Abstract
Objective
To describe return to normal function, productivity, and satisfaction of patients with moderate or severe migraine attacks treated with combined sumatriptan/naproxen sodium, sumatriptan alone, naproxen sodium alone, or placebo.
Patients, design, and setting
Patients in 2 identical, US, phase 3, randomized, double-blind, parallel-group, placebo-controlled, single-dose, multicenter studies treated a single moderate or severe migraine attack with sumatriptan/naproxen sodium (85 mg sumatriptan formulated with RT Technology and 500 mg naproxen sodium in a single-tablet formulation), sumatriptan, naproxen sodium, or placebo.
Main outcome measures
Ability to function (not impaired, mildly impaired, severely impaired, or required bed rest) was collected in diary cards completed immediately prior to treatment, every 30 minutes for the first 2 hours, and hourly from 2 to 24 hours while awake. Patients completed the Productivity Assessment Questionnaire (PAQ) 24 hours after study drug administration. The Patient Perception of Migraine Questionnaire (PPMQ) was administered at screening and 24 hours post treatment to capture patient satisfaction.
Results
Compared with the other groups, the sumatriptan/naproxen sodium group reported significantly higher levels of normal or mildly impaired functioning as early as 2 and 4 hours after dosing. They also demonstrated greater reductions in workplace productivity loss compared with placebo in both studies, and were consistently more satisfied with their treatment compared with patients in other treatment groups and compared with their usual medications.
Conclusions
Treatment with sumatriptan/naproxen sodium allowed significantly more subjects to return to normal or mildly impaired functioning more quickly, and sumatriptan/naproxen sodium patients were significantly more satisfied with their treatment compared with other treatment groups. Overall productivity loss was significantly reduced following use of sumatriptan/naproxen sodium.
Introduction
Migraine, as defined by the International Headache Society (IHS), affects about 18% of women and 6% of men in the United States.[1,2] Worldwide, typically 10% of the adult population suffers from the disease with the same relative proportions of women to men as in the United States. The intensity and duration of symptoms render many migraine sufferers unable to function or to perform work and nonwork activities.[2–4] Indeed, migraines have long been recognized as a major cause of work absenteeism and impaired productivity.[5–9] In a population survey, more than one third (38.2%) of physician-diagnosed migraine patients missed at least 1 workday; 80.1% missed at least 1 day of household work; and 64.9% missed at least 1 day of family or social activity in the previous 3 months.[10] Productivity losses for migraine patients have been well documented[6, 11–14] and have been estimated to cost between $5.6 and $17 billion per year.[9,11] Furthermore, Lipton and colleagues[15] confirmed the relationship between work-related disability and health-related quality of life (HRQOL), showing significant differences in HRQOL for patients with mild, moderate, and severe levels of disability from headache.
Although the exact cause of migraine is unknown, most experts agree that there may be multiple mechanisms causing the well-known symptom complex of migraine. Most experts also agree that these symptoms are the result of vasodilation of the cranial meningeal blood vessels and inflammation of the surrounding nerves.[16] In addition, untreated migraine attacks last 4-72 hours, and many attacks that are initially relieved appear to recur within 24 hours, indicating the need for long-acting therapy.[17–19] These facts led clinicians and scientists to combine a 5HT1 agonist drug that has known, selective vasoconstrictive and acute anti-inflammatory properties and efficacy in migraine (sumatriptan) with a long-acting anti-inflammatory/analgesic (naproxen) in order to concurrently target serotonin dysmodulation and inflammation. A fixed-dose, single-tablet formulation of sumatriptan 85 mg, as the succinate, formulated with RT Technology and naproxen sodium 500 mg (hereafter sumatriptan/naproxen sodium) has been developed for the acute treatment of migraine. RT Technology is a fast-disintegrating, rapid-release formulation designed to facilitate tablet disintegration and drug dispersion in the stomach. This can mitigate the effects of gastric stasis that often accompany migraine.[20] The complementary mechanisms of these compounds, coupled with the long-acting duration of naproxen, have been shown in multiple studies to provide a treatment response superior to monotherapy without an increase in adverse events.[16,21,22]
Patient satisfaction in regard to therapy is important to measure because it may influence treatment decisions, patient compliance, and health outcomes.[2,23,24] Furthermore, it can be viewed as an indicator of quality of care and may also reflect aspects of effectiveness and safety.[25] Although sometimes measured as a single item, the importance of measuring multiple attributes valued by patients is increasingly recognized. Considering a variety of treatment attributes, such as overall effectiveness, duration of effect, number of doses required, and relief of pain and symptoms, provides the information needed for optimal treatment decisions.
