Abstract
Objective
Evaluation of fetal cerebral cortex sulcation is important for the prenatal diagnosis of neuronal migration disorders. Although abnormal sylvian fissure morphological features are frequently observed in these conditions, the diagnosis of an abnormal sylvian fissure relies on subjective interpretation of ultrasonographic images. This study was performed to develop an objective ultrasonographic parameter for sylvian fissure evaluation.
Methods
This cross-sectional study included normal singleton pregnancies without fetal anomalies. Using multi-planar, 3-dimensional ultrasonography, the sylvian fissure midpoint was identified. The sylvian fissure-to-parietal bone distance (SPB) was measured from the midpoint to the inner surface of the parietal bone, perpendicular to the falx cerebri. Bland-Altman plots were used to determine intraobserver and interobserver agreement. Regression analysis was used to evaluate the correlation between SPB measurements and gestational age.
Results
Two hundred (99%) of 202 pregnancies had a visible sylvian fissure, identifiable as early as 12 weeks of gestation. The mean SPB values at 12 and 41 weeks was 2.1 mm and 14.3 mm, respectively. Intraobserver and interobserver mean differences between paired measurements were 0.01 mm (95% limits of agreement, -0.41 to 0.43 mm) and 0.05 mm (95% limits of agreement, -1.79 to 1.90 mm), respectively. A linear correlation was observed between the SPB and gestational age (multiple R=0.91; R2=0.82 [SPB = -2.85 + 0.42 × gestational age]).
Conclusions
(1) The SPB can be reproducibly measured from 12 weeks of gestation to term; and (2) a strong positive correlation was observed between the SPB and gestational age.
Keywords: fetal brain, sylvian fissure, 3-dimensional ultrasonography
INTRODUCTION
The normal process of cerebral cortical development has been found to follow a predictable timetable throughout gestation.1,2 Anatomic studies have demonstrated the increasing complexity of the fetal brain surface development in utero as gestational age advances.3,4 Indeed, the pattern of development of fetal brain sulcation has been subjectively described by both magnetic resonance imaging and 2-dimensional (2D) neurosonography. However, developmental patterns described with the use of these imaging modalities generally lag two to four weeks when compared to anatomic studies.5-9 For example, Lan et al6 reported the earliest visualization of the sylvian fissure by magnetic resonance imaging at 15 weeks, whereas Toi et al9 reported visualization of this structure for the first time by 2D ultrasonography at 15-16 weeks.
The sylvian fissure is the deepest lateral cerebral fissure, separating the temporal from the frontal lobes of the brain. Initially depicted as a smooth depression on the lateral surface of the brain hemisphere, the sylvian fissure can be identified in anatomic specimens by the third month of gestation.10 A recent study based on subjective evaluation of different cerebral fissures and sulci by 2D ultrasonography described the characteristic morphologic pattern of development of the sylvian fissure with increasing gestational age.9 This particular pattern of development of the sylvian fissure in fetuses without brain anomalies was characterized by: (1) a smooth margined indentation between 15 and 17 weeks; (2) the development of obtuse angular margins at the site of the developing circular sulcus between 17 and 20 weeks; and (3) a change from obtuse to acute angles after 24 weeks.
In a different study from the same group, Fong et al11 retrospectively reviewed prenatal ultrasonographic examinations of 7 fetuses with lissencephaly associated with Miller-Dieker syndrome. By applying this specific pattern of sylvian fissure maturation, as well as the developmental patterns for other sulci described previously, the authors found that all cases of lissencephaly had delayed cortical development when compared with the normal brain development expected for the same gestational age. The sylvian fissure, in particular, was visualized in all fetuses after 20 weeks and found to consistently exhibit delayed maturation. The earliest diagnosis of lissencephaly was made at 23 weeks,11 challenging previous ultrasound studies that reported the prenatal diagnosis of lissencephaly only at 27 to 28 weeks of gestation.12,13
Because lissencephaly is associated with a wide spectrum of cerebral anomalies, and ultrasonographic analysis of fetal brain sulcation remains subjective in nature, only severe forms of lissencephaly can be detected by prenatal ultrasonography, whereas milder degrees of cerebral involvement (e.g. pachygyria) may be difficult to diagnose. This is particularly true for those fetuses examined before 20 weeks (around the time that a routine ultrasonographic examination is performed), in whom a subjective diagnosis of delayed cortical development may be impossible due to the normally smooth, shallow aspect of the sylvian fissure in the early second trimester.9,11
The aims of this study were: (1) to develop an objective ultrasonographic parameter with which to assess the sylvian fissure throughout gestation; and (2) to determine the gestational age range for visualization of the sylvian fissure by 3-dimensional (3D) ultrasonography.
