Abstract
A new environment-sensitive fluorophore, 6-N,N-dimethylamino-2,3-naphthalimide (6DMN) was introduced in the δ-selective opioid agonist H-Dmt-Tic-Glu-NH2 and in the μ-selective opioid agonist endomorphin-2 (H-Tyr-Pro-Phe-Phe-NH2). Environment sensitive fluorophores are a special class of chromophores that generally exhibit a low quantum yield in aqueous solution, but become highly fluorescent in nonpolar solvents or when bound to hydrophobic sites in proteins or membranes. New fluorescent δ-selective irreversible antagonists [H-Dmt-Tic-Glu-NH-(CH2)5-CO-Dap(6DMN)-NH2(1) and H-Dmt-Tic-Glu-Dap(6DMN)-NH2)] (2) were identified as potential fluorescent probes showing properties suitable for studies of distribution and internalization of δ-opioid receptors by confocal laser scanning microscopy.
Introduction
Fluorescence spectroscopy has become one of the most valuable tools for the development of new probes for biochemical research,1, 2 being extensively used for monitoring ions,3 small molecules,4 and biological processes,5 such as protein folding,6 protein-protein interactions,7 and phosphorylation events.8 While many fluorescence applications rely on the use of intrinsic fluorophores, the development of new extrinsic fluorophores remains an essential element for the design of new fluorescent probes. Environment-sensitive fluorophores are a special class of chromophores whose spectroscopic behaviour depends on the physicochemical properties of its immediate environment.9 These molecules generally exhibit a low quantum yield in aqueous solution, but become highly fluorescent in non polar solvents or when bound to hydrophobic sites in proteins or membranes. Particularly useful are the solvatochromic fluorophores that display sensitivity to the polarity of the local environment, such as 2-propionyl-6-dimethylaminonaphthalene (PRODAN),10 4-dimethylamino phtalimide (4-DMAP),11 and 4-amino-1,8-naphthaimide derivatives.12 Prodan and derivatives (Aladan, an alanine derivative of 6-dimethylamino-2-acyl-naphthalene) 13, 14 still constitute the most widely used environment-sensitive fluorophores, regardless of certain limitations mainly resulting from the relatively intense fluorescence even in aqueous environments.
Peptide ligands for opioid receptors were previously labelled with fluorescent functionalities, such as rhodamine,15 pyrene,16 dansyl,17, 18 and fluorescein.19 20 These groups can be readily attached to a free carboxylic acid or an amino group on the peptides in one of two ways: (i) to a side chain functional group of a noncritical residue; or (ii) by extending the peptide backbone in a manner that has minimal influence on receptor binding. 21 Other studies show that a non-peptidic fluorescent probe, derived from the naltrindole template for the δ-opioid receptor, is a potent δ-opioid receptor antagonist in the mouse vas deferens (MVD, a smooth muscle assay) and binds to the δ-opioid receptor with relatively high affinity (Ki = 1 nM) and selectivity.20 However, with the exception of the arylacetamide-derived fluorescent ligands,22 none of these compounds have been reported as molecular probes nor was their selectivity for any of the major opioid receptor types (δ, μ, κ) studied.
The synthesis of the μ-opioid peptide [Dmt1]DALDA (H-Dmt-D-Arg-Phe-Lys-NH2) containing dansyl or anthranoyl fluorophores was reported by Schiller et al.17, 18 and who subsequently described the synthesis of a fluorescent δ-opioid ligand containing the environment-sensitive amino acid Aladan (H-Tyr-Tic-Aladan-Phe-OH) [Ki(δ) = 2.56 nM, selectivity μ/δ = 7930, Φ = 0.150 in Tris-HCl buffer pH 6.6]. 14 Furthermore, our group reported the synthesis of a δ-selective tripeptide containing fluorescein at the C-terminal (H-Dmt-Tic-Glu-NH-(CH2)5-NH-C(=S)-NH-fluorescein [Ki(δ) = 0.035 nM, selectivity μ/δ = 4370, Φ = 0.227 in Tris-HCl buffer pH 6.6]. 23
Recently, Imperiali et al. published the synthesis of the new environment sensitive fluorophore 6-N,N-dimethylamino-2,3-naphthalimide (6DMN) and of the corresponding N-protected amino acid Fmoc-α-amino-β-(6-N,N-dimethylamino napthalimide) propanoic acid (Fmoc-Dap(6DMN)-OH).24 They demonstrated that the corresponding model compound methyl 2-(6-(dimethylamino)-1,3-dioxo-1H-benzo[f]isoindol-2(3H)-yl)acetate (6DMN-Gly-OMe) had a red shifted fluorescence in polar protic environments; i.e., maximum emission intensity shifted from 491 nm in toluene to 592 nm in water and the fluorescence quantum yield (Φ) decreased more than 100-fold from chloroform (0.225) to water (0.002). The 6DMN fluorophore combines some of the advantageous fluorescence properties of PRODAN with the extreme sensitivity to the local polarity exhibited by the 4-aminophthalimide family of the environment-sensitive fluorophores. 11 This type of environment-sensitive molecule could be useful in confocal microscopy studies where flurescence will be higher for opioid-receptor interactions and lower for non-specific binding.