Although triptans are noted for their productivity benefits,[12, 26–29] it is unclear whether the addition of naproxen to sumatriptan will provide additional benefit in this regard. In 2 well-controlled studies, we evaluated the effect of sumatriptan formulated with RT Technology (sumatriptan) 85 mg and naproxen sodium 500 mg (naproxen), a unique fixed-dose, single-tablet formulation (sumatriptan/naproxen sodium) vs each component of this combination or placebo on patient ratings of their ability to function, their productivity, and their ratings of satisfaction with treatment attributes.
Methods
Study Designs
We conducted 2 identical, phase 3, randomized, double-blind, parallel-group, placebo-controlled, single-dose, multicenter, US migraine studies to measure ability to function, productivity, and satisfaction. Patients were randomized to receive sumatriptan/naproxen sodium (85 mg sumatriptan formulated with RT Technology and 500 mg naproxen sodium) in a unique fixed-dose, single-tablet formulation; 85 mg sumatriptan formulated with RT Technology (sumatriptan); 500 mg naproxen sodium (naproxen); or placebo. Patients included men or nonpregnant, nonlactating women, 18-65 years of age, with a demonstrated history (≥ 6 months) of migraine headaches (IHS criteria 1.1 or 1.2) who had their first migraine before age 50 and had experienced an average of 2-6 moderate or severe migraine attacks per month in the previous 3 months.
Both studies included a screening visit, at-home treatment of a single migraine attack and a follow-up visit 1-5 days following treatment. Patients completed pain, ability to function, and symptom assessments via a diary card immediately prior to taking the study drug for treatment of a moderate or severe migraine (baseline). After taking the study drug, patients completed assessments every 30 minutes for the first 2 hours, hourly from 2 to 4 hours, and then hourly while awake through 24 hours post dose. Patients were allowed to take rescue medication, if necessary, 2 hours after taking the study drug. The studies were performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocols were approved by ethics committees, and all patients provided written informed consent. More information on study designs and safety and efficacy assessments is available elsewhere.[30]
Analyses were conducted with the intent-to-treat population, which included all patients treating a moderate or severe migraine with study drug who recorded at least 1 posttreatment pain assessment.
Measures
Ability to Function
Ability to function, defined as the ability to perform work or usual activities, was measured from 0 to 24 hours with a 4-point categorical scale (not impaired, mildly impaired, severely impaired, and required bed rest). The number of patients in the sumatriptan/naproxen sodium group who were functioning normally (ie, not impaired) was compared at 1 through 5 hours with the other treatment groups with a Cochran-Mantel-Haenszel test with pooled investigator site (small sites pooled) as the strata. Post hoc analyses of the median time to first response of normal functioning and the median time to sustained response (continual responses of normal functioning from first report through 24 hours post dosing) were compared with a log-rank test with censoring at 24 hours. Patients who reported normal functioning at initial dosing were excluded from these analyses.
Productivity
Proper selection of an instrument that captures patient-reported productivity data is important to ensure strong internal validity and reliability. When selecting an instrument, one must first determine the research question. We chose the Productivity Assessment Questionnaire (PAQ) in order to quantify productivity-related impairment, rather than to qualify to what extent migraines, or their treatment, affect individual functional domains. The PAQ has been used effectively in previous migraine research.[7, 31–33] It also provides useful data that can be incorporated into economic models.
Patients completed the PAQ 24 hours after study drug administration. Patients recorded the number of hours missed from work and hours worked with symptoms; they also rated their percent effectiveness while working with symptoms during the 24 hours after taking the study drug. Lost work productivity was then calculated as hours missed from work plus the hours worked with symptoms, adjusted for percent effectiveness.[8] Lost work productivity was not calculated if the patient was not scheduled to work when the migraine attack occurred. Lost nonwork activity time was collected and calculated similarly. Total disability was the sum of lost work productivity and lost nonpaid activity time. Each of these productivity parameters was summarized by treatment group and compared statistically for sumatriptan/naproxen sodium vs other treatment groups with the Wilcoxon rank-sum test controlling for pooled investigator site as strata.
Satisfaction
Patient satisfaction was captured at screening and 24 hours post treatment with the Patient Perception of Migraine Questionnaire (PPMQ),[34] using 8 attributes of migraine medications on a 7-point Likert scale ("very dissatisfied" to “very satisfied”). Posttreatment satisfaction ratings were compared statistically with the Wilcoxon rank-sum test controlling for pooled investigator site as strata. In addition, the change in satisfaction scores from screening was calculated so that positive scores indicate improvement.