Materials and Methods
Study Population
This cross-sectional study included singleton pregnancies from our clinical database and digital library of ultrasonographic images from January 2001 to June 2005. All patients had uncomplicated pregnancies, no fetal structural or chromosomal anomalies, and fetal size appropriate for gestational age. Ultrasonography was performed for estimation of gestational age, anatomy, and/or fetal size evaluation. Fetal gestational age was based upon the first day of the last normal menstrual period and was confirmed by either a first-trimester (crown-rump length measurement) or early second-trimester (head circumference, biparietal diameter, femur length, and abdominal circumference) ultrasonography if menstrual history was unreliable. After delivery, all infants underwent postnatal follow-up, and no brain abnormalities were identified. All patients provided written informed consent before participation in the study. The study protocol was approved by the Institutional Review Board of the National Institute of Child Health and Human Development, as well as the Human Investigation Committees of both Wayne State University and William Beaumont Hospital.
Volume Acquisition and Measurements
Transabdominal ultrasonography was performed in all patients. Three-dimensional volume data sets of the fetal brain were acquired with motorized curved array transducers (2-5 or 4-8 MHz; Medison 530 [Medison Co, Ltd, Seoul, Korea] and Voluson 730 Expert [General Healthcare, Milwaukee, WI]) and stored on removable digital media for further analysis. Volume data sets were acquired by transverse sweeps through the fetal head, using as a reference point for the initial acquisition, the plane at which the biparietal diameter was measured. Images were examined using either 3D View version 4.5 or 4D View version 5.0 software (GE Healthcare). The sylvian fissure was identified in the original axial plane of acquisition. After adjusting the volume data set to obtain perfectly aligned axial, coronal, and sagittal planes, the reference dot was positioned at the midpoint of the sylvian fissure. Correct placement of the reference dot was confirmed in both the axial and coronal planes, and the image was then magnified as much as the reviewing software allowed. The sylvian fissure-to-parietal bone distance (SPB) was measured from the midpoint to the inner surface of the parietal bone, perpendicular to the falx cerebri (Figure 1). All measurements were performed by 1 physician (P.M.). A second examiner (J.P.K.), blinded to both the original manipulation of the volume data set and the measurements obtained by the first examiner, was randomly assigned 40 volume data sets to evaluate interobserver agreement. Each examiner measured the SPB 3 times. The mean of 3 values from Examiner 1 was used to perform the regression analysis. To evaluate intraobserver agreement, comparisons were made between the second and third measurements of Examiner 1. For the interobserver agreement analysis, the means of the second and third measurements of each examiner were compared.
Figure 1.
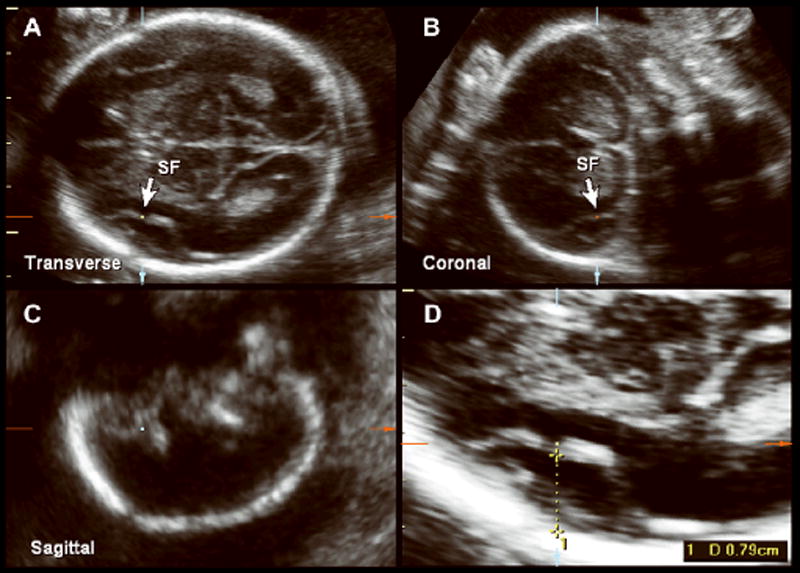
Multiplanar 3D volume of the fetal head at 22 weeks of gestation, demonstrating a transverse acquisition plane in which measurement of the SPB was taken (A), and coronal (B) (c) and sagittal (C) planes that confirmed identification of the sylvian fissure (SF). D, Magnification and measurement of the SPB.
