3%g | −0.1 |
| NPH (HS or AM+HS) + lispro (PP) [309] | 63%f | 12.0%g | −0.1 | ||
| Ratner[60] | 28 wks | Glargine (HS) + RHI (PP) [256] | 18.2%f (.0116) | 65.1h (<.05) | −0.16 −0.21 |
| NPH (HS or AM+HS) + RHI (PP) [262] | 27.1%f | 101.2h | |||
| Ashwell[62] | 32 wks (16 × 2 crossover) | Glargine (PM) + lispro (PP) [51] | 41%f, Mo 1 (.001) | 65%f, Mos 2-4 (.013) | 7.5i (<.001) |
| NPH (PM or AM+PM) + RHI (PP) [51] | 62%f, Mo 1 | 82%f, Mos 2–4 | 8.0i | ||
| Porcellati[63] | 1 year | Glargine (AD) + lispro (PP) [61] NPH 4 times daily + lispro (PP) [60] | 1.2j (<.05) 3.2j | −0.4 (<.05) 0.0 | |
| Hermansen[64] | 18 wks | Detemir (AM + HS) + aspart (PP) [298] | 4.0k | −0.50 (<.001) −0.28 | |
| NPH (AM+HS) + RHI (PP) [297] | (<.001) 9.2k | ||||
| Home[65] | 16 wks | Detemir (AM + HS) + aspart (PP) [139] | 34%f (.035) | −0.85 | |
| Detemir Q12 hours + aspart (PP) [137] | 44%f | (.002)l (3-way) | −0.82 −0.65 (.027)n | ||
| NPH (AM+HS) + aspart (PP) [132] | 50%f (<.001)m | ||||
| Russell-Jones[66] | 6 mos | Detemir (HS) + RHI (PP) [491] | 70.6%f | −0.06 | |
| NPH (HS) + RHI (PP) [256] | Dete(Detemir risk 26% lower than NPH; .003) | +0.06 | |||
| 72.9%f | |||||
| Pieber[67] | 16 wks | Detemir (AM + AD) + aspart (PP) [139] | 60%f | −0.43 | |
| Detemir (AM + HS) + aspart (PP) [132] | 51%f | −0.49 | |||
| NPH (AM+HS) + aspart (PP) [129] | 60%f | −0.39 | |||
| Kolendorf[68] | 32 wks (16 × 2 crossover) | Detemir (AM + HS) + aspart (PP) [125] | 6k (<.0001) | 3.4k;g (<.001) | −0.3 |
| NPH (AM+HS) + aspart (PP) [128] | 12k | 6.9k;g | −0.3 | ||
| Pieber[69] | 26 wks | Detemir (AM + AD) + aspart (PP) [161] | Detemir had 32% lower risk than glargine (<.05) | −0.6 | |
| Glargine (HS) + aspart (PP) [159] | −0.6 | ||||
| Pediatric Parallel-Group Comparative Trials | |||||
| Murphy[70] | 32 wks (16 × 2 crossover) Peds ages 12-18 | Glargine (HS) + lispro (PP) [25] | 8 of 25 nights (<.05) 14 of 25 nights | 8.7i 9.1i | |
| NPH (HS) + RHI (PP) [25] | |||||
| Schober[72] | 28 wks; Peds ages 5-16 | Glargine (HS) + RHI (PP) [174] | 12.6%f 17.7%f | +0.28 | |
| NPH (HS or AM+HS) + RHI (PP) [175] | +0.27 | ||||
| Robertson[71] | 26 wks; Peds aged 11.9 ± 2.8 yrs | Detemir (QD or BID) + aspart (PP) [232] | 4.5k (.011) | 8.0 at end | |
| NPH (QD or BID) + aspart (PP) [115] | 7.1k | 8.0 at end | |||
Analytic methods for event comparisons differ from trial to trial; P values are listed when comparison is significant
A1C values are change from baseline unless otherwise indicated
events per 100 patient-days
moderate
severe
patients with 1 or more episode
patients with episodes confirmed with blood glucose measurements
episodes per 100 patient-years
A1C value at end of treatment
episodes/patient/month
episodes/patient-year
ANOVA comparison of 3 treatment groups together
compared with AM + HS detemir
compared with pooled detemir groups.
T1DM = type 1 diabetes mellitus; N = number of patients; HS = at bedtime; PP = preprandial; RHI = regular human insulin; AD = dinnertime; M = moderate; S = severe; QD = once daily; BID = twice daily; RR = relative risk. Method of literature review and selection of trials included: Table 3 & Table 4 include data identified by searching PubMed, Adis, and BIOSIS literature databases. English-language clinical trials published between 2000 and 2006 using the following search strategy were identified: “(detemir OR glargine) AND nocturnal AND (hypoglycemia OR hypoglycaemia) AND diabetes.” Further, to capture trials that may not have specified “nocturnal” in the title or abstract text but still reported nocturnal hypoglycemia data, a supplemental search of PubMed using “(detemir OR glargine) AND (nocturnal OR hypoglycemia OR hypoglycaemia) AND diabetes” was performed. Reviews from 2004 to 2006 were included and scanned to identify missing trials. Publications (n = 307: 151 PubMed; 104 Adis; 52 BIOSIS) were evaluated and exclusions were made as follows: duplication (or abstracts since published in full), review older than 2004, letters, commentaries, case reports, simulations, cost or economic evaluations, subgroup analyses, patient-information summaries, trials involving CSII, nonparallel comparisons (eg, to a prestudy period), trials <16 weeks' duration, comparisons to analog-containing premixes, conducted with noncommercially available analog formulations, or studies conducted with insulin preparations that are no longer commercially available.
