Abstract
An increase in the level of the c-Jun transcription factor and of its phosphorylation has previously been shown to be essential for nerve growth factor (NGF) withdrawal-induced apoptosis of rat sympathetic neurons (SCG). The Rho-like GTPases Cdc42 and Rac1 are involved in the regulation of a number of cellular processes, including activation of the c-Jun NH2-terminal kinase (JNK) pathway. Therefore, we have investigated the role of these GTPases in this process. Overexpression of activated Rac1 or Cdc42 in SCG neurons maintained in the presence of NGF induced apoptosis, whereas expression of dominant negative mutants of Cdc42 or Rac1 blocked apoptosis following NGF withdrawal. Cdc42 activation produced an increase in the level of c-Jun and of its phosphorylation. Furthermore, Cdc42-induced death was prevented by coexpressing the c-Jun dominant negative FLAGΔ169. Thus, Cdc42 appears to function as an initiator of neuronal cell death by activating a transcriptional pathway regulated by c-Jun.
Neuronal apoptosis or programmed cell death (PCD) is a crucial process occurring not only during normal development and tissue turnover but also in pathological situations such as stroke, Alzheimer’s, and Huntington’s diseases (1, 2). Neuronal PCD involves the activation of a number of enzymes and genes and is regulated by specific growth factors, such as neurotrophins (NT), which promote survival of particular neuronal populations (3, 4) by binding to specific cell surface receptors (for review, see ref. 5). Removal of these survival factors activates or de-represses signaling pathways that eventually lead to apoptosis. Recently, a great deal of progress has been made in understanding these pathways. One of the most significant observations is that neuronal apoptosis requires gene transcription (6), and some of the transcription factors activated during induction of neuronal apoptosis have been identified. When rat sympathetic neurons (SCG) were deprived of nerve growth factor (NGF) the level of the c-Jun transcription factor specifically and significantly increased, suggesting that AP-1 activity is part of the transcriptional program required for neuronal cell death. Phosphorylation of c-Jun on serines 63 and 73 in the transactivation domain enhances its transcriptional activity (7, 8), and an increase in c-Jun NH2-terminal kinase (JNK) activity has been observed soon after NGF withdrawal from these cells (9). In addition, Xia et al. (10) showed that in PC12 cells, NGF withdrawal led to activation of JNK and the p38/HOG1 mitogen-activated protein kinase whereas the extracellular-regulated activated-kinase (ERK) signaling pathway was inhibited. Finally, the functional importance of the activation of c-Jun has been demonstrated in studies in which apoptosis of SCG neurons after NGF withdrawal could be blocked by microinjection of anti-c-Jun antibodies or overexpression of a c-Jun dominant negative mutant (11, 12). Altogether, these findings indicate that the pathways regulating both the level of the c-Jun protein and its phosphorylation are important in inducing neuronal death. Therefore, upstream regulators of these pathways could be potential candidates as neuronal death mediators, and it would be of great interest to identify them.
An increasing number of kinases that activate the stress-activated protein kinases SAPK/JNK and p38 kinase pathways have been identified. These include the mitogen-activated protein kinase kinases (MEKKs) (13–15), the p21-activated protein kinases (PAKs) (16–19), the mixed lineage kinase (MLK3, also called SPRK and PTK-1) (20–22), the germinal center kinase (GCK) (23), the transforming growth factor β-activated kinases (TAKs), the Nck interacting protein (NIK) (24), and the apoptosis signal-regulating kinase (ASK1) (25). Furthermore, several groups have studied upstream regulators of these kinases and they have provided evidence that the Rho-like GTPases Cdc42 and Rac1 are involved. Indeed, PAK1 and MKL3 have been shown to be activated by Cdc42 and Rac1 (23–29). These GTPases, therefore, are now thought to be involved in a wide variety of cellular responses including cytoskeletal changes, cellular transformation, inflammatory responses, cell motility, and cytokinesis (30–33).
Altogether, these findings prompted us to investigate the role of Rac1 and Cdc42 in the induction of SCG cell death (6, 34, 35). These cells are difficult to transfect, and we adopted the approach of microinjecting them with a variety of expression plasmids designed to activate or inhibit particular neuronal signaling pathways. We report here that activated Cdc42 or Rac1 can induce apoptosis of SCG neurons via activation of c-Jun whereas their dominant negative counterparts protect them against NGF withdrawal-induced death.