Statistical Analysis
Regression analysis was used to determine whether there was a relationship between the SPB and gestational age. Linear, quadratic and cubic models were applied, and the model with the best fit was chosen (SPSS 12.0; SPSS Inc, Chicago, IL; MedCalc for Windows, version 8.1.0.0, MedCalc Software, Mariakerke, Belgium). Bland-Altman plots (difference of paired variables versus their average) were used to assess intraobserver and interobserver agreement.14 Ninety-five percent limits of agreement and the mean difference for paired observations were calculated.
RESULTS
A total of 202 consecutive patients who met the eligibility criteria were included. Sylvian fissure identification and measurement were possible in 200 (99%) of the 202 patients from 12 to 41 weeks. The mean measurement ranged from 2.1 mm at 12 weeks to 14.3 mm at 41 weeks 5 days (Figures 2 and 3). Among the 2 patients in whom the sylvian fissure could not be identified, 1 was at 16 weeks 3 days and the other at 17 weeks 5 days. In both data sets, the resolution of the images was not sufficiently clear to identify the structure.
Figure 2.
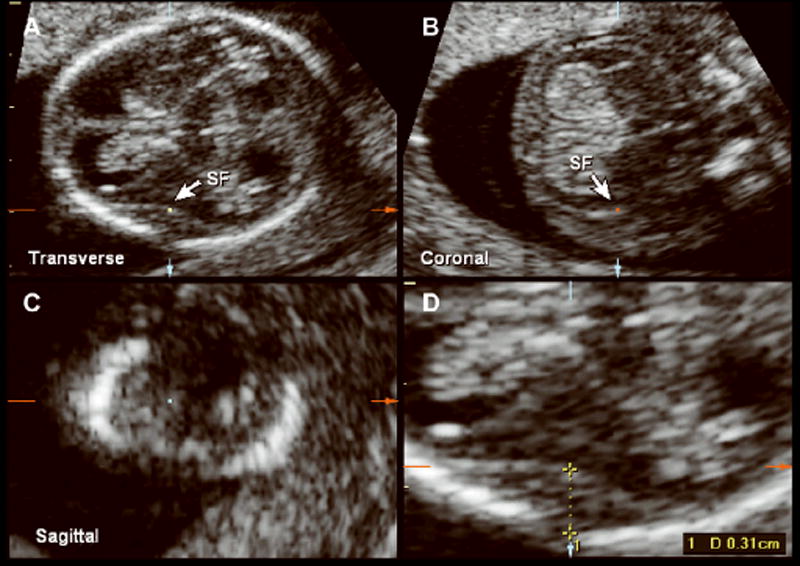
Multiplanar volume acquisition of the fetal head at 12 weeks 4 days of gestation depicting the transverse(A), coronal (B), and sagittal (C) planes with identification of the sylvian fissure (SF). D, Measurement of the SPB at 12 weeks of gestation.
Figure 3.