Table 4.
Nocturnal Hypoglycemia and Glycemic Control in Clinical Trials Comparing Insulin Analogs With a Human Insulin-Containing Comparator Regimen in Adults With T2DM
| Reference | Length | Treatments (Regimen) [N] | Nocturnal Hypoglycemia (P value)a | % A1Cb (P value) | |
|---|---|---|---|---|---|
| Riddle[73] | 24 wks | Glargine (HS) + OADs (various) [367] | 4.0c (<.001) 6.9c | 1.3c;d (<.002) | −1.65 −1.59 |
| NPH (HS) + OADs (various) [389] | 2.5c;d | ||||
| Fritsche[74] | 24 wks | Glargine (HS) + glimepiride (QD) [229] | 23%e 17%e | −0.96 (.008)f −1.24 −0.84 | |
| Glargine (AM) + glimepiride (QD) [237] | 38%e | (<.001)g | (.0002)g | ||
| NPH (HS) + glimepiride [232] | (3-way) | (3-way) | |||
| Massi Benedetti[75] | 1 year | Glargine (HS) + OADs (various) [289] | 12%e | −0.46 | |
| NPH (HS) + OADs (various) [281] | (.002)24%e | −0.38 | |||
| Janka[76] | 24 wks | Glargine (AM) + OADs (glimepiride [QD]+ metformin [APT]) [177] | 0.51h (.0449) | −1.64 | |
| Human premix 70/30 (AM + AD) [187] | 1.04h | (.003) −1.31 | |||
| Yki-Jarvinen[77] | 9 mos | Glargine (HS) + metformin (APT)[61] NPH (HS) + metformin (APT) [49] | 43%i (.08) 59%i | −1.99 −2.10 | |
| Rosenstock[78] | 28 wks | Glargine (QD) + RHI (PP) [259] | 26.5%e (.0136) | −0.41 −0.59 | |
| NPH (QD or BID) + RHI (PP) [259] | 35.5%e | ||||
| Raslova[79] | 22 wks | Detemir (QD or BID) + aspart (PP) [195] NPH (QD or BID) + RHI (PP) [200] | 14.9%e 17.5%e | −0.65 −0.58 | |
| Haak[80] | 26 wks | Detemir (QD or BID) + aspart (PP) [341] NPH (QD or BID) + aspart (PP) [164] | 15.8%e 23.6%e | −0.2 −0.4 | |
| Hermansen[81] | 24 wks | Detemir (AM + PM) + OADs (APT) [237] | Detemir had 55% lower risk than NP (<.001) | −1.8 −1.9 | |
| NPH (AM + PM) + OADs (APT) [238] | |||||
Analytic methods for event comparisons differ from trial to trial; P values are listed when comparison is significant
A1C values are change from baseline unless otherwise indicated
events per patient-year
patients with major nocturnal hypoglycemia episodes confirmed with blood glucose measurements ≤ 3.1 mmol/L
patients with 1 or more episode
insulin glargine AM vs insulin glargine HS
compared with both analog-containing treatment groups
episodes per patient-year
symptomatic episodes of which 98% (glargine) and 93% (NPH) were confirmed nocturnal in study weeks 1–12
insulin glargine AM vs NPH.
T2DM = type 2 diabetes mellitus; OAD = oral antidiabetic drug; N = number of patients; HS = at bedtime; APT = as prior therapy; RHI = regular human insulin; AD = dinnertime; M = moderate; S = severe; QD = once daily; BID = twice daily.
Patients With T1DM
The safety benefit of a long-acting insulin analog with regard to nocturnal hypoglycemia was first described by Pieber and colleagues.[82] Over a 4-week treatment period, nocturnal hypoglycemia risk was 19% lower (P = .0037) with insulin glargine (preliminarily known as HOE 901) than with NPH insulin once daily but was not compared with NPH insulin twice daily. However, because effects on nocturnal hypoglycemia are harder to detect in trials of short duration, the remainder of this review focuses on trials lasting 16 weeks or longer.
Insulin Glargine Trials in Patients With T1DM
Four randomized trials (1863 patients in total) of up to 30 weeks' duration compared the effects of insulin glargine at bedtime with NPH insulin administered either at bedtime[57] or morning plus bedtime.[58–60] In 2 of the larger trials,[58,59] the basal insulin was supplemented with prandial regular human insulin (RHI) or insulin lispro, respectively. Both trials reported no change in A1C from baseline and similar rates of nocturnal hypoglycemia with NPH and glargine. Two other trials,[57,60] however, found better or comparable reductions in A1C, respectively, as well as significantly less nocturnal hypoglycemia in patients treated with insulin glargine than with NPH. The benefit of the basal insulin was seen regardless of the prandial insulin used (RHI or lispro).