Results
Study Population
The populations for studies 1 and 2 were primarily women (88% and 86%), white (89% and 88%), nonsmokers (87% and 84%), with a mean age of approximately 40 years; more than 75% of patients reported a history of migraines without aura. Approximately 31% of patients in both studies had used oral sumatriptan; 37% or 41% had used nonsteroidal anti-inflammatory drugs (NSAIDs); and 11% or 12% had used narcotics. Between one fourth and one third of patients also reported typically using over-the-counter drugs. The treatment groups were similar with regard to all demographics, type of previous medication use, and migraine history characteristics in both studies.[30]
Ability to Function
Before treatment, most (50% to 60%) patients in Study 1 reported that their ability to function was severely impaired or that bed rest was required, and 40% and 50% of patients in Study 2 reported similar impairment. At hours 2 through 5 in both studies, significantly more patients in the sumatriptan/naproxen sodium group reported no impairment compared with the naproxen and placebo groups (Figure 1). In addition, in Study 2, significantly more patients in the sumatriptan/naproxen sodium group also reported no impairment compared with the sumatriptan group at hours 2 through 5.
Figure 1.
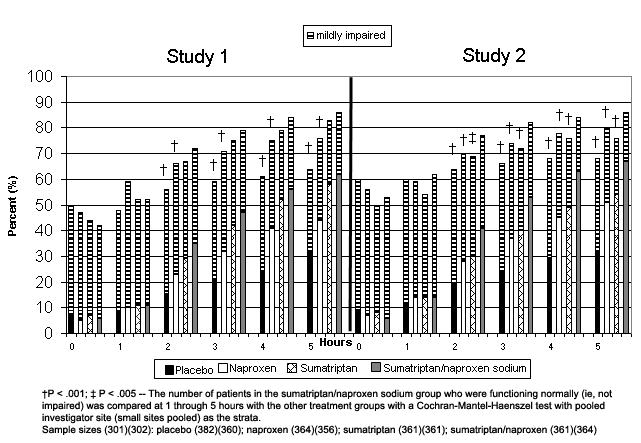
Proportion of patients reporting normal functioning or mild impairment from baseline to 5 hours post treatment.
† P < .001; ‡ P < .005 – The number of patients in the sumatriptan/naproxen sodium group who were functioning normally (ie, not impaired) was compared at 1 through 5 hours with the other treatment groups with a Cochran-Mantel-Haenszel test with pooled investigator site (small sites pooled) as the strata.
Sample sizes (301)(302): placebo (382)(360); naproxen (364)(356); sumatriptan (361)(361); sumatriptan/naproxen (361)(364)
Figure 2 shows that sumatriptan/naproxen sodium patients reported normal functioning more quickly than other groups. Excluding patients who reported normal functioning at dosing, we found that the median time to first report of normal function in Study 1 was 4 hours for the sumatriptan/naproxen sodium group compared with 4, 7, and 11 hours for the sumatriptan, naproxen (P < .001), and placebo groups (P < .001), respectively (Table). In Study 2, the median time to first report of normal function was 3 hours for the sumatriptan/naproxen sodium group compared with 5, 5, and 11 hours for the sumatriptan (P = .002), naproxen (P < .001), and placebo groups (P < .001), respectively. Similarly, the time to sustained report of normal functioning was significantly shorter for the sumatriptan/naproxen sodium group compared with the naproxen and placebo groups in Study 1 and significantly shorter than all 3 groups in Study 2 (Table).
Figure 2.
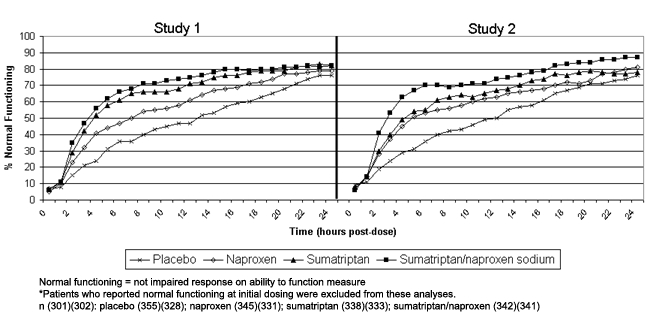
Proportion of patients reporting normal functioning at each hour following treatment.*
Normal functioning = not impaired response on ability to function measure
*Patients who reported normal functioning at initial dosing were excluded from these analyses.
n (301)(302): placebo (355)(328); naproxen (345)(331); sumatriptan (338)(333); sumatriptan/naproxen (342)(341)
Table.