MATERIALS AND METHODS
Cell Culture.
Sympathetic neurons were isolated from the superior cervical ganglia of 1-day-old Sprague–Dawley rats as described in ref. 11. SCG neurons were plated on 13-mm-diameter glass coverslips coated with polylysine and laminin at a density of 8,000–10,000 cells per coverslip. They were then cultured in DMEM (GIBCO/BRL) supplemented with 10% fetal calf serum (Globepharm), 2 mM glutamine, penicillin, streptomycin, 20 mM each of fluordeoxyuridine and uridine (basic culture medium), and 50 ng/ml NGF.
Antibodies.
The anti-c-Jun antibody was raised against a GST-c-Jun protein, encompassing amino acids 1–58 (36), and the anti-phospho-c-Jun antibody was raised against a phospho-peptide, encompassing amino acids 57–68 of c-Jun, with phospho-serine 63 (9).
Construction of Expression Vectors.
The 0.6-kb BamHI-EcoRI fragments of the pGex2T-Cdc42 and -Rac1 mutants were subcloned into the mammalian expression vector pRK5 downstream of a cytomegalovirus promoter and in-frame with a c-myc epitope tag. DNA for microinjection was purified on two CsCl gradients.
Microinjection.
After 5–7 days in culture, the neurons were microinjected with DNA mixtures by using a Zeiss Axiovert 135M microscope with an Eppendorf transjector (model 5246) and micromanipulator (model 5171). DNA was injected directly into the nucleus with needles pulled on a horizontal electrode puller (Camden Instruments, Leicester, U.K., model 773). Routinely, 50–80% of neurons survived the injection.
Survival Assay.
To assess the number of viable cells, 70,000 kDa Texas Red Dextran at a final concentration of 5 mg/ml was injected, along with the DNA, to detect the injected cells. Five to 24 hr after injection, the cells were refed, or, in the case of NGF withdrawal experiments, the medium was replaced with basic culture medium supplemented with an anti-NGF antibody (Boehringer) at 100 ng/ml. The number of Texas Red-positive cells was scored and assigned as the 100% value. After appropriate times, the number of Texas Red-positive cells was scored again and the percentage of survival was assessed. In the case of NGF withdrawal experiments, the cells were stained with calcein AM (0.5 μM final concentration), which fluoresces green in live cells. Only the Texas Red-positive cells that were calcein positive and had a normal morphology were scored as viable. All experiments were counted blind.
Expression Studies.
To verify the expression of the proteins of interest, guinea pig IgG was coinjected at a final concentration of 5 mg/ml. After appropriate times, the cells were fixed and permeabilized with 50% methanol/50% acetone for 20 min at −20°C and incubated with the 9E10 anti-c-myc antibody (Boehringer), diluted 1:250, to check for expression of the tagged proteins, and blocked with 50% normal goat serum in 1% BSA in PBS. They were stained finally with a rhodamine-conjugated anti-guinea pig IgG antibody diluted 1:100 to detect the injected cells and with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. The coverslips were mounted with Citifluor and viewed on a Nikon Microphot FXA fluorescence microscope.
Immunofluorescence Staining.
To detect c-Jun or phospho-c-Jun in immunofluorescence experiments, the cells were fixed and permeabilized 24 hr after injection as in ref. 11, blocked with 50% normal goat serum, and incubated with a 1:100 dilution of an anti-c-Jun antibody or a monoclonal anti-phospho-c-Jun antibody diluted 1:5,000. They were then stained with an FITC-conjugated secondary antibody and an anti-guinea pig IgG antibody to detect the injected cells as described above. Only the cells showing a clear increase over background staining were scored positive. To examine nuclear morphology, cells were stained with Hoechst dye (Hoechst 33342, Sigma) at 10 mg/ml.
TUNEL Analysis.
When indicated, TUNEL analysis was performed 16 hr after injection. Cells were fixed with 3% paraformaldehyde and permeabilized with 0.5% Triton X-100. They were then incubated with a TUNEL reaction mixture (In Situ Cell Death Detection Kit, Boehringer). Finally, the cells were stained to detect the injected cells as described above.
RESULTS
Activation of Rac1 or Cdc42 Induces Neuronal Apoptosis.