One 32-week crossover trial compared bedtime insulin glargine plus mealtime insulin lispro to twice-daily NPH plus RHI.[62] The insulin glargine-lispro combination provided better glycemic control in terms of A1C by 0.5% compared with the NPH-RHI combination (P < .001).[62] Nocturnal hypoglycemia occurred less frequently with insulin glargine/lispro (41% to 65% of patients had at least 1 episode) than with NPH/RHI (62% to 82%) during month 1 (P = .001) and months 2-4 (P = .013) of the study, respectively. Although this trial was performed in a limited number of patients and was of relatively short duration, its results suggest that pairing a basal analog with a prandial analog may optimize the benefit of reducing nocturnal hypoglycemia over an all-human insulin-containing regimen.
A year-long trial comparing insulin glargine at dinnertime vs intensive 4-times-daily NPH (each with insulin lispro at meals) reported a larger reduction in A1C with insulin glargine/lispro (0.4% difference; P < .05) and fewer nocturnal hypoglycemia episodes per patient-month than with NPH/lispro (1.2 vs 3.2; P < .05).[63] Thus, most trials with insulin glargine showed similar glycemic control and similar or lower rates of nocturnal hypoglycemia as compared with NPH insulin.
Insulin Detemir Trials in Patients With T1DM
Insulin detemir was also the focus of 4 large comparative clinical efficacy trials (2150 total T1DM patients) reporting nocturnal hypoglycemia data.[64–67] In 3 trials (16-18 weeks' duration) insulin detemir was administered AM/bedtime,[64,65,67] AM/dinner,[67] or every 12 hours,[65] with insulin aspart at mealtimes. Comparisons were made to NPH (AM/bedtime) with mealtime RHI[64] or insulin aspart.[65,67] Glycemic control observed with insulin detemir was superior to NPH in 2 trials by 0.22% (P < .001) and 0.18% (P = .027).[64,65] The difference in nocturnal hypoglycemia incidence was most evident in the trial by Hermansen and coworkers,[64] which compared an all-analog vs an all-human insulin-containing regimen. Moreover, insulin detemir administered AM/bedtime resulted in a 53%[65] to 55%[64] lower risk for nocturnal hypoglycemia compared with NPH (P < .001). The risk for nocturnal hypoglycemia with every-12-hour insulin detemir was 26% lower than with NPH.[65] In another study,[67] nocturnal hypoglycemia incidence was similar using AM/dinner or AM/bedtime insulin detemir, despite providing significantly better A1C control over NPH. A fourth large trial compared daily bedtime insulin detemir with NPH over 6 months. With both groups using RHI as the mealtime insulin, glycemic control was similar; however, nocturnal hypoglycemia risk was 26% lower in patients treated with insulin detemir (P = .003).[66]
A 32-week, 2-way, crossover study of 130 patients[68]compared insulin detemir (AM/bedtime) with NPH (AM/bedtime), both using mealtime insulin aspart. Effects on glycemic control, hypoglycemia incidence, and variability of in-home SMBG levels were measured. Although mean reductions in A1C (0.3%) were comparable across treatments, the relative risk of experiencing nocturnal hypoglycemia was 50% lower in patients treated with insulin detemir. Less intrapatient variability in BG was observed in most trials comparing insulin detemir with NPH (eg, there was less variability in BG before breakfast[65]or dinner,[64] as well as less fluctuation of BG over 24 hours).[66] The lower variability with insulin detemir and consistent BG response potentially explains the generally lower nocturnal hypoglycemia risk compared with NPH at similar or better glycemic control. Additionally, compared with NPH, the favorable or neutral effects on weight gain in most trials with insulin detemir[8,64,66,67,81] and in one trial with insulin glargine[78] might result from more predictable time-action profiles and less need for prophylactic bedtime snacking.
Consistent with data suggesting that there is less within-patient variability of insulin detemir compared with both insulin glargine and NPH,[55] results from a randomized trial comparing insulin detemir (AM/bedtime) vs insulin glargine (bedtime) reported that severe and nocturnal hypoglycemia were lower by 72% and 32%, respectively, in insulin detemir-treated patients.[69] With insulin aspart used as the prandial insulin in both treatment arms, overall hypoglycemic episodes and glycemic control achieved were comparable, with A1C changes from baseline of −0.6% in both groups after 26 weeks.
Most clinical trials evaluating insulin detemir in T1DM patients reported comparable or better glycemic control with less incidence of nocturnal hypoglycemia than with NPH as the basal insulin.[64-66,68] In one trial, insulin detemir[69] demonstrated less nocturnal hypoglycemia compared with insulin glargine at equivalent glycemic control. When insulin detemir is paired with a rapid-acting prandial insulin analog, the beneficial effects on reducing nocturnal hypoglycemia appear more prominent.