Time to Reporting of Normal Functioning (Hours)
| N | Time to First Report of Normal Functioning* Median (95% CI) | P Value† vs Sumatriptan/Naproxen Sodium | Time to Sustained Report of Normal Functioning* Median (95% CI) | P Value† vs Sumatriptan/Naproxen Sodium | ||
|---|---|---|---|---|---|---|
| Study 1 | ||||||
| Sumatriptan/naproxen sodium | 342 | 4 (3-5) | 5 (4-7) | |||
| Sumatriptan | 338 | 4 (4-5) | ns | 7 (6-9) | ns | |
| Naproxen | 345 | 7 (6-8) | < .001 | 11 (8-13) | .005 | |
| Placebo | 355 | 11 (9-13) | < .001 | 16 (14-18) | < .001 | |
| Study 2 | ||||||
| Sumatriptan/naproxen sodium | 341 | 3 (3-4) | 4 (3-4) | |||
| Sumatriptan | 333 | 5 (4-6) | .002 | 8 (7-12) | .001 | |
| Naproxen | 331 | 5 (5-7) | < .001 | 9 (7-11) | < .001 | |
| Placebo | 328 | 11 (9-13) | < .001 | 14 (12-16) | < .001 | |
Normal functioning = not impaired response on ability to function measure
Log-rank test controlling for censoring at 24 hours; subjects reporting normal functioning (not impaired) at dosing (time = 0) were excluded.
CI = confidence interval; ns = not statistically significant
Productivity
The sumatriptan/naproxen sodium group experienced greater reductions in productivity in both studies (Figure 3). Total lost productivity was 33% and 27% lower, on average, in the sumatriptan/naproxen sodium group (4.7 and 4.5 hours) compared with the placebo group (7.0 and 6.2 hours; P < .001) and 16% and 17% lower compared with the naproxen group (5.6 and 5.4 hours; P = .016) for studies 1 and 2, respectively. In Study 2, the sumatriptan/naproxen sodium group was 20% lower compared with the sumatriptan group (5.6 hours; P = .002).
Figure 3.
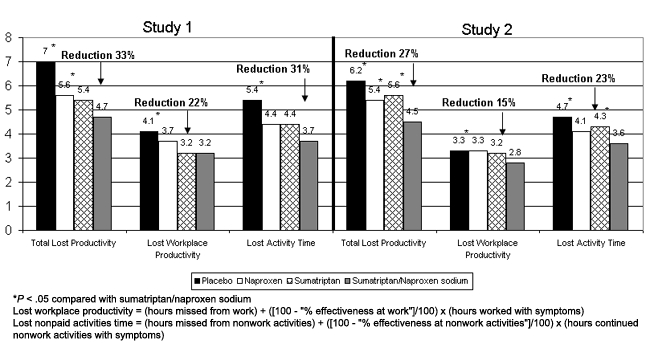
Mean productivity loss during 24 hours post treatment.
*P < .05 compared with sumatriptan/naproxen sodium
Lost workplace productivity = (hours missed from work) + ([100 – “% effectiveness at work”]/100) × (hours worked with symptoms)
Lost nonpaid activities time = (hours missed from nonwork activities) + ([100 – “% effectiveness at nonwork activities”]/100) × (hours continued nonwork activities with symptoms)
For workplace productivity, the sumatriptan/naproxen sodium group reported a mean of 3.2 hours of lost work productivity compared with 4.1 hours for the placebo group in Study 1 (P = .024) and 2.8 vs 3.3 hours (P = .008) in Study 2. For lost activity time, the sumatriptan/naproxen sodium group reported losing 3.7 hours compared with 5.4 hours reported by the placebo group (P < .001) in Study 1, and a loss of 3.6 hours compared with 4.7 for the placebo group (P = .005) in Study 2.
Satisfaction
Patients in the sumatriptan/naproxen sodium group were significantly more satisfied with their treatment 24 hours post treatment than the other treatment groups in both studies (Figure 4). The sumatriptan/naproxen sodium group was significantly more likely to report being satisfied or very satisfied with their treatment on each of the 8 treatment attributes compared with all other treatments in both studies – with the exception of drowsiness in Study 1, in which treatment was not significantly better than placebo (P ≤ .001). In Study 1, the overall satisfaction with effectiveness was 50% with sumatriptan/naproxen sodium treatment, 41% with sumatriptan, 35% with naproxen, and 21% with placebo; for Study 2, overall satisfaction with effectiveness was 53% with sumatriptan/naproxen sodium, 42% with sumatriptan, 35% with naproxen, and 19% with placebo.
Figure 4.
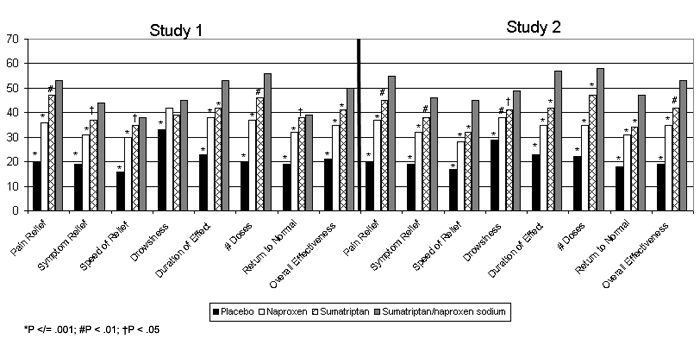
Percentage of subjects satisfied/very satisfied with treatment 24 hours post dosing.