In this correspondence we evaluate the opportunities offered by this new fluorescent amino acid starting from the data collected with our reference compound H-Dmt-Tic-Glu-NH-(CH2)5-NH-C(=S)-NH-fluorescein.23 We report the synthesis, biological evaluation and in situ visualization of δ opioid receptors in mouse neuroblastoma cells of μ- and δ-selective opioid ligands derived from endomorphin-2 25 and the Dmt-Tic pharmacophore, respectively (Table 1). 26
Table 1.
Receptor binding and functional bioactivity.
| Receptor affinity a(nM) | Functional bioactivity (nM) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Structure | Kiδ | Kiμ | μ/δ | δ/μ | MVD ICb50 | MVD Kce | GPI ICb50 |
| 0.035 d | 152 d | 4370 d | Irreversible antagonist d | >1000 d | ||||
| 1 | 0.158±0.027 (3) | 41.3±1.09 (3) | 261 | Irreversible antagonist > 0.3 nM | 244±35 | |||
| 2 | H-Dmt-Tic-Glu-Dap(6DMN)-NH2 | 7.80±0.29 (4) | 7880±1139 (4) | 1010 | Irreversible antagonist > 10 nM | Partial agonist (max 40%) IC50 = 100 nM | ||
| 3 | 668.4 ±123 (6) | 251.7±90 (6) | 2.7 | Partial agonist (max 20%) IC50 = 200 nM | 120±8 | |||
| 4 | 5937±1396 (4) | 244.5±14 (5) | 24 | Partial agonist (max 29%) IC50 = 300 nM | 253±15 | |||
| 5 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2 | 839±38 (3) | 3420±817 (3) | 4.1 | Partial agonist (max 22%) IC50 = 500nM | 389±12 | ||
Chemistry
Peptides, including the fluorescent-sensitive amino acid (1, 2, 4, 5), were synthesized using standard Fmoc solid phase peptide synthesis (SPPS), cleaved/deprotected from the solid support, and purified by HPLC as detailed in the experimental section. Compound 3 containing fluorescein was prepared by standard solution peptide synthesis as reported in Scheme 1 (Supporting Information). Boc-Tyr-Pro 27 was condensed with H-Phe-Phe-OBzl 28 via WSC/HOBt. After C-terminal benzyl ester deprotection by catalytic hydrogenation (Pd/C, 10%), N-Z-1,5-pentanediamine was condensed (WSC/HOBt). N-Z deprotection by catalytic hydrogenation (Pd/C, 10%) gave the intermediate Boc-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH2 suitable for the reaction with fluorescein 5-isothiocyanate.23 Final N-Boc-deprotection with TFA gave the final crude product (3) that was purified by preparative HPLC as detailed in supporting information.