Long-acting Insulin Analogs and Nocturnal Hypoglycemia in Children With T1DM
Studies in children and adolescents have often employed CSII as an alternative to multiple daily injections with successful control over glycemia, including less severe hypoglycemia and nocturnal hypoglycemia.[50] Two randomized trials with insulin glargine and one with insulin detemir were identified in younger patients.[70–72] One was a randomized, 32-week, crossover trial[70] comparing bedtime insulin glargine/lispro with NPH/RHI in 28 adolescents. A1C levels were comparable between treatments but nocturnal hypoglycemia incidence was 42% lower in the glargine/lispro group (P < .05; χ2). Another 6-month study of children and adolescents found that bedtime insulin glargine provided significantly better glycemic control with less severe nocturnal hypoglycemia than once- or twice-daily NPH.[72] Finally, a 26-week trial comparing insulin detemir/aspart to NPH/aspart in children and adolescents[71] found a 0.8% decline in A1C in both arms and a 36% lower nocturnal hypoglycemia risk in the insulin detemir group (P = .011). Thus, as in adults with T1DM, children treated with insulin glargine or insulin detemir (especially when paired with a prandial insulin analog) can expect to have fewer episodes of nocturnal hypoglycemia while maintaining as good or better glycemic control as with NPH insulin.
Patients With T2DM
In T2DM patients, insulin therapy is often initiated by adding a basal insulin to an OAD regimen. Therefore, most trials in patients with T2DM comparing the long-acting insulin analogs with traditional human insulin include therapy with OADs (Table 4). Few studies in T2DM compare basal-bolus regimens using multiple daily injections,[78–80] and of those, all but one[79] controlled for the prandial insulin. Thus, there is limited information about the relative risk for nocturnal hypoglycemia in all-analog vs all-human insulin-containing regimens in patients with T2DM.
Insulin Glargine Trials of Patients With T2DM
In treat-to-target trials, patients are generally instructed to use simple dosing algorithms to achieve a target fasting plasma glucose (FPG) level. In one such trial,[73] overweight patients taking OADs were randomly allocated to add insulin glargine or NPH as a basal insulin and instructed to titrate their insulin doses to an FPG < 100 mg/dL. Although A1C declined significantly and comparably in both groups, nocturnal hypoglycemia episodes were 42% less frequent in insulin glargine-treated patients compared with NPH-treated patients (4.0 vs 6.9 events per patient-year, respectively; P < .001). Overall hypoglycemia was also reduced in patients receiving insulin glargine.
In patients receiving glimepiride plus either AM or bedtime insulin glargine or bedtime NPH insulin, nocturnal hypoglycemia risk was lowest in the group randomly assigned to AM insulin glargine and highest in the bedtime NPH group.[74] Moreover, AM insulin glargine provided better glycemic control than bedtime insulin glargine or NPH. In patients continuing on OADs (which previously had been used alone or were combined with insulin therapy), treatment for 1 year with either once-daily insulin glargine or NPH reduced A1C equally, but twice as many patients on NPH experienced nocturnal hypoglycemia (overall population = 578; P = .002).[75] Similar results were observed in the insulin-naive subset of patients entering this trial (n = 426).[83] Another 24-week study comparing AM insulin glargine plus OADs to premixed human insulin 70/30 twice daily (no OADs) in insulin-naive patients found that insulin glargine/OADs provided better glycemic control (P = .0003) with fewer nocturnal hypoglycemia episodes (0.51 vs 1.04 per patient-year; P = .0449) than did premixed human insulin 70/30.[76] Overall hypoglycemic episodes were also much lower (P < .0001) in the insulin glargine-OAD group (4.07 vs 9.87 per patient-year). A recent study[77] compared insulin glargine/metformin with NPH/metformin in patients with poorly controlled T2DM. Patients with hypoglycemic symptoms were asked to confirm the episode with SMBG. After 9 months of treatment, both groups had significant reductions in A1C (-1.99% and −2.10%). The rates of confirmed hypoglycemia were 5.0 vs 7.7 episodes per patient-year in insulin glargine- and NPH-treated patients, respectively, of which 98% and 93% of episodes were nocturnal. Finally, in patients with T2DM who had completely transitioned to insulin therapy (ie, not on OADs), once-daily insulin glargine and once-daily or twice-daily NPH provided comparable reductions in A1C with 25% less nocturnal hypoglycemia in the insulin glargine-treated patients (P = .0136).[78] Thus, administration of insulin glargine to patients with T2DM generally reduces the incidence of nocturnal hypoglycemia and provides similar glycemic control compared with conventional human or premixed human insulin formulations, and does so whether patients are on insulin alone or whether they remain on OADs.
Insulin Detemir Trials in Patients With T2DM
Because insulin detemir is a newer therapeutic agent than insulin glargine, fewer clinical studies have been completed to date in patients with T2DM. However, available data on the incidence of nocturnal hypoglycemia in patients treated with insulin detemir are promising. Nocturnal hypoglycemia occurred 38% and 8% less frequently despite providing similar glycemic control in studies comparing insulin detemir plus mealtime insulin aspart vs NPH/RHI[79] or NPH/aspart,[80] respectively. Hermansen and colleagues[81] recently completed a large (N = 475) trial comparing insulin detemir with NPH, both in combination with OADs. After 24 weeks, significant, comparable decreases in A1C were seen in both arms (-1.8% and −1.9%, respectively), and overall and nocturnal hypoglycemia were 47% and 55% lower, respectively, in insulin detemir vs NPH groups (P < .001) even though A1C was decreased to approximately 7%. Additional studies with insulin detemir in T1DM and T2DM patients are ongoing, and because of this insulin's predictable time-action profile, these studies are expected to yield similar favorable results.