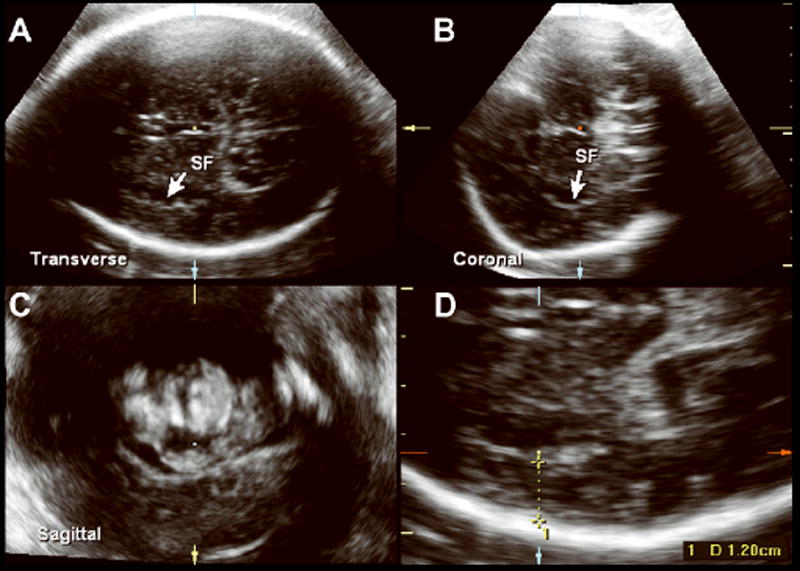
Multiplanar volume acquisition of the fetal head at 36 weeks of gestation depicting the transverse(A), coronal (B), and sagittal (C) planes with identification of the sylvian fissure (SF). D, Measurement of the SPB at 36 weeks of gestation.
The mean intraobserver difference between measurements was 0.01 mm, with 95% limit of agreement ranging from -0.41 to 0.43 mm (Figure 4). The mean difference in measurements between observers was 0.05 mm, and the 95% limits of agreement were 1.79 to 1.90 mm (Figure 5).
Figure 4.
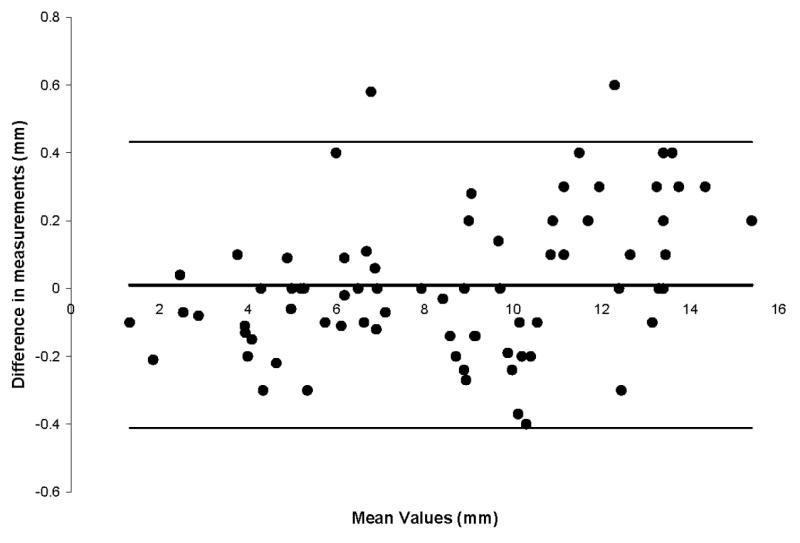
Bland-Altman plot for intraobserver variation. The mean difference between measurements was 0.01 mm. The 95% limits of agreement ranged from -0.41 to 0.43 mm.
Figure 5.
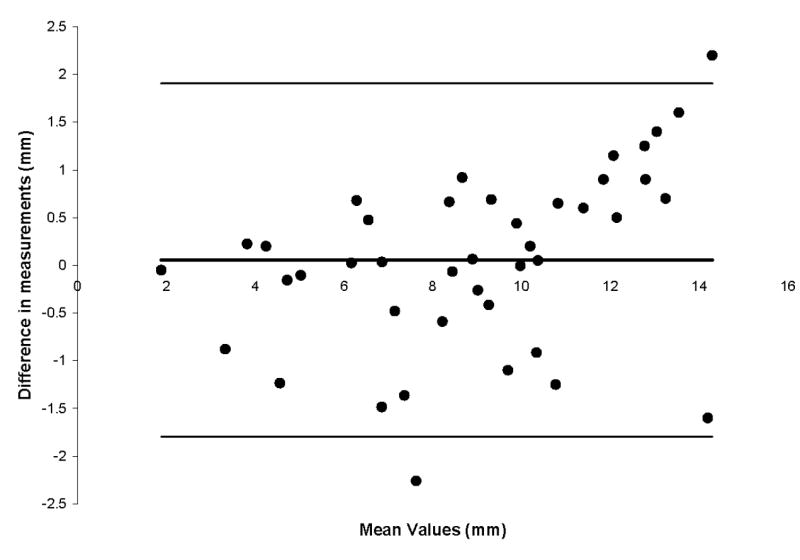
Bland-Altman plot for interobserver variation. The mean difference in measurements was 0.05 mm. The 95% limits of agreement ranged from -1.79 to 1.90mm.