*P ≤ .001; #P < .01; †P < .05
In addition to overall effectiveness, the largest difference between sumatriptan/naproxen sodium and other treatment groups was in patient satisfaction with the duration of effect and number of doses required for treatment (Figure 5). At least 10% more patients in the sumatriptan/naproxen sodium group reported that they were satisfied or very satisfied compared with sumatriptan, and approximately 30% or more were satisfied or very satisfied compared with the placebo group. In Study 1, roughly 15%, and in Study 2, approximately 20% more patients in the sumatriptan/naproxen sodium group were satisfied or very satisfied compared with naproxen.
Figure 5.
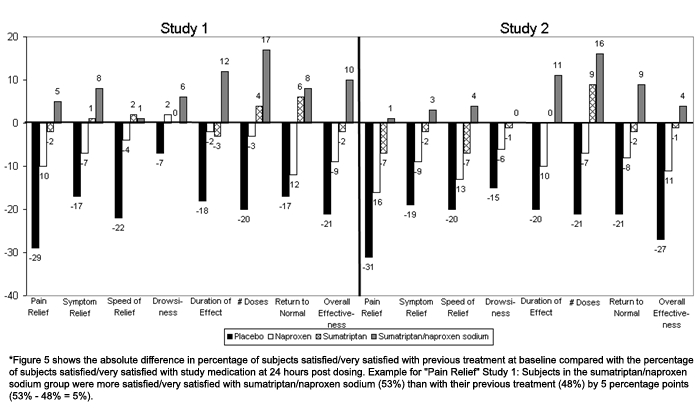
Change in the percent of subjects satisfied/very satisfied with study treatment compared with previous treatment.
*Figure 5 shows the absolute difference in percentage of subjects satisfied/very satisfied with previous treatment at baseline compared with the percentage of subjects satisfied/very satisfied with study medication at 24 hours post dosing. Example for “Pain Relief” Study 1: Subjects in the sumatriptan/naproxen sodium group were more satisfied/very satisfied with sumatriptan/naproxen sodium (53%) than with their previous treatment (48%) by 5 percentage points (53% − 48% = 5%).
Satisfaction ratings were also compared with previous treatments reported by patients during the screening visit. Compared with previous treatment, the sumatriptan/naproxen sodium group reported higher rates of satisfaction for duration of effect (increase of 12% for Study 1 and 11% for Study 2), the number of doses needed for relief of symptoms (increase of 17% for Study 1 and 16% for Study 2), and the time it took to return to normal (increase of 8% for Study 1 and 9% for Study 2). In Study 1, overall effectiveness (increase of 10%), symptom relief (increase of 8%), and pain relief (increase of 5%) were also higher for the sumatriptan/naproxen sodium group compared with previous treatment.
Discussion
Treatment with sumatriptan/naproxen sodium allowed significantly more subjects to return to normal or mildly impaired functioning more quickly compared with the components or placebo groups. Two thirds of the sumatriptan/naproxen sodium group (patients reporting normal functioning at dosing were excluded; Figure 2) reported normal functioning by 5 or 6 hours, and 80% reached normal functioning by 15 or 17 hours, which was maintained through the 24-hour observation period with 1 exception (79% at 17 hours). By contrast, it took 14 hours for two thirds of the patients in the naproxen group to report sustained normal functioning and 18 or 20 hours for patients in the placebo group. Neither the naproxen, sumatriptan, nor placebo group achieved 80% normal functioning during the observation period in either study.
Our findings also suggest that employers and patients may expect similar or improved productivity, on average, with sumatriptan/naproxen sodium compared with treatment with sumatriptan alone. Sumatriptan, as well as other triptans, has demonstrated reductions in productivity losses in other studies.[26–29] The sumatriptan/naproxen sodium group experienced 22% and 15% reductions in lost workplace productivity compared with placebo, yielding similar or numerically better results compared with sumatriptan patients. When lost activity time and total lost productivity were considered, the sumatriptan/naproxen sodium group was somewhat better (Study 1) or significantly better (Study 2) than the sumatriptan group. These findings suggest that employers and patients may expect similar or improved productivity, on average, with sumatriptan/naproxen sodium than previously experienced by sumatriptan treatments.