With few exceptions, trials in patients with either T1DM or T2DM have demonstrated that at glycemic control comparable to that provided by NPH, the risk for nocturnal hypoglycemia is lower with long-acting insulin analogs. Two meta-analyses provide an excellent overall perspective on these trends. In one analysis of 4 insulin glargine trials (N = 2304 T2DM patients)[84] confirmed episodes of nocturnal hypoglycemia were 29% fewer than in NPH-treated patients, with equivalent reductions in A1C (1%) from baseline. Similarly, using data from phase 3 and phase 4 trials with insulin glargine to perform a meta-regression analysis (N = 1786 T2DM patients), the analog was predicted to lower A1C by 0.87% compared with NPH at an equivalent incidence of nocturnal hypoglycemia.[85]
Conclusion
Intensive therapy should achieve glycemic control while avoiding severe and nocturnal hypoglycemia as much as possible. Physiologic, behavioral, and pharmacologic factors contribute to nocturnal hypoglycemia risk but the latter 2 factors are modifiable. Frequent episodes of nocturnal hypoglycemia can exacerbate hypoglycemia unawareness and lead to a dangerous cycle in which symptoms may not be apparent until BG levels are dangerously low. Judicious choice of pharmacotherapy with an insulin regimen that offers more physiologic and predictable time-action profiles than traditional human basal insulin can lower nocturnal hypoglycemia risk without compromising glycemic control. Can long-acting insulin analogs help overcome the challenge of nocturnal hypoglycemia? On the basis of this review of clinical trial results, insulin detemir or insulin glargine may be the best option to provide basal insulin coverage in patients who do not choose or require CSII.
Figure 1.
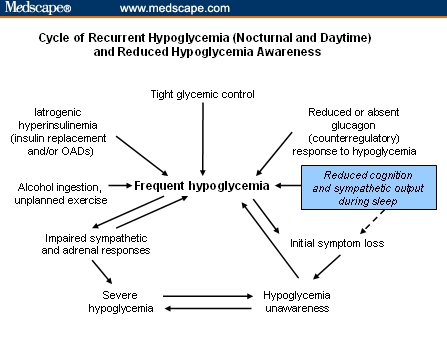
The cycle of frequent hypoglycemia, illustrating the impact of impaired counterregulation that exists in diabetes. Sleep, with reduced cognition and sympathetic output, and other behavioral factors contribute. Frequent hypoglycemic episodes, whether daytime or nighttime, can lead to impaired sympathoadrenal responses, symptom loss, and hypoglycemia unawareness, and eventually, may lead to severe hypoglycemia. Figure adapted from Fanelli et al.[16] Copyright John Wiley & Sons Limited. Reproduced with permission.
Acknowledgments
I wish to thank Kathryn J. Lucchesi, PhD, RPh, for her editorial assistance in the preparation of this manuscript. Kathryn has disclosed no relevant financial relationships.
Funding Information
The author acknowledges Novo Nordisk for funding that helped support the preparation of this manuscript.
Footnotes
Readers are encouraged to respond to the author at Sbrunton@pceconsortium.org or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
References
- 1.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4–S36. [PubMed] [Google Scholar]
- 2.American Association of Clinical Endocrinologists. The American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management–2002 update. Endocr Pract. 2002;8(suppl 1):40–82. [PubMed] [Google Scholar]
- 3.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 4.Nordfeldt S, Ludvigsson J. Fear and other disturbances of severe hypoglycaemia in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:83–91. doi: 10.1515/jpem.2005.18.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Matyka KA. Nocturnal hypoglycaemia in children: the effects on cognitive function. Diabetes Nutr Metab. 2002;15:390–394. [PubMed] [Google Scholar]
- 6.Allen KV, Frier BM. Nocturnal hypoglycemia: clinical manifestations and therapeutic strategies toward prevention. Endocr Pract. 2003;9:530–543. doi: 10.4158/EP.9.6.530. [DOI] [PubMed] [Google Scholar]
- 7.Dunn CJ, Plosker GL, Keating GM, McKeage K, Scott LJ. Insulin glargine: an updated review of its use in the management of diabetes mellitus. Drugs. 2003;63:1743–1778. doi: 10.2165/00003495-200363160-00007. [DOI] [PubMed] [Google Scholar]
- 8.Home P, Kurtzhals P. Insulin detemir: from concept to clinical experience. Expert Opin Pharmacother. 2006;7:325–343. doi: 10.1517/14656566.7.3.325. [DOI] [PubMed] [Google Scholar]
- 9.Braunstein SN, White JR. Trends in the management of type 2 diabetes: an emerging role for insulin. J Managed Care Pharm. 2005;11:S2–S11. doi: 10.18553/jmcp.2005.11.s1-b.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo PJ. The case for insulin treatment early in type 2 diabetes. Cleve Clin J Med. 2004;71:385–392. 394. doi: 10.3949/ccjm.71.5.385. [DOI] [PubMed] [Google Scholar]