Regression analysis of the SPB by gestational age was best described by a linear regression model: SPB = -2.85 + 0.43 × gestational age (R = 0.91; R2 = 0.82) (Figure 6). The expected SPB values by gestational age are shown in Table 1.
Figure 6.
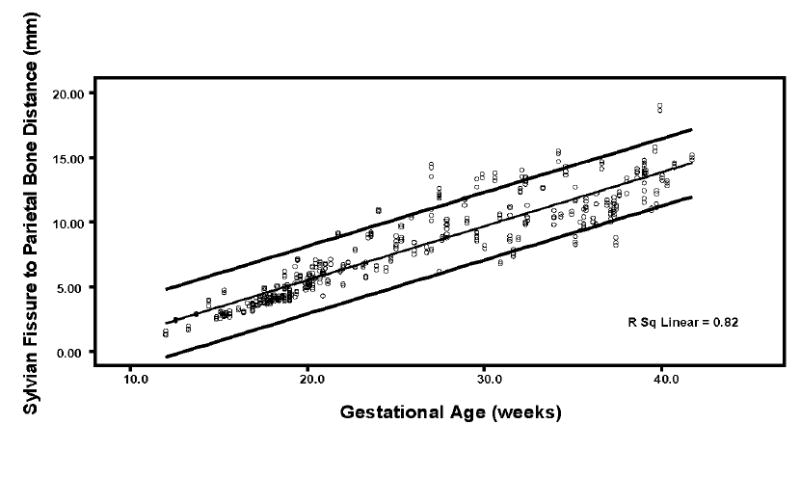
Scatterplot of the measurements of the SPB. The bottom, middle, and top lines represent the 5th, 50th, and 95th percentiles, respectively.
Table 1.
Expected SPB Values by Gestational Age (5th, 50th, and 95th Percentiles)
| SPB, mm | |||
|---|---|---|---|
| Gestational age, wk | 5th percentile | 50th percentile | 95th percentile |
| 12 | 1.74 | 2.15 | 2.66 |
| 13 | 2.05 | 2.57 | 3.09 |
| 14 | 2.46 | 2.99 | 3.51 |
| 15 | 2.87 | 3.41 | 3.94 |
| 16 | 3.28 | 3.82 | 4.36 |
| 17 | 3.69 | 4.24 | 4.79 |
| 18 | 4.10 | 4.66 | 5.21 |
| 19 | 4.51 | 5.07 | 5.64 |
| 20 | 4.92 | 5.49 | 6.06 |
| 21 | 5.32 | 5.91 | 6.49 |
| 22 | 5.73 | 6.32 | 6.91 |
| 23 | 6.14 | 6.74 | 7.34 |
| 24 | 6.55 | 7.16 | 7.76 |
| 25 | 6.96 | 7.58 | 8.19 |
| 26 | 7.37 | 7.99 | 8.61 |
| 27 | 7.78 | 8.41 | 9.04 |
| 28 | 8.19 | 8.83 | 9.46 |
| 29 | 8.60 | 9.24 | 9.89 |
| 30 | 9.01 | 9.66 | 10.31 |
| 31 | 9.41 | 10.08 | 10.74 |
| 32 | 9.82 | 10.49 | 11.16 |
| 33 | 10.23 | 10.91 | 11.59 |
| 34 | 10.64 | 11.33 | 12.01 |
| 35 | 11.05 | 11.75 | 12.44 |
| 36 | 11.45 | 12.16 | 12.86 |
| 37 | 11.87 | 12.58 | 13.29 |
| 38 | 12.28 | 13.00 | 13.71 |
| 39 | 12.69 | 13.40 | 14.14 |
| 40 | 13.10 | 13.83 | 14.56 |
| 41 | 13.50 | 14.25 | 14.99 |
DISCUSSION
Improvements in ultrasound technology have increased the possibility of recognizing fetal brain structures with better resolution and at earlier gestational ages. Worthen et al15 initially assessed the relationship of cortical sulcal development with gestational age and proposed a grading system based on the complexity of structural development. This provided initial descriptive data showing the increase in general ultrasonographic sulcal complexity with gestational age, supporting prior anatomical findings,3 but not identifying specific structures. Bernard et al16 compared the ultrasonographic detection of intracranial structures in 70 fetuses ranging from 10 to 40 weeks with anatomic sections from 43 fetuses. Identification of the sylvian fissure was not noted until 21 weeks of gestation. In a retrospective trial of 337 transvaginal sonograms, Monteagudo and Timor-Tritsch8 evaluated the development of the fetal cortex with description of the gestational ages at which targeted sulci appeared. The sylvian fissure was consistently identified at 18 weeks of gestation. Toi et al9 prospectively examined 46 fetuses with targeted transabdominal neurosonography from 15.6 to 29.6 weeks of gestation. The sylvian fissure was identified in 1 fetus at 15 to 16 weeks of gestation and in all fetuses at 17 weeks of gestation in later. These studies, however, were all performed with 2D ultrasonography, in contrast to this study, which used 3D multiplanar imaging.