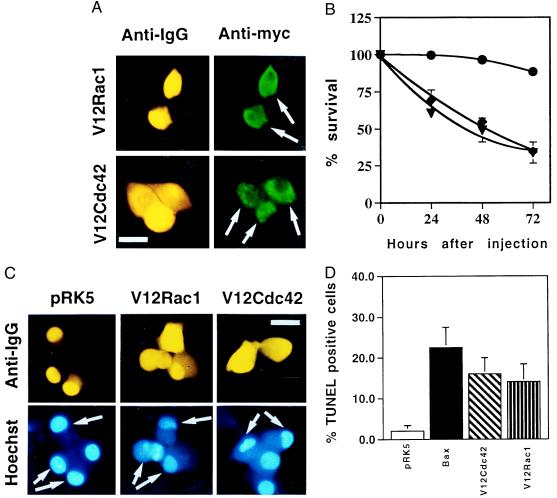
To study the function of Rac1 and Cdc42 in SCG neurons, their activated forms, the V12 mutants, or the empty expression vector pRK5 were microinjected, at 0.1 mg/ml, into SCG neurons cultured for 6 days in the presence of NGF. The glycine-to-valine mutation at position 12 results in decreased intrinsic GTPase activity, and the V12 mutant is believed to exist predominantly in an active GTP-bound conformation and to interact continuously with its downstream effectors (37). Three to 4 hr later, the cells were stained with an anti-c-myc antibody to check the level of Rac1 and Cdc42 expression: more than 70–80% of the injected cells clearly expressed the GTPases, which were localized throughout the cell body (Fig. 1A). The effect of Rac1 and Cdc42 on the survival of SCG neurons maintained in the presence of NGF was then determined. Expression of the activated forms of either Rac1 or Cdc42 resulted in the rapid induction of cell death, with about 70% of the cells dying by 72 hr (Fig. 1B). As additional controls, we microinjected the dominant negative mutants N17Cdc42 and N17Rac1 [the threonine-to-asparagine mutation produces an inactive GDP-bound GTPase, which apparently competes with the wild-type protein for a common pool of regulatory proteins (38, 39)]. These mutants had no effect on the survival of SCG neurons maintained in the presence of NGF (data not shown). Furthermore, we looked at the effect of activated Ras and RhoA on the survival of SCG neurons in the presence of NGF. D12Ras slightly reduced the number of live cells, whereas V14RhoA had no effect at all (data not shown). This indicates that specific expression of the activated forms of Cdc42 or Rac1 is sufficient to induce neuronal death, even in the presence of a potent survival agent.
Figure 1.
Activated Cdc42 and Rac1 induce neuronal apoptosis. (A) The activated V12 mutants of Cdc42 and Rac1 were subcloned into the pRK5 mammalian expression vector and tagged with a myc epitope. Sympathetic neurons (SCG neurons), cultured for 5–7 days in the presence of NGF, were microinjected with 0.1 mg/ml DNA and 5 mg/ml guinea pig IgG, to follow the injected cells. For each experiment, 200 cells were microinjected. Four to 24 hr after injection, the cells were stained to check for expression of Rac1 and Cdc42 as described in Materials and Methods. (Bar = 100 μm.) The white arrows indicate the expressing cells. (B) Survival of SCG neurons injected with V12Cdc42 (triangles), V12Rac1 (squares), or pRK5 (circles). Twenty-four, 48, and 72 hr later, the percentage of surviving cells was assessed as described in Material and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments ± SEM. (C) Morphology of the cells microinjected with V12Cdc42, V12Rac1, or pRK5. Six-day-old SCG neurons were injected with 0.3 mg/ml DNA together with guinea pig IgG and stained with Hoechst 24 hr after injection. White arrows indicate the injected cells: only the cells overexpressing the constitutively active forms of Rac1 and Cdc42 displayed clearly pyknotic nuclei. (Bar = 100 μm.) (D) TUNEL analysis of SCG neurons microinjected with V12Cdc42, V12Rac1, pRK5, or Bax. Six-day-old SCG neurons were microinjected with 0.3 mg/ml V12 Rac1, V12Cdc42, pRK5 DNA, or 0.05 mg/ml Bax DNA. TUNEL analysis was performed 16 hr later. The results are the means of three independent experiments ± SEM.