Results and Discussion
In vitro opioid activity profile
Receptor binding and functional bioactivities are reported in Table 1. In comparison to the reference δ-selective fluorescent compound derivative,23 1 is characterized by the substitutions of 1,5-pentanediamine—introduced to reduce potential interference with opioid receptors—with 6-aminohexanoic acid (the same number of methylene units) and fluorescein by the new fluorescent probe Dap(6DMN). These modifications are detrimental for binding and selectivity at δ-opioid receptors; in fact, affinity for δ-opioid receptors decreased 4.5 fold, while that for μ-opioid receptors increased 3.7-fold yielding a loss of selectivity of about 17-fold. Unexpectedly, 2 (the analogue without the pentamethylene spacer) decreased δ affinity 49-fold, but increased selectivity 3.9 fold (Kδi= 7.8 nM, selectivity = 1010) relative to 1. Compounds 3-5 depict three attempts to synthesize fluorescent derivatives selective for μ opioid receptors; only a few examples are reported in the literature. In fact, the least active but non-selective μ -opioid peptides synthesized were reported by Schiller et al.; 17, 18 the fluorescent analogues of [Dmt1 ]DALDA were endowed of high μ affinity (Kμi ranging from 0.508 to 0.589 nM) but exhibited very low selectivities (Kδi/Kμi = 3.2-6.1). Cmpd 4 coincides with 1 where the δ selective tripeptide Dmt-Tic-Glu is substituted by the μ selective tetrapeptide Tyr-Pro-Phe-Phe corresponding to the sequence of endomorphin-2. This new analogue (4), in spite of its low μ affinity (Kμi = 244.5 nM), is characterized by a selectivity (Kδi/Kμi = 24) which is higher than other published μ selective compounds.29 On the other hand, 3 is comparable to the reference compound where the δ selective tripeptide Dmt-Tic-Glu was replaced by the μ selective tetrapeptide Tyr-Pro-Phe-Phe. The endomorphin-2 analogue(5) [H-Tyr-Pro-Dap(6DMN)-Phe-NH2] derives from a similar modification reported in the δ selective tetrapeptide TIPP, where Phe3 was substituted by the fluorescent amino acid Aladan to yield [Aladan3 ]TIPP.14 Considering this modification, we substituted Dap(6DMN) in the third position of the endomorphin-2 sequence; however, both these two compounds (4, 5) had negligible affinity and selectivity for μ- and δ-opioid receptors.
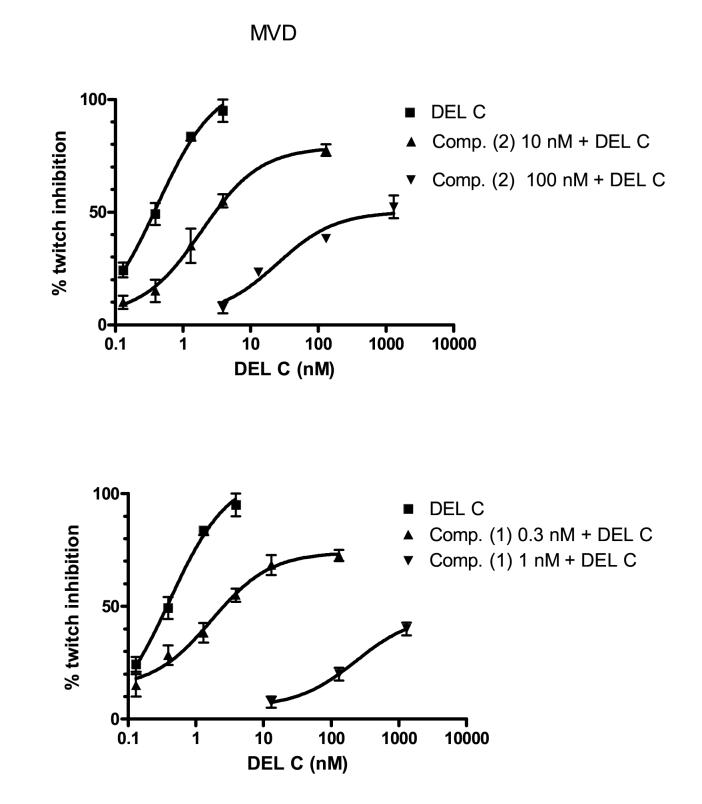
Compounds 1-5 were tested for functional bioactivity in MVD and GPI preparations (Table 1). Interestingly, these fluorescent derivatives (1 and 2) and the reference compound in the MVD assay demonstrated non-equilibrium antagonist activity (Figs. 1 and 2). The log dose-response curves of deltorphin C (δ agonist) in the presence of increasing concentrations of 1 or 2 reduced the apparent efficacy and Hill slope [deltorphin C = 1.1; +0.3 nM (1) = 0.7 and at +1 nM (1) = 0.5; at +10 nM (2) = 0.9 and at +100 nM (2) = 0.6]. Both compounds bind tightly and dissociated very slowly in the tissue preparation; δ antagonism could not be reversed by washing the tissue with a drug-free solution for over a 3 h period of time. Moreover, the longer the compound remained in contact with the tissue, the greater was the magnitude of the observed δ antagonism. Compound 1 containing the pentamethylene spacer between the opioid sequence and the fluorescent amino acid exhibited a δ-irreversible antagonism at a concentration as low as 0.3 nM, whereas the corresponding derivative lacking the pentamethylene spacer (2) began at a 30-fold higher concentration (10 nM)confirming the importance of the spacer between the opioid pharmacophore and the fluorophore.