Applications of 3D ultrasonography continue to evolve as this technology becomes increasingly available in clinical practice. Among the potential benefits of 3D ultrasonography, the possibility of navigating the fetal brain in 3 orthogonal planes of a section has been proposed to facilitate the evaluation of the human fetal brain.17;18 This observation has been endorsed by the recommendations of an American Institute of Ultrasound in Medicine concensus conference in 2005,19 which stated that although not the standard of care, 3D ultrasonography can be helpful in the evaluation of a variety of obstetric areas, including the fetal central nervous system. With 3D ultrasonography, the ability to correctly determine the multiple planes at which a landmark can be visualized also allows for standardization of measurement and evaluation.
There are few reports in the literature of comparison between 2D and 3D ultrasonographic visualization of the fetal brain. In a recent review of the clinical use of 3D/4D imaging in obstetrics, the authors describe 2 studies that directly compared 2D and 3D ultrasonographic examination of either normal or abnormal fetal brains.20 Both studies demonstrated an increase in the accuracy of diagnosis and visualization of structures using 3D imaging.21;22 Pilu et al23 compared the use of 2D median planes and median planes reconstructed via 3D ultrasonography in the evaluation of midline structures in both normal and anomalous fetal brains. Three-dimensional median planes correlated well with 2D images, and 3D ultrasonography was noted to be a more rapid and easier method for obtaining median plane views. The ultrasonographic findings of both modalities correlated with anatomic findings. The authors, however, did note that in normal fetuses without brain anomalies, 2D views appeared to be of superior quality. A number of studies support the utility of 3D ultrasonography in central nervous system evaluation.22;24-28 However, most of the literature involves obtaining 3D data sets via transvaginal ultrasonography. In a recent study, Correa et al29 described the use of transabdominal 3D ultrasonography for fetal neurosonography. Fetal brain volume data sets of 202 prospectively enrolled patients from 16 to 24 weeks were evaluated for visualization of major cerebral anatomical structures, including various sulci. The investigators found that the use of volume datasets obtained transabdominally (as in the current study) is feasible for examination of the fetal brain. In fact, the sylvian fissure was visualized in 65% of fetuses at 16 to 17 weeks of gestation, which is similar to what has been reported previously. Our study included fetal sonograms from an earlier gestational age than in any previous studies. Using the described technique, we were able to visualize the sylvian fissure as early as 12 weeks of gestation.