- 11.Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 12.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 13.U.K. Prospective Diabetes Study Group. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 14.Piche M-E, Arcand-Bosse J-F, Despres J-P, et al. What is a normal glucose value? Differences in indexes of plasma glucose homeostasis in subjects with normal fasting glucose. Diabetes Care. 2004;27:2470–2477. doi: 10.2337/diacare.27.10.2470. [DOI] [PubMed] [Google Scholar]
- 15.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 16.Fanelli CG, Porcellati F, Pampanelli S, Bolli GB. Insulin therapy and hypoglycaemia: the size of the problem. Diabetes Metab Res Rev. 2004;20:S32–S42. doi: 10.1002/dmrr.514. [DOI] [PubMed] [Google Scholar]
- 17.Matyka KA. Sweet dreams?–nocturnal hypoglycemia in children with type 1 diabetes. Pediatric Diabetes. 2002;3:74–81. doi: 10.1034/j.1399-5448.2002.30203.x. [DOI] [PubMed] [Google Scholar]
- 18.DCCT Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med. 1991;90:450–459. [PubMed] [Google Scholar]
- 19.Yki-Jarvinen H. Insulin therapy in type 2 diabetes: role of the long-acting insulin glargine analogue. Eur J Clin Invest. 2004;34:410–416. doi: 10.1111/j.1365-2362.2004.01356.x. [DOI] [PubMed] [Google Scholar]
- 20.Davis RE, Morrissey M, Peters JR, et al. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. 2005;21:1477–1483. doi: 10.1185/030079905X61929. [DOI] [PubMed] [Google Scholar]
- 21.Malone JK, Beattie SD, Campaigne BN, et al. Therapy after single oral agent failure: adding a second oral agent or an insulin mixture? Diabetes Res Clin Pract. 2003;62:187–195. doi: 10.1016/j.diabres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Devries JH, Wentholt IM, Masurel N, et al. Nocturnal hypoglycaemia in type 1 diabetes-consequences and assessment. Diabetes Metab Res Rev. 2004;20(suppl 2):S43–S46. doi: 10.1002/dmrr.513. [DOI] [PubMed] [Google Scholar]
- 23.Gabriely I, Shamoon H. Hypoglycemia in diabetes: common, often unrecognized. Cleve Clin J Med. 2004;71:335–342. doi: 10.3949/ccjm.71.4.335. [DOI] [PubMed] [Google Scholar]
- 24.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52:1195–1203. doi: 10.2337/diabetes.52.5.1195. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman FR, Austin J, Neinstein A, et al. Nocturnal hypoglycemia detected with the Continuous Glucose Monitoring System in pediatric patients with type 1 diabetes. J Pediatr. 2002;141:625–630. doi: 10.1067/mpd.2002.129175. [DOI] [PubMed] [Google Scholar]
- 26.Gertzman J, White B, Streja D. Severity of hypoglycemia and hypoglycemia unawareness are associated with the extent of unsuspected nocturnal hypoglycemia. Diabetes. 2005;52(suppl 1):A146. [Google Scholar]
- 27.Bell SJ, Forse RA. Nutritional management of hypoglycemia. Diabetes Educ. 1999;25:41–47. doi: 10.1177/014572179902500106. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald MJ. Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care. 1987;10:584–588. doi: 10.2337/diacare.10.5.584. [DOI] [PubMed] [Google Scholar]
- 29.Galassetti P, Mann S, Tate D, et al. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab. 2001;280:E908–E917. doi: 10.1152/ajpendo.2001.280.6.E908. [DOI] [PubMed] [Google Scholar]
- 30.Kerr D, Macdonald IA, Heller SR, Tattersall RB. Alcohol causes hypoglycaemic unawareness in healthy volunteers and patients with type 1 (insulin-dependent) diabetes. Diabetologia. 1990;33:216–221. doi: 10.1007/BF00404799. [DOI] [PubMed] [Google Scholar]
- 31.Kolaczynski JW, Caro JF. Insulin resistance: site of the primary defect or how the current and the emerging therapies work. J Basic Clin Physiol Pharmacol. 1998;9:281–294. doi: 10.1515/jbcpp.1998.9.2-4.281. [DOI] [PubMed] [Google Scholar]
- 32.Turner BC, Jenkins E, Kerr D, Sherwin RS, Cavan DA. The effect of evening alcohol consumption on next-morning glucose control in type 1 diabetes. Diabetes Care. 2001;24:1888–1893. doi: 10.2337/diacare.24.11.1888. [DOI] [PubMed] [Google Scholar]
- 33.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes. 1993;42:1233–1237. doi: 10.2337/diab.42.9.1233. [DOI] [PubMed] [Google Scholar]
- 34.Perros P, Deary IJ. Long-term effects of hypoglycaemia on cognitive function and the brain in diabetes. In: Frier BM, Fisher BM, editors. Hypoglycaemia in Clinical Diabetes. New York: John Wiley & Sons, Ltd; 1999. pp. 187–210. [Google Scholar]
- 35.Brands AMA, Biessels GJ, de Haan EHF, Kappelle LJ, Kessels RPC. Effects of type 1 diabetes on cognitive performance. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 36.Matyka K, Ford-Adams M, Dunger DB. Hypoglycaemia and counterregulation during childhood. Horm Res. 2002;57(suppl 1):85–90. doi: 10.1159/000053322. [DOI] [PubMed] [Google Scholar]