To further characterize the death induced by Rac1 and Cdc42, we examined the morphology of the dying cells by Hoechst staining. Twenty-four hours after injection, the cells had clearly started to display pyknotic nuclei, a typical feature of apoptosis (Fig. 1C). This result was confirmed by TUNEL analysis performed on these cells and on cells overexpressing Bax, which is known to be a strong inducer of apoptosis under these conditions (40). After 16 hr, about 20% of the cells overexpressing Bax or V12Cdc42 and about 10% of the cells overexpressing V12Rac1 were TUNEL positive versus only 2% of pRK5-overexpressing cells. Altogether, these observations show that constitutive expression of activated forms of Rac1 or Cdc42 can induce neuronal cell death, presumably by activating a pro-apoptotic signaling cascade.
Cdc42 Is Required for NGF Withdrawal-Induced Apoptosis.
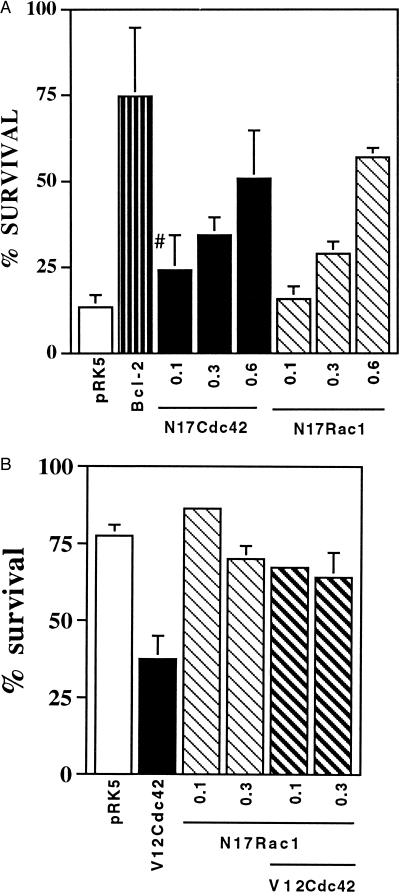
To determine whether apoptosis initiated by NGF deprivation results in the activation of Cdc42 or Rac1, we microinjected the dominant negative mutants N17Cdc42 and N17Rac1. More than 60% of the injected cells expressed the protein of interest (data not shown). Four to 5 hr after injection, the cells were withdrawn from NGF, and the percentage of surviving cells was assessed 48 hr later. Both N17Cdc42 and N17Rac1 protected the SCG neurons from NGF withdrawal-induced death in a dose-dependent manner. These results demonstrate that not only can activated Cdc42 and Rac1 induce neuronal apoptosis, but also that they are required for NGF withdrawal-induced neuronal cell death (Fig. 2A).
Figure 2.
N17Rac1 and N17Cdc42 can prevent NGF withdrawal-induced neuronal death. (A) SCG neurons, cultured for 5–7 days, were microinjected with increasing concentrations of N17Rac1 (hatched bars), N17Cdc42 (solid bars), 1.0 mg/ml pRK5 (negative control, open bar), or 0.05 mg/ml of Bcl-2 (positive control, striped bar). Twenty-four hours later the cells were withdrawn from NGF and left for an additional 48 hr. Cell survival was assessed by calcein staining as described in Material and Methods. The results are the means of four independent experiments ± SEM. #, the P value of N17Cdc42 at 0.1 mg/ml is <0.01. (B) Sympathetic neurons (SCG neurons), cultured for 5–7 days in the presence of NGF, were coinjected with 0.1 mg/ml V12Cdc42 (solid bar) and increasing concentrations of N17Rac1 (hatched bars) or 0.4 mg/ml pRK5 (open bar). Forty-eight hours later, the percentage of surviving cells was assessed as described in Material and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments ± SEM.
To assess the hierarchy, if any, between Cdc42 and Rac1, we coinjected V12Cdc42 with N17Rac1 or V12Rac1 with N17Cdc42. The cells were maintained in the presence of NGF, and 48 hr later the percentage of surviving cells was assessed. Fig. 2B shows that N17Rac1 blocked V12Cdc42-induced death whereas N17Cdc42 had no effect on V12Rac1-induced death (data not shown), suggesting that in neurons as in other cells Cdc42 is upstream of Rac1 (32, 41). Therefore, we decided to focus our studies on the mechanism of Cdc42-induced apoptosis.
Activation of Cdc42 Results in an Increase in the Level of c-Jun Protein and of Its Phosphorylation.