In conclusion, this study demonstrates that: (1) the sylvian fissure can be identified from as early as 12 weeks of gestation with the use of 3D multiplanar ultrasonography; (2) the SPB can be reproducibly measured; and (3) it increases linearly with advancing gestational age. Because abnormal subjective assessment of sylvian fissure morphologic features is associated with neuronal migration disorders such as lissencephaly,11 the SPB may prove to be a useful and objective parameter for identifying patients at risk for cortical dysmaturation, potentially at an earlier gestational age than previously described.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Marin-Padilla M. Prenatal and early postnatal ontogenesis of the human motor cortex: a golgi study. I. The sequential development of the cortical layers. Brain Res. 1970;23:167–83. doi: 10.1016/0006-8993(70)90037-5. [DOI] [PubMed] [Google Scholar]
- 2.Naidich TP, Grant JL, Altman N, Zimmerman RA, Birchansky SB, Braffman B, et al. The developing cerebral surface. Preliminary report on the patterns of sulcal and gyral maturation--anatomy, ultrasound, and magnetic resonance imaging. Neuroimaging Clin N Am. 1994;4:201–40. [PubMed] [Google Scholar]
- 3.Dorovini-Zis K, Dolman CL. Gestational development of brain. Arch Pathol Lab Med. 1977;101:192–95. [PubMed] [Google Scholar]
- 4.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 5.Garel C, Chantrel E, Brisse H, Elmaleh M, Luton D, Oury JF, et al. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol. 2001;22:184–89. [PMC free article] [PubMed] [Google Scholar]
- 6.Lan LM, Yamashita Y, Tang Y, Sugahara T, Takahashi M, Ohba T, et al. Normal fetal brain development: MR imaging with a half-Fourier rapid acquisition with relaxation enhancement sequence. Radiology. 2000;215:205–10. doi: 10.1148/radiology.215.1.r00ap05205. [DOI] [PubMed] [Google Scholar]
- 7.Levine D, Barnes PD. Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology. 1999;210:751–58. doi: 10.1148/radiology.210.3.r99mr47751. [DOI] [PubMed] [Google Scholar]
- 8.Monteagudo A, Timor-Tritsch IE. Development of fetal gyri, sulci and fissures: a transvaginal sonographic study. Ultrasound Obstet Gynecol. 1997;9:222–28. doi: 10.1046/j.1469-0705.1997.09040222.x. [DOI] [PubMed] [Google Scholar]
- 9.Toi A, Lister WS, Fong KW. How early are fetal cerebral sulci visible at prenatal ultrasound and what is the normal pattern of early fetal sulcal development? Ultrasound Obstet Gynecol. 2004;24:706–15. doi: 10.1002/uog.1802. [DOI] [PubMed] [Google Scholar]
- 10.Gray H. Anatomy of the Human Body. Philadelphia: Lea and Febiger; 1918. Ref Type: Internet Communication. [Google Scholar]
- 11.Fong KW, Ghai S, Toi A, Blaser S, Winsor EJ, Chitayat D. Prenatal ultrasound findings of lissencephaly associated with Miller-Dieker syndrome and comparison with pre- and postnatal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2004;24:716–23. doi: 10.1002/uog.1777. [DOI] [PubMed] [Google Scholar]
- 12.McGahan JP, Pilu G, Nyberg DA. Cerebral malformations. In: Nyberg DA, McHahan JP, Pretorius DH, Pilu G, editors. Diagnostic Imaging of Fetal Anomalies. Philadelphia: Lippincott Williams and Willkins; 2003. pp. 221–290. Ref Type: Generic. [Google Scholar]
- 13.Saltzman DH, Krauss CM, Goldman JM, Benacerraf BR. Prenatal diagnosis of lissencephaly. Prenat Diagn. 1991;11:139–43. doi: 10.1002/pd.1970110302. [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 15.Worthen NJ, Gilbertson V, Lau C. Cortical sulcal development seen on sonography: relationship to gestational parameters. J Ultrasound Med. 1986;5:153–56. doi: 10.7863/jum.1986.5.3.153. [DOI] [PubMed] [Google Scholar]