Figure 1.
Functional bioactivity of compounds 1 and 2 on MVD. Inhibition of DEL C induced twitch by (2) (10 and 100 nM) and (1) (0.3 and 1 nM) were conducted as given in the experimental section using MVD preparations.
Figure 2.
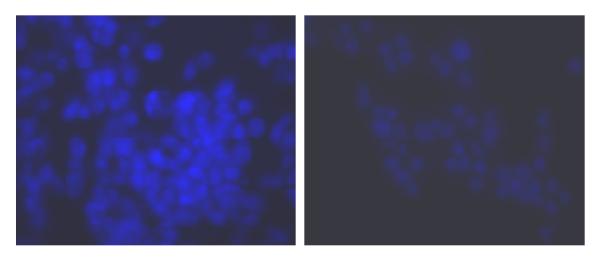
Microscopic visualization of compound 1 in NG108-15 cells: (left) NG108-15 cells with fluorescence compound; (right) non-specific binding obtained by preincubation with the opioid receptor antagonist naloxone.
In the GPI assay, 1 and 2 are endowed with weak agonist or partial agonist activities, which is in agreement with the affinity data and selectivity Compound 3, projected as an analogue of the reference compound modified to have potential μ-opioid selectivity in addition to 4 and 5, failed to elicit opioid activity, confirming the difficulty in obtaining biologically functional fluorescent μ ligands based on an endomorphin scaffold.
Fluorescence Detection
Visualization of δ-opioid receptors in situ with our fluorescent probe was obtained by incubating (15 min at 35 °C) 1 (20 nM) with the NG108-15 (mouse N18 neuroblastoma × rat C6 glioma) cell line, which expresses mouse δ-opioid receptors. Fig. 2 shows the fluorescent photomicrograph obtained using 1 in absence (left panel) or in presence (right panel) of the non-specific opioid-receptor antagonist naloxone (20 μM); preincubation with naloxone (10 min) essentially abolished the fluorescence bound to δ-opioid receptors. This illustrates the advantage of using environment-sensitive fluorophores with low Φ in the “unbound” state and results in a lower background signal from the free probe, therefore greater sensitivity and spatial resolution.
Fluorescence quantum yield measurements
In order to probe the environment affecting the sensitivity of the fluorescent peptides, the emission spectra and the fluorescent quantum yields of 1 were recorded in solvents of different polarity: water (Tris-HCl buffer at pH = 6.6, acetonitrile and dichloromethane. Fluorescence quantum yields were calculated with respect to quinine sulphate in 0.5M H2SO4 as the standard (Φ = 0.546).30 Steady state fluorescence parameters of 1 and 2 are reported in Table 2. Solutions of both the samples and the reference compound were prepared by dilution in the appropriate solvent from the primary solutions whose absorbance was below 0.2 at the same excitation wavelength (350 nm). Fluorescent measurements were taken for each solution with the same instrument parameters and the fluorescence spectra were corrected for instrumental response before integration. The integrated corrected emission spectra values were plotted against the absorbance and data used least squares fit to a straight line. The slope of the best-fit line was assumed to be proportional to the emission quantum yield. The yield for each sample was calculated as detailed in the experimental section. For samples in aqueous solution, the correction for refractive index was assumed to be insignificant. The spectroscopic behaviour of 1 resembled that of the model compound 6DMN-GlyOMe reported by Imperiali et al.;24 the lower energy absorption maxima, around 380 nm, showed only a small dependence on solvent polarity, while emission maxima followed the trend observed for 6DMN-GlyOMe in CH3CN and CH2Cl2. The emission maxima found in water at pH = 6.6 for 1 and 2 (λem= 467 and 464 nm respectively) are significantly blue shifted with respect to those expected on the basis of the previously reported behaviour of the fluorophore in protic solvents and water at neutral pH. This could be attributed to the role of protonation inside the compound, which might have a strong influence on the electronic structure of the excited state.