- 37.Boland E, Monsod T, Delucia M, et al. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 38.Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 39.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23:1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 40.Carroll MF, Burge MR, Schade DS. Severe hypoglycemia in adults. Rev Endocr Metab Disord. 2003;4:149–157. doi: 10.1023/a:1022990003161. [DOI] [PubMed] [Google Scholar]
- 41.Taira M, Takasu N, Komiya I, Taira T, Tanaka H. Voglibose administration before the evening meal improves nocturnal hypoglycemia in insulin-dependent diabetic patients with intensive insulin therapy. Metabolism. 2000;49:440–443. doi: 10.1016/s0026-0495(00)80005-0. [DOI] [PubMed] [Google Scholar]
- 42.Yale JF. Nocturnal hypoglycemia in patients with insulin-treated diabetes. Diabetes Res Clin Pract. 2004;65(suppl 1):S41–S46. doi: 10.1016/j.diabres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Bolli GB, Perriello G, Fanelli CG, De Feo P. Nocturnal blood glucose control in type I diabetes mellitus. Diabetes Care. 1993;16(suppl 3):71–89. doi: 10.2337/diacare.16.3.71. [DOI] [PubMed] [Google Scholar]
- 44.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4:673–682. doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- 45.Lindstrom T, Olsson PO, Arnqvist HJ. The use of human ultralente is limited by great intraindividual variability in overnight plasma insulin profiles. Scand J Clin Lab Invest. 2000;60:341–347. doi: 10.1080/003655100750019242. [DOI] [PubMed] [Google Scholar]
- 46.Eli Lilly and Company. Re: Discontinuation of Humulin [R]U ULTRALENTE [R] (human insulin [rDNA origin] extended zinc suspension) and Humulin [R]L LENTE [R] (human insulin [rDNA origin] zinc suspension) [Dear Doctor Letter] 2005 Available at: www.fda.gov/CDER/drug/shortages/HumulinDr.pdf Accessed December 3, 2006.
- 47.Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagedorn (NPH) insulin in pens. Lancet. 1999;354:1604–1607. doi: 10.1016/S0140-6736(98)12459-5. [DOI] [PubMed] [Google Scholar]
- 48.Oiknine R, Bernbaum M, Mooradian AD. A critical appraisal of the role of insulin analogues in the management of diabetes mellitus. Drugs. 2005;65:325–340. doi: 10.2165/00003495-200565030-00003. [DOI] [PubMed] [Google Scholar]
- 49.Heinemann L, Sinha K, Weyer C, et al. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet Med. 1999;16:332–338. doi: 10.1046/j.1464-5491.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- 50.Weinzimer SA, Doyle EA, Tamborlane WV. Disease management in the young diabetic patient: glucose monitoring, coping skills, and treatment strategies. Clin Pediatr. 2005;44:393–403. doi: 10.1177/000992280504400503. [DOI] [PubMed] [Google Scholar]
- 51.Weinzimer SA, Doyle EA, Steffen AT, Sikes KA, Tamborlane WV. Rediscovery of insulin pump treatment of childhood type 1 diabetes. Minerva Med. 2004;95:85–92. [PubMed] [Google Scholar]
- 52.Doyle Boland EA, Steffen AT, Tamborlane WV. Case study: contrasting challenges of insulin pump therapy in a toddler and adolescent with type 1 diabetes. Diabetes Educ. 2005;31:584–590. doi: 10.1177/0145721705278888. [DOI] [PubMed] [Google Scholar]
- 53.Goldman-Levine JD, Lee KW. Insulin detemir–a new basal insulin analog. Ann Pharmacother. 2005;39:502–507. doi: 10.1345/aph.1E334. [DOI] [PubMed] [Google Scholar]
- 54.Chapman TM, Perry CM. Insulin detemir: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs. 2004;64:2577–2595. doi: 10.2165/00003495-200464220-00008. [DOI] [PubMed] [Google Scholar]
- 55.Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–1620. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 56.Scholtz HE, Pretorius SG, Wessels DH, Becker RH. Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia. 2005;48:1988–1995. doi: 10.1007/s00125-005-1916-y. [DOI] [PubMed] [Google Scholar]
- 57.Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Intern Med J. 2005;35:536–542. doi: 10.1111/j.1445-5994.2005.00902.x. [DOI] [PubMed] [Google Scholar]
- 58.Home PD, Rosskamp R, Forjanic-Klapproth J, Dressler A. A randomized multicentre trial of insulin glargine compared with NPH insulin in people with type 1 diabetes. Diabetes Metab Res Rev. 2005;21:545–553. doi: 10.1002/dmrr.572. [DOI] [PubMed] [Google Scholar]
- 59.Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care. 2000;23:1666–1671. doi: 10.2337/diacare.23.11.1666. [DOI] [PubMed] [Google Scholar]
- 60.Ratner RE, Hirsch IB, Neifing JL, et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. Diabetes Care. 2000;23:639–643. doi: 10.2337/diacare.23.5.639. [DOI] [PubMed] [Google Scholar]