- 16.Bernard C, Droulle P, Didier F, Gerard H, Larroche JC, Plenat F, et al. Echographic aspects of cerebral sulci in the ante- and perinatal period. J Radiol. 1988;69:521–32. [PubMed] [Google Scholar]
- 17.Goncalves LF, Nien JK, Espinoza J, Kusanovic JP, Lee W, Swope B, et al. What does 2-dimensional imaging add to 3- and 4-dimensional obstetric ultrasonography? J Ultrasound Med. 2006;25:691–99. doi: 10.7863/jum.2006.25.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteagudo A, Timor-Tritsch IE, Mayberry P. Three-dimensional transvaginal neurosonography of the fetal brain: ‘navigating’ in the volume scan. Ultrasound Obstet Gynecol. 2000;16:307–13. doi: 10.1046/j.1469-0705.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 19.Benacerraf BR, Benson CB, Abuhamad AZ, Copel JA, Abramowicz JS, Devore GR, et al. Three- and 4-dimensional ultrasound in obstetrics and gynecology: proceedings of the american institute of ultrasound in medicine consensus conference. J Ultrasound Med. 2005;24:1587–97. doi: 10.7863/jum.2005.24.12.1587. [DOI] [PubMed] [Google Scholar]
- 20.Goncalves LF, Lee W, Espinoza J, Romero R. Three- and 4-dimensional ultrasound in obstetric practice: does it help? J Ultrasound Med. 2005;24:1599–624. doi: 10.7863/jum.2005.24.12.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller GM, Weiner CP, Yankowitz J. Three-dimensional ultrasound in the evaluation of fetal head and spine anomalies. Obstet Gynecol. 1996;88:372–78. doi: 10.1016/0029-7844(96)00207-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang PH, Ying TH, Wang PC, Shih IC, Lin LY, Chen GD. Obstetrical three-dimensional ultrasound in the visualization of the intracranial midline and corpus callosum of fetuses with cephalic position. Prenat Diagn. 2000;20:518–20. doi: 10.1002/1097-0223(200006)20:6<518::aid-pd860>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Pilu G, Segata M, Ghi T, Carletti A, Perolo A, Santini D, et al. Diagnosis of midline anomalies of the fetal brain with the three-dimensional median view. Ultrasound Obstet Gynecol. 2006;27:522–29. doi: 10.1002/uog.2751. [DOI] [PubMed] [Google Scholar]
- 24.Cohen LS, Sankpal R, Endres L. Transabdominal three-dimensional volume imaging of the fetal brain at 18--24 weeks’ gestation. Int J Gynaecol Obstet. 2001;72:145–50. doi: 10.1016/s0020-7292(00)00353-2. [DOI] [PubMed] [Google Scholar]
- 25.Hata T, Yanagihara T, Matsumoto M, Hanaoka U, Ueta M, Tanaka Y, et al. Three-dimensional sonographic features of fetal central nervous system anomaly. Acta Obstet Gynecol Scand. 2000;79:635–39. [PubMed] [Google Scholar]
- 26.Lai TH, Chang CH, Yu CH, Kuo PL, Chang FM. Prenatal diagnosis of alobar holoprosencephaly by two-dimensional and three-dimensional ultrasound. Prenat Diagn. 2000;20:400–03. doi: 10.1002/(sici)1097-0223(200005)20:5<400::aid-pd839>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Pooh RK, Pooh K. Transvaginal 3D and Doppler ultrasonography of the fetal brain. Semin Perinatol. 2001;25:38–43. doi: 10.1053/sper.2001.22895. [DOI] [PubMed] [Google Scholar]
- 28.Timor-Tritsch IE, Monteagudo A, Mayberry P. Three-dimensional ultrasound evaluation of the fetal brain: the three horn view. Ultrasound Obstet Gynecol. 2000;16:302–06. doi: 10.1046/j.1469-0705.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 29.Correa FF, Lara C, Bellver J, Remohi J, Pellicer A, Serra V. Examination of the fetal brain by transabdominal three-dimensional ultrasound: potential for routine neurosonographic studies. Ultrasound Obstet Gynecol. 2006;27:503–08. doi: 10.1002/uog.2750. [DOI] [PubMed] [Google Scholar]