Table 2.
Steady state fluorescence parameters of compounds 1 and 2.
| No. | λem (nm) | λem, corr (nm) | Φ |
|---|---|---|---|
| Reference | 520 a | 522 a | 0.24±0.01 a |
| 464 a | 464 a | 0.012±0.001 a | |
| 1 | 557 b | 571 b | 0.194±0.01 b |
| 534 c | 541 c | 0.396±0.01 c | |
| 467 a | 467 a | 0.022±0.001 a | |
| 2 | 554 b | 567 b | 0.192±0.01 b |
| 531 c | 538 c | 0.394±0.01 c |
Conclusion
The incorporation of the amino acid H-Dap(6DMN)-OH containing the environment-sensitive fluorophore 6-N,N-dimethylamino-2.3-naphthalimide (6DMN) into compounds containing the δ-opioid Dmt-Tic pharmacophore antagonist yielded selective probes (Kδi/Kμi = 260 and 1000 relative to the μ-opioid receptor) that bound as apparent irreversible antagonists to δ-opioid receptors in membranes of NG108-15 cells. The maintenance of the opioid binding properties and fluorescence parameters indicated that the fluorophore had weak effect on δ-opioid receptor interaction. Our fluorescent conjugates 1 and 2 could be applied as a specific probe to study the in vitro localization of δ-opioid receptors in tissues, receptor internalization, and trafficking in live cells in real time by use of confocal microscopy. 29 It is important to emphasize that environment-sensitive fluorophores become highly fluorescent in either nonpolar solvents or when bound to hydrophobic sites in proteins or membranes. These environment-sensitive molecules could be useful in confocal microscopy studies where flurescence will be higher for interactions between an opioid ligand and its receptor and lower for non-specific binding.
Experimental Section
All peptide synthesis reagents and amino acid derivatives were purchased from GL Biochem (Shanghai) and Novabiochem. C-terminal amide peptides were synthesized on Fmoc-PAL-PEG-PS resin from Applied Biosystems, and all other chemicals were purchased from Aldrich or Fluka. High-performance liquid chromatography was performed using an Agilent 1100 series Liquid Chromatograph Mass Spectrometer system. Analytical HPLC was run using LiChrospher RP-18 (5 μm) 4,6 × 150 mm analytical column from Merck. Purification of peptides was made on a ZORBAX ODS C18, 94 × 250 mm reverse-phase column. The standard gradient for analytical and preparative HPLC used was 90:10 to 20:80 over 30 minutes (water:acetonitrile, 0.1% TFA). Electrospray Ionization Mass Spectrometry (ESI/MS) was performed with an Agilent 1100 Series LC/MSD model in positive scan mode using direct injection of the purified peptide solution into the MS. 1H NMR (δ) spectra were measured, when not specified, in DMSO-d6(Bruker AC-200 spectrometer) and peaks are parts per million downfield from tetramethylsilane (internal standard). TLC was performed on precoated plates of silica gel F254 (Merck, Darmstadt, Germany) using the following solvent systems: (A) 1-butanol/AcOH/H2O (3:1:1, v/v/v), (B) CH2Cl2/methanol/toluene (17:1:2); ninhydrin (1%, Merck), fluorescamine (Hoffman-La Roche), and chlorine reagents used as sprays. Melting points were determined on a Kofler apparatus and are uncorrected. Optical rotations were determined at 10 mg/mL in methanol with a Perkin-Elmer 241 polarimeter with a 10 cm water-jacketed cell.