Finally, our data suggest that, for treating moderate or severe migraine headaches, patients will, on average, be more satisfied with sumatriptan/naproxen sodium than with placebo or the individual components of the active medication. With only 1 exception (drowsiness in Study 1), the sumatriptan/naproxen group included a significantly higher proportion of patients reporting that they were very satisfied or satisfied with treatment attributes compared with sumatriptan, naproxen, or placebo patients in both studies. Furthermore, these satisfaction ratings were an improvement over baseline ratings of their usual medications, particularly in terms of duration of effect, number of doses needed for symptom relief, speed of return to usual activities, and overall medication effectiveness.
These findings were consistent with primary efficacy results from each of the studies.[30] Both studies demonstrated that sumatriptan/naproxen sodium, a safe and effective acute treatment for migraine, provided a superior 2-hour pain relief and overall 2-hour symptom-free response compared with placebo and was superior to its components, sumatriptan 85 mg and naproxen sodium 500 mg, for sustained pain-free response. Furthermore, sumatriptan/naproxen sodium was superior to sumatriptan for sustained pain relief, 2- to 24-hour sustained migraine relief, lower use of rescue medication, longer median time until rescue in patients who took rescue medication, and less recurrence of migraines.
Although these findings were consistent across 2 randomized, well-controlled clinical studies, they are based on the treatment of a single moderate or severe migraine attack. In an open-label, 12-month study in a clinical trial setting, patients treated 24,486 moderate or severe migraine attacks with sumatriptan/naproxen sodium.[35] In 60% of attacks, patients were pain-free at 2 hours, and in an additional 21% of attacks, patients characterized their pain as mild. Moreover, functioning as measured by the Migraine Specific Quality of Life Questionnaire, Version 2.1 (MSQ)[36] was improved for 3 months of treatment and sustained over the course of the study. Patients also were more satisfied with sumatriptan/naproxen sodium compared with previous treatment; these levels of satisfaction persisted over the year-long study.[35]
Further studies that impose fewer restrictions on the selection of patients and involve sumatriptan/naproxen sodium as a routine part of treating episodic, acute migraine will be needed to determine whether the advantages of sumatriptan/naproxen sodium treatment observed in the investigation reported here will be sustained through repeated measures.
Efficacy and patient-reported outcomes, such as productivity and satisfaction, have not been compared in patients taking sumatriptan/naproxen sodium vs patients taking commercially available tablets of sumatriptan and naproxen at the same time. In other disease areas, compliance has been improved when 1 medication is taken rather than 2 medications.[37–40]
The distinct pharmacokinetic profile of sumatriptan/naproxen sodium may contribute to its superior efficacy relative to sumatriptan monotherapy because its administration as a single fixed-dose tablet of sumatriptan/naproxen sodium significantly alters the pharmacokinetics of both components. Although 3 oral doses (25 mg, 50 mg, and 100 mg) of sumatriptan succinate tablets are available in the United States, sumatriptan 85 mg was chosen for the combination tablet in order to balance the efficacy and safety profile. Pharmacokinetic data have demonstrated that sumatriptan peak concentrations (Cmax) and early exposure (area under the curve [AUC] 0-2) are similar following administration of a single fixed-dose tablet of sumatriptan/naproxen sodium relative to a sumatriptan 100-mg tablet formulated with RT Technology.[41] In addition, the time to maximum concentration (Tmax) of sumatriptan is approximately 50 minutes earlier with sumatriptan/naproxen sodium than that of commercially available sumatriptan 100 mg formulated with RT Technology, and the Tmax of naproxen is delayed with sumatriptan/naproxen sodium compared with naproxen alone.[41,42] These findings suggest the potential for extended clinical benefits from the compounded formulation, with an improved sustained pain-relief profile and lower risk for early headache recurrence.
Conclusion
Treatment with sumatriptan/naproxen sodium allowed significantly more subjects to return to normal or mildly impaired functioning more quickly, and sumatriptan/naproxen sodium patients were significantly more satisfied with their treatment compared with other treatment groups. Overall productivity loss in the combination of paid work and activities outside paid work was significantly reduced following use of sumatriptan formulated with RT Technology and naproxen sodium (sumatriptan/naproxen sodium) in a single-tablet formulation.
Acknowledgments
The authors thank Pat Ray Reese, PhD, of Reese Associates Consulting, LLC and Kelley Friel, MA, for their writing and editing services.
Footnotes
Readers are encouraged to respond to the author at wesleyhead@aol.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
Contributor Information
Stephen Landy, Wesley Neurology Clinic, Memphis, Tennessee Author's email: wesleyhead@aol.com.
Sarah E. DeRossett, GlaxoSmithKline, Research Triangle Park, North Carolina.
Alan Rapoport, The New England Center for Headache, Stamford, Connecticut; Clinical Professor of Neurology, David Geffen School of Medicine at UCLA, Los Angeles, California.