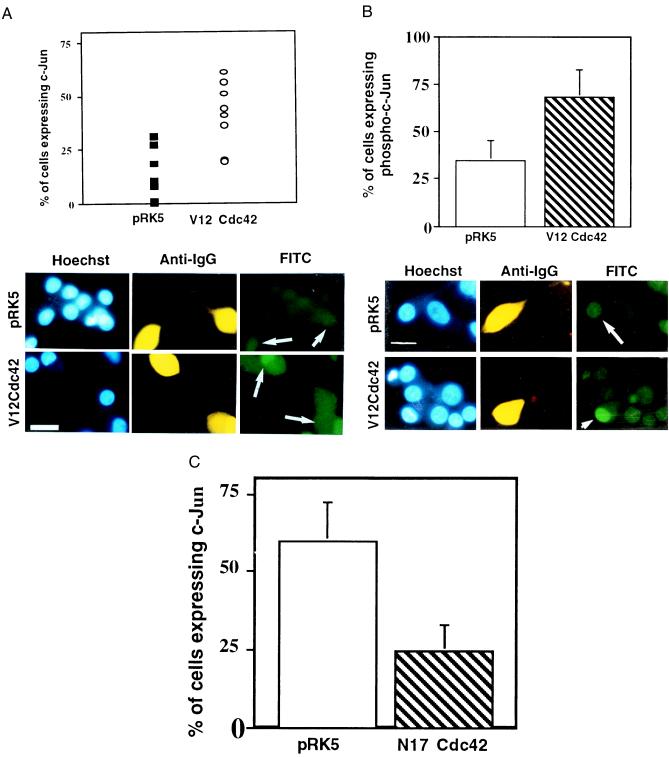
We next wanted to determine whether activated Cdc42 induces an increase in the level of the c-Jun protein and of its phosphorylation, as occurs after NGF withdrawal (11). Cells microinjected with either V12Cdc42 or the empty expression vector pRK5 were labeled with a specific anti-c-Jun antibody. Cells injected with pRK5 did not show any increase in c-Jun expression compared with noninjected cells (Fig. 3A), whereas cells injected with V12Cdc42 showed a clear increase in levels of nuclear c-Jun. In a similar manner, these cells were stained with an anti-phospho-c-Jun antibody, specific for phosphorylated serine 63 (9). Again, the pRK5-injected cells did not display increased nuclear phospho-c-Jun staining compared with background. However, V12Cdc42 induced a significant enhancement of the nuclear staining (Fig. 3B). This suggests that activation of Cdc42 results in increased expression of the c-Jun protein and of its phosphorylation in neuronal cells. Our results corroborate with the very recent findings of Perona et al. (42), showing that the Rho family GTPases efficiently induce the transcriptional activity of c-Jun.
Figure 3.
Activation of Cdc42 results in an increase in the level of c-Jun protein and of its phosphorylation. (A) V12Cdc42 or pRK5 (0.3 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, which were maintained in the presence of NGF. Twenty-four hours after injection, the cells were fixed, permeabilized, and stained with Hoechst dye (Left), a rhodamine-conjugated anti-guinea pig IgG antibody to detect the injected cells (Center), and an anti-c-Jun antibody (Right). Only the cells in which c-Jun staining was clearly above background were scored as positive. The results are presented as a scatter plot (Upper). The data are the means ± SEM of six independent experiments. Cdc42 induced a 2-fold increase in the percent of cells expressing c-Jun (white arrows) (P < 0.02). (Bar = 100 μm.) (B) V12Cdc42 or pRK5 (0.3 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, which were kept in the presence of NGF. Twenty-four hours after injection, the percent of cells expressing phospho-c-Jun was assessed. Only the cells in which phospho-c-Jun staining was clearly above background were scored positive. The results are presented as a bar graph (Upper). The data are the means ± SEM of four independent experiments; P < 0.03. Cdc42 is capable of inducing a significant increase in the level of phospho-c-Jun in the injected cells (white arrows). (Bar = 100 μm.) (C) N17Cdc42 or pRK5 (0.6 mg/ml) was microinjected into 5- to 7-day-old SCG neurons, together with guinea pig IgG. The neurons were withdrawn from NGF 4–6 hr after injection. Twenty-four hours later, the percentage of cells expressing nuclear c-Jun was assessed. The results were represented as a bar graph. The data are the means ± SEM of six independent experiments. N17Cdc42 can block the induction of the increase in the level of c-Jun that is normally observed after NGF withdrawal (white arrows). (Bar = 100 μm.)