Peptide synthesis used standard SPPS protocols on a 0.02 to 0.05 mmol scale using a 0.21 mmol/g loading PAL-PEG-PS solid support. Amino acids were purchased as protected Fmoc amino acids with the standard side chain protecting scheme: Fmoc-6-aminohexanoic acid, Fmoc-Glu(OtBu)-OH, Fmoc-Tic-OH, Fmoc-Phe-OH, Fmoc-Pro-OH. Boc-Tyr(tBu)-OH and Boc-Dmt-OH 31 were used as N-terminal amino acids. The synthesis of Fmoc-Dap(6DMN)-OH fluorescent building block was made using previously reported procedures.24 Amino acids were manually coupled in four-fold excess using a mixture of N-[(1H-benzotriazol-1-yl)(dimethylamino)methylene]-N-methylmethanaminium (HBTU) and 1-hidroxybenzotriazole (HOBt) and N,N-diisopropylethylamine (DIEA) in DMF as activating agents. Coupling of Fmoc-Dap(6DMN)-OH was performed using 2-(1H-7-aza-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate methanaminium (HATU) but otherwise using the same protocol as the other amino acids. Each amino acid was activated for two minutes in DMF before being added onto the resin. Peptide bond-forming couplings were conducted for 1 h and monitored using the TNBS test.32 Deprotection of the temporal Fmoc protecting group was made treating the resin with 20% piperidine in DMF solution for 15 minutes.
Peptides were cleaved from the resin, and side-chain protecting groups were simultaneously removed by treatment with the following cleavage mixture: 50 μL dichloromethane, 25 μL triisopropyl silane, 25 μL water and 950 μL TFA (1 mL of mixture/50 mg of resin) for 1.5-2 h at room temperature. All peptides were precipitated with diethyl ether (4 °C) and further purified by reverse-phase HPLC.
Deprotection
To 0.05 mmol of Fmoc-HN-(aa)n on a solid support, piperidine (3 mL, 20% in DMF) was added and nitrogen was passed through the mixture for 15 minutes. The resin was then filtered and washed with DMF (3 × 3 mL × 3 min) and TNBS test was run with a small resin sample to confirm that the deprotection was successful.
Coupling
Fmoc-aa-OH (0.2 mmol) was dissolved in HOBt/HBTU solution (1 mL 0.2 M HBTU, 0.2M HOBt in DMF) and DIEA (1.5 mL 0.195 M solution in DMF) was added, the resulting mixture was activated for 2 min, and then added over the previously deprotected resin. Nitrogen was passed through the resin suspension for 1 h, when TNBS test of a small resin sample was negative. The resin was then washed with DMF (3 × 3 mL × 3 min) and subjected to the following deprotection/coupling cycles in a similar way.
Coupling of Fmoc-Dap(6DMN)-OH
Fmoc-Dap(6DMN)-OH (55 mg, 0.1 mmol) and HATU ( 38 mg, 0.1 mmol) were dissolved in DMF (0.5 mL) and DIEA (0.75 mL 0.195 M solution in DMF) was added, the resulting mixture was activated for 2 minutes, and then added over the resin. Nitrogen was passed through the resin suspension for 1 h, when TNBS test of a small resin sample was negative. The resin was then washed with DMF (3 × 3 mL × 3 min) and recoupled with Fmoc-Dap(6DMN)-OH following the same protocol described above. The resin was then subjected to the following deprotection/coupling cycles using the HOBt/HBTU coupling scheme.
Cleavage
An aliquot of 0.05 mmol of resin bound peptide dried overnight was placed in a 50 mL flask, to which was added 5 mL of the cleavage cocktail (250 μL CH2Cl2, 125 μL water, 125 μL TIS, TFA to 5 mL), and the resulting mixture was shaken for 2 h, the resin was filtered and the TFA filtrate was concentrated under an argon current until a volume of 2 mL, and added over ice-cold ethyl ether (40 mL). After 5 min, the peptide was centrifuged and washed again with 20 mL of ice-cold ether. The solid residue was dried under Ar and redissolved in acetonitrile/water 1:1 (2 mL), and purified by preparative reverse-phase HPLC. The collected fractions were lyophilized and stored at -20 °C.