John Rothrock, The University of Alabama at Birmingham.
Michael H. Ames, GlaxoSmithKline, Research Triangle Park, North Carolina.
Susan A. McDonald, GlaxoSmithKline, Research Triangle Park, North Carolina.
Steven P. Burch, GlaxoSmithKline, Research Triangle Park, North Carolina.
References
- 1.Lipton RB, Stewart WF, Von KM. Burden of migraine: societal costs and therapeutic opportunities. Neurology. 1997;48:S4–S9. doi: 10.1212/wnl.48.3_suppl_3.4s. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 3.Turner-Bowker DM, Bayliss MS, Ware JE, Jr, Kosinski M. Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual Life Res. 2003;12:1003–1012. doi: 10.1023/a:1026179517081. [DOI] [PubMed] [Google Scholar]
- 4.Mannix LK. Epidemiology and impact of primary headache disorders. Med Clin North Am. 2001;85:887–895. doi: 10.1016/s0025-7125(05)70349-7. [DOI] [PubMed] [Google Scholar]
- 5.Von KM, Stewart WF, Simon DJ, Lipton RB. Migraine and reduced work performance: a population-based diary study. Neurology. 1998;50:1741–1745. doi: 10.1212/wnl.50.6.1741. [DOI] [PubMed] [Google Scholar]
- 6.Stang P, Cady R, Batenhorst A, Hoffman L. Workplace productivity. A review of the impact of migraine and its treatment. Pharmacoeconomics. 2001;19:231–244. doi: 10.2165/00019053-200119030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Weaver MB, Mackowiak JI, Solari PG. Triptan therapy impacts health and productivity. J Occup Environ Med. 2004;46:812–817. doi: 10.1097/01.jom.0000135606.43950.d2. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz BS, Stewart WF, Lipton RB. Lost workdays and decreased work effectiveness associated with headache in the workplace. J Occup Environ Med. 1997;39:320–327. doi: 10.1097/00043764-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999;159:813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache. 2001;41:638–645. doi: 10.1046/j.1526-4610.2001.041007638.x. [DOI] [PubMed] [Google Scholar]
- 11.Osterhaus JT, Gutterman DL, Plachetka JR. Healthcare resource and lost labour costs of migraine headache in the US. Pharmacoeconomics. 1992;2:67–76. doi: 10.2165/00019053-199202010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kwong WJ, Taylor FR, Adelman JU. The effect of early intervention with sumatriptan tablets on migraine-associated productivity loss. J Occup Environ Med. 2005;47:1167–1173. doi: 10.1097/01.jom.0000174296.46911.79. [DOI] [PubMed] [Google Scholar]
- 13.Stewart WF, Lipton RB, Simon D. Work-related disability: results from the American migraine study. Cephalalgia. 1996;16:231–238. doi: 10.1046/j.1468-2982.1996.1604231.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 15.Lipton RB, Liberman JN, Kolodner KB, Bigal ME, Dowson A, Stewart WF. Migraine headache disability and health-related quality-of-life: a population-based case-control study from England. Cephalalgia. 2003;23:441–450. doi: 10.1046/j.1468-2982.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith TR, Sunshine A, Stark SR, Littlefield DE, Spruill SE, Alexander WJ. Sumatriptan and naproxen sodium for the acute treatment of migraine. Headache. 2005;45:983–991. doi: 10.1111/j.1526-4610.2005.05178.x. [DOI] [PubMed] [Google Scholar]
- 17.Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment? Headache. 2002;42(suppl1):3–9. doi: 10.1046/j.1526-4610.2002.0420s1003.x. [DOI] [PubMed] [Google Scholar]
- 18.Aurora SK. Headache recurrence as a criterion for assessing efficacy of triptans: a perspective. Headache. 2002;42:70–79. doi: 10.1046/j.1526-4610.2002.02016.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–658. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 20.Kori SH, Sandefer EP, McDonald SA, Page RC, Doll WJ. Improved absorption of sumatriptan tablets formulated with RT Technology[TM] (SumaRT) Cephalalgia. 2005;25:933. [Google Scholar]
- 21.Silberstein SD, Stark S, DeRossett SE, Taylor D, McDonald SA, Lener S. Superior clinical benefits of a new single-tablet formulation of sumatriptan formulated with RT technology and naproxen sodium. Neurology. 2006;66(suppl2):A254–255. [Google Scholar]
- 22.Brandes JL, Cady RK, Smith TR, Lener S, Zhang Y, Alexander WJ. Interim analysis of the long-term safety study of a new single-tablet formulation of sumatriptan formulated with RT Technology and naproxen sodium. Program and abstract of the 12th Congress of the International Headache Society; October 9-12, 2005; Kyoto, Japan. Abstract. [Google Scholar]