We also examined the effect of N17Cdc42 on the induction of c-Jun that occurs after NGF withdrawal. Five- to 6-day-old SCG neurons were microinjected with N17Cdc42 or pRK5. Twenty-four hours after injection, the cells were withdrawn from NGF and the level of c-Jun was examined by immunofluorescence. Sixty percent of the cells injected with pRK5 expressed high levels of nuclear c-Jun, whereas only 25% of the cells injected with N17Cdc42 were positive for c-Jun staining (Fig. 3C). Thus, expression of N17Cdc42 decreased c-Jun levels, suggesting that Cdc42 activity is necessary for c-Jun induction after NGF deprivation.
Cdc42-Induced Apoptosis Requires AP-1 Activity.
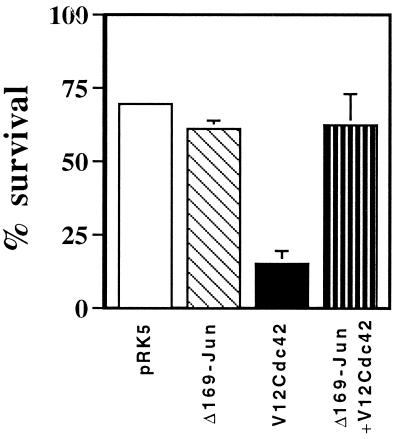
Accumulation of phosphorylated c-Jun in the nucleus upon activation of Cdc42 should lead to increased AP-1 activity. To determine whether AP-1 activity was necessary for Cdc42-induced apoptosis, we coinjected neurons with V12Cdc42 and FLAGΔ169, a mutant of c-Jun that lacks the amino-terminal transactivation domain and acts as a dominant inhibitor of AP-1 activity (11). Coexpression of FLAGΔ169 fully blocked Cdc42-induced cell death in the presence of NGF (Fig. 4). This suggests that Cdc42 activation of neuronal apoptosis requires AP-1 activity.
Figure 4.
Cdc42-induced apoptosis requires AP-1 activity. pCDFLAGΔ169 (0.4 mg/ml), 0.1 mg/ml V12Cdc42, and 70 kDa Texas Red Dextran were coinjected into SCG neurons. The cells were maintained in the presence of NGF, and the percentage of surviving cells was assessed 48 hr later. The results are the means of three independent experiments ± SEM. FLAGΔ169 blocked V12Cdc42-induced death.
DISCUSSION
Taken together, the findings described in this study demonstrate a role for the small GTP-binding proteins Cdc42 and Rac1 in the induction of apoptosis of primary sympathetic neurons. Most importantly, our results show that activated Cdc42 is required for NGF withdrawal-induced neuronal death. Our observations also suggest that Cdc42 induces neuronal cell death via an increase of the phosphorylated active form of c-Jun, suggesting that Cdc42 signals via JNK in neuronal cells as it does in other cell types (43, 44). These results corroborate with the very recently published studies by Chuang et al. (45) showing that Cdc42 could initiate an apoptotic signal in Jurkat T lymphocytes.
N17Rac1 reverses the induction of death by V12Cdc42 whereas N17Cdc42 had no effect on V12Rac1-induced death, suggesting that Cdc42 lies upstream of Rac1 as it has been shown in fibroblasts (32, 41). How the removal of NGF activates Cdc42 or Rac1 is unclear and remains a topic for future studies.
The requirement for Cdc42 in neuronal apoptosis suggests that Cdc42 is a key component of the cell death machinery in sympathetic neurons and that activation of Cdc42 is one of the early events in the death-signaling pathway. Whether Cdc42 only activates the death machinery or also inhibits components of the survival pathway remains to be elucidated. The experiments described herein suggest a target for compounds designed to block neuronal cell death.
Acknowledgments
We thank Karen Philpott, Jonathan Ham, Paul Lang, Nathalie Lamarche, and Alan Hall for helpful discussions and critical reading of the manuscript. We are also grateful to Mike Olson and Alan Hall for providing the Rac1 and Cdc42 constructs. Finally, we thank Jonathan Whitfield, Mary-Jane MacCarthy, and Galia Rimon for technical support and advice.