- 61.Kudva YC, Basu A, Jenkins GD, et al. Randomized controlled clinical trial of glargine versus ultralente insulin in the treatment of type 1 diabetes. Diabetes Care. 2005;28:10–14. doi: 10.2337/diacare.28.1.10. [DOI] [PubMed] [Google Scholar]
- 62.Ashwell S, Amiel S, Bilous R, et al. Improvement in HbA1c with insulin glargine + insulin lispro in comparison with NPH insulin+unmodified human insulin in people with type 1 diabetes. Diabetes. 2003;52(suppl 1):A442. [Google Scholar]
- 63.Porcellati F, Rossetti P, Pampanelli S, et al. Better long-term glycaemic control with the basal insulin glargine as compared with NPH in patients with type 1 diabetes mellitus given meal-time lispro insulin. Diabet Med. 2004;21:1213–1220. doi: 10.1111/j.1464-5491.2004.01323.x. [DOI] [PubMed] [Google Scholar]
- 64.Hermansen K, Fontaine P, Kukolja KK, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia. 2004;47:622–629. doi: 10.1007/s00125-004-1365-z. [DOI] [PubMed] [Google Scholar]
- 65.Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care. 2004;27:1081–1087. doi: 10.2337/diacare.27.5.1081. [DOI] [PubMed] [Google Scholar]
- 66.Russell-Jones D, Simpson R, Hylleberg B, Draeger E, Bolinder J. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther. 2004;26:724–736. doi: 10.1016/s0149-2918(04)90072-0. [DOI] [PubMed] [Google Scholar]
- 67.Pieber TR, Draeger E, Kristensen A, Grill V. Comparison of three multiple injection regimens for type 1 diabetes: morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabet Med. 2005;22:850–857. doi: 10.1111/j.1464-5491.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- 68.Kolendorf K, Ross GP, Pavlic-Renart I, et al. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in type 1 diabetes. Diabet Med. 2006;23:729–735. doi: 10.1111/j.1464-5491.2006.01862.x. [DOI] [PubMed] [Google Scholar]
- 69.Pieber TR, Treichel HC, Hompesch B, et al. Comparison of insulin detemir and insulin glargine in subjects with Type 1 diabetes using intensive insulin therapy. Diabet Med. 2007 doi: 10.1111/j.1464-5491.2007.02113.x. Mar 22; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Murphy NP, Keane SM, Ong KK, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care. 2003;26:799–804. doi: 10.2337/diacare.26.3.799. [DOI] [PubMed] [Google Scholar]
- 71.Robertson KJ, Schoenle E, Gucev Z, et al. Insulin detemir compared with NPH insulin in children and adolescents with type 1 diabetes. Diabet Med. 2007;24:27–34. doi: 10.1111/j.1464-5491.2007.02024.x. [DOI] [PubMed] [Google Scholar]
- 72.Schober E, Schoenle E, Van DJ, Wernicke-Panten K. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15:369–376. doi: 10.1515/jpem.2002.15.4.369. [DOI] [PubMed] [Google Scholar]
- 73.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 74.Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952–959. doi: 10.7326/0003-4819-138-12-200306170-00006. [DOI] [PubMed] [Google Scholar]
- 75.Massi Benedetti M, Humburg E, Dressler A, Ziemen M. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Horm Metab Res. 2003;35:189–196. doi: 10.1055/s-2003-39080. [DOI] [PubMed] [Google Scholar]
- 76.Janka HU, Plewe G, Riddle MC, et al. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28:254–259. doi: 10.2337/diacare.28.2.254. [DOI] [PubMed] [Google Scholar]
- 77.Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442–451. doi: 10.1007/s00125-005-0132-0. [DOI] [PubMed] [Google Scholar]
- 78.Rosenstock J, Schwartz SL, Clark CM, Jr., et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- 79.Raslova K, Bogoev M, Raz I, et al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. [Correction appears in Raslova K, et al. Diabetes Res Clin Pract. 2006;72:112] Diabetes Res Clin Pract. 2004;66:193–201. doi: 10.1016/j.diabres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Haak T, Tiengo A, Draeger E, Suntum M, Waldhausl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56–64. doi: 10.1111/j.1463-1326.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 81.Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 82.Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care. 2000;23:157–162. doi: 10.2337/diacare.23.2.157. [DOI] [PubMed] [Google Scholar]
- 83.Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000;23:1130–1136. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 84.Rosenstock J, Dailey G, Massi-Benedetti M, et al. Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care. 2005;28:950–955. doi: 10.2337/diacare.28.4.950. [DOI] [PubMed] [Google Scholar]
- 85.Yki-Jarvinen H. The relationship between glycemic control and hypoglycemia using insulin glargine versus NPH insulin: A meta-regression analysis in type 2 diabetes. Diabetes. 2003;52:A149–A150. [Google Scholar]