- 23.Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care: relationship to illness attitudes and outcomes. J Gen Intern Med. 1989;4:506–511. doi: 10.1007/BF02599549. [DOI] [PubMed] [Google Scholar]
- 24.Taylor TR. Understanding the choices that patients make. J Am Board Fam Pract. 2000;13:124–133. doi: 10.3122/15572625-13-2-124. [DOI] [PubMed] [Google Scholar]
- 25.Patrick DL, Martin ML, Bushmell DM, Pesa J. Measuring satisfaction with migraine treatment: expectations, importance, outcomes, and global ratings. Clin Ther. 2003;25:2920–2935. doi: 10.1016/s0149-2918(03)80345-4. [DOI] [PubMed] [Google Scholar]
- 26.Becker WJ. Are the triptans for migraine therapy worth the cost? Can J Neurol Sci. 2000;27:111–115. [PubMed] [Google Scholar]
- 27.Legg RF, Sclar DA, Nemec NL, Tarnai J, Mackowiak JI. Cost benefit of sumatriptan to an employer. J Occup Environ Med. 1997;39:652–657. doi: 10.1097/00043764-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Cortelli P, Dahlof C, Bouchard J, et al. A multinational investigation of the impact of subcutaneous sumatriptan. III: workplace productivity and non-workplace activity. Pharmacoeconomics. 1997;11(suppl1):35–42. doi: 10.2165/00019053-199700111-00006. [DOI] [PubMed] [Google Scholar]
- 29.Davies GM, Santanello N, Gerth W, Lerner D, Block GA. Validation of a migraine work and productivity loss questionnaire for use in migraine studies. Cephalalgia. 1999;19:497–502. doi: 10.1046/j.1468-2982.1999.019005497.x. [DOI] [PubMed] [Google Scholar]
- 30.Brandes JL, Kudrow D, Stark S, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297:1443–1454. doi: 10.1001/jama.297.13.1443. [DOI] [PubMed] [Google Scholar]
- 31.Adelman JU, Sharfman M, Johnson R, et al. Impact of oral sumatriptan on workplace productivity, health-related quality of life, healthcare use, and patient satisfaction with medication in nurses with migraine. Am J Manag Care. 1996;2:1407–1416. [Google Scholar]
- 32.Mushet GR, Miller D, Clements B, Pait G, Gutterman DL. Impact of sumatriptan on workplace productivity, nonwork activities, and health-related quality of life among hospital employees with migraine. Headache. 1996;36:137–143. doi: 10.1046/j.1526-4610.1996.3603137.x. [DOI] [PubMed] [Google Scholar]
- 33.Schulman EA, Cady RK, Henry D, et al. Effectiveness of sumatriptan in reducing productivity loss due to migraine: results of a randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2000;75:782–789. doi: 10.4065/75.8.782. [DOI] [PubMed] [Google Scholar]
- 34.Davis KH, Black L, Sleath B. Validation of the Patient Perception of Migraine Questionnaire. Value Health. 2002;5:421–429. doi: 10.1046/J.1524-4733.2002.55120.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith T, Blumenthal H, Diamond M, et al. Sumatriptan/naproxen sodium for migraine: efficacy, health-related quality of life and satisfaction outcomes. Headache. 2007;47:683–692. doi: 10.1111/j.1526-4610.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the migraine-specific quality of life questionnaire (MSQ Version 2.1) Headache. 2000;40:204–215. doi: 10.1046/j.1526-4610.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 37.Swanson J. Compliance with stimulants for attention-deficit/hyperactivity disorder: issues and approaches for improvement. CNS Drugs. 2003;17:117–131. doi: 10.2165/00023210-200317020-00004. [DOI] [PubMed] [Google Scholar]
- 38.Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loghman-Adham M. Medication noncompliance in patients with chronic disease: issues in dialysis and renal transplantation. Am J Manag Care. 2003;9:155–171. [PubMed] [Google Scholar]
- 40.Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25:2289–2304. doi: 10.1016/s0149-2918(03)80220-5. [DOI] [PubMed] [Google Scholar]
- 41.Walls C, Byrd S, Hickmott F, et al. Early sumatriptan pharmacokinetics following a sumatriptan 100 mg RT Technology[TM] tablet and a fixed single-tablet formulation of sumatriptan 85 mg RT Technology[TM] and 500 mg naproxen sodium. Cephalalgia. 2006;26:1395. [Google Scholar]
- 42.Kori S, Littlefield D, Taylor D, Wargin B, Haberer L, Lener S. Pharmacokinetics of a single-tablet formulation of sumatriptan RT Technology and naproxen sodium. Cephalalgia. 2005;25:933. [Google Scholar]