ABBREVIATIONS
- NGF
nerve growth factor
- JNK
c-Jun NH2-terminal kinase
- FITC
fluorescein isothiocyanate
References
- 1.Oppenheim R W. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 2.Linnik M D. Rest Neurol Neurosci. 1996;9:219–225. doi: 10.3233/RNN-1996-9404. [DOI] [PubMed] [Google Scholar]
- 3.Barde Y. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 4.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan D R, Miller F D. Curr Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 6.Martin D P, Schmidt R E, DiStefano P S, Lowry O H, Carter J G, Johnson E M. J Cell Biol. 1988;106:829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karin M, Hunter T. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 8.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Nature (London) 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 9.Eilers, A., Whitfield, J., Babij, C., Rubin, L. L. & Ham, J. (1998), J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 10.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 11.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 12.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M., Jr J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blank J L, Gerwins P, Elliott E M, Sather S, Johnson G L. J Biol Chem. 1996;271:5361–5368. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 14.Hirai S, Osada S, Spyrou G, Ohno S. Oncogene. 1996;12:641–650. [PubMed] [Google Scholar]
- 15.Yan M, Dal T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton T J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 16.Lim L, Manser E, Leung T, Hall C. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- 17.Bagrodia S, Dérijard B, Davis R J, Cerione R A. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 18.Brown J L, Stowers L, Baer M, Trejo J A, Coughlin S, Chant J. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 19.Frost J A, Xu S, Hutchinson M R, Marcus S, Cobb M H. Mol Cell Biol. 1996;16:3707–3713. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teramoto H, Coso O A, Miyata H, Igishi T, Miki T, Gutkind J S. J Biol Chem. 1996;271:27225–27338. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- 21.Rana A, Gallo K, Godowski P, Hirai S-I, Ohno S, Zon L, Kyriakis J M, Avruch J. J Biol Chem. 1996;271:19025–19028. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- 22.Tibbles L A, Ing Y-L, Kiefer F, Chan J, Iscove N, Woodgett J R, Lassam N J. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 23.Pombo C M, Kehrl H, Sanchezl L, Katz P, Avruch J, Zon L I, Woodgett J R, Force T, Kyriakis J M. Nature (London) 1995;377:750–754. doi: 10.1038/377750a0. [DOI] [PubMed] [Google Scholar]
- 24.Su Y-C, Han J, Xu S, Cobb M, Skolnik E Y. EMBO J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 26.Manser E, Chong C, Zhao Z, Leung T, Michael G, Lim L. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevitch R J, Bokoch G M. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 28.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. Nature (London) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 29.Martin G A, Bollag G, McCormick F, Abo A. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vojtek A B, Cooper J A. Cell. 1995;82:527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 31.Symons M. Trends Biochem Sci. 1996;21:178–180. [PubMed] [Google Scholar]
- 32.Nobes C D, Hall A. Cell. 1995;51:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 33.Hall A. Mol Biol Cell. 1992;3:475–479. doi: 10.1091/mbc.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deckwerth T L, Johnson E M. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards S N, Buckmaster A E, Tolkowski A M. J Neurochem. 1991;57:2140–2143. doi: 10.1111/j.1471-4159.1991.tb06434.x. [DOI] [PubMed] [Google Scholar]
- 36.Lallemand D, Spyrou G, Yaniv M, Pfarr C M. Oncogene. 1997;14:819–830. doi: 10.1038/sj.onc.1200901. [DOI] [PubMed] [Google Scholar]
- 37.Diekmann D, Brill S, Garrett M D, Totty N, Hsuan J, Monfries C, Hall C, Lim L, Hall A. Nature (London) 1991;351:400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- 38.Feig L A, Cooper G G M. Mol Cell Biol. 1988;8:2472–2478. doi: 10.1128/mcb.8.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 40.Verkrellis K, McCarthy M-J, Watson A, Whitfield J, Rubin L L, Ham J. Development. 1997;124:1239–1249. doi: 10.1242/dev.124.6.1239. [DOI] [PubMed] [Google Scholar]
- 41.Kozma R, Ahmed S, Best A, Lim L. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal J C. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 43.Coso O A, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 44.Minden A, Lin A, Claret F-X, Abo A, Karin M. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 45.Chuang T-H, Hanh K M, Lee J-D, Danley D E, Bokoch G M. Mol Biol Cell. 1997;8:1687–1698. doi: 10.1091/mbc.8.9.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]