H-Dmt-Tic-Glu-NH-(CH2)5-(C=O)-Dap(6DMN)-NH2(1)
Rf(A) 0.72; HPLC K′ 7.91; mp 161-163 °C; [α]20 -7.8; MH+ D 920; 1H-NMR (DMSO-d6) δ 1.29-1.57 (m, 6H), 2.06-3.20 (m, 24H), 3.95-5.16 (m, 8H), 6.29-8.42 (m, 11H).
H-Dmt-Tic-Glu-Dap(6DMN)-NH2(2)
Rf(A) 0.65; HPLC K′ 7.38; mp 157-159 °C; [α]20 -9.2; MH+ D 807; 1 H-NMR (DMSO-d6) δ 2.06-3.17 (m, 20H), 3.95-5.16 (m, 8H), 6.29-8.42 (m, 11H).
TFA. H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S)-NH-fluorescein (3)
Rf(A) 0.66; HPLC K′ 7.26; mp 153-155 °C; [α]20D-15.1; MH+ 1047; 1 H-NMR (DMSO-d6) δ 1.29-1.55 (m, 6H), 1.92-2.34 (m, 4H), 2.92-3.29 (m, 8H), 3.41-3.51 (m, 4H), 3.95-4.92 (m, 4H), 6.11-7.28 (m, 23H).
H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-Dap(6DMN)-NH2(4)
Rf(A) 0.74; HPLC K′ 7.96; mp 165-167 °C; [α]20D-13.8; MH+ 995; 1 H-NMR (DMSO-d6) δ 1.29-1.57 (m, 6H), 1.92-2.34 (m, 6H), 2.85-3.20 (m, 14H), 3.41-3.51 (m, 2H), 3.95-5.16 (m, 7H), 6.68-8.37 (m, 19H). H-Tyr-Pro-Dap(6DMN)-Phe-NH2(5). Rf(A) 0.69; HPLC K′ 7.48; mp 160-162 °C; [α]20D -14.3; MH+ 735; 1 H-NMR (DMSO-d6) δ 1.92-2.34 (m, 4H), 2.92-3.17 (m, 10H), 3.41-3.51 (m, 2H), 3.95-5.16 (m, 6H), 6.68-8.42 (m, 14H). Elemental analysis of compounds 1-5 is reported in Table 3 (Supporting Information).
Competitive Receptor Binding Assays
These assays were conducted as described in detail elsewhere using rat brain synaptosomes (P2 fraction).31, 33-35 Membrane preparations were preincubated to eliminate endogenous opioid peptides and stored at -80 °C in buffered 20% glycerol.34, 36 Each analogue was analyzed in duplicate using five to eight dosages of peptide and independent repetitions with different synaptosomal preparations (n values are listed in Table 1 in parentheses and the results are listed as the mean ± SE). Unlabeled peptide (2 μM) was used to determine non-specific binding in the presence of 1.9 nM [3 H]deltorphin C (45.0 Ci/mmol, Perkin-Elmer, Boston, MA; KD= 1.4 nM) for δ-opioid receptors, and for μ-opioid receptors, 3.5 nM [3 H]DAMGO (50.0 Ci/mmol, Amersham Biosciences, Buckinghamshire, U.K.; KD= 1.5 nM). Glass fiber filters (Whatman GFC) were soaked in 0.1% polyethylenimine to enhance the signal/noise ratio of the bound radiolabeled-synaptosome complex, and the filters washed thrice in ice-cold buffered BSA.34 The affinity constants (Ki) were calculated according to Cheng and Prusoff.37
Functional Bioactivity in Isolated Organ Preparations
Preparations of myenteric plexus-longitudinal muscle obtained from male guinea pig ileum (GPI, enriched in μ-opioid receptors) and preparations of MVD (containing δ-opioid receptors) were used for field stimulation with bipolar rectangular pulses of supramaximal voltage.38 Agonists were evaluated for their ability to inhibit the electrically evoked twitch. The biological potency of the compounds was compared against the activity of the μ-opioid receptor agonist dermorphin in GPI and with MVD for the δ-opioid receptor measured agonist deltorphin C. The results are expressed as the IC50 obtained from dose-response curves (Prism, GraphPad). To evaluate antagonism, compounds were added to the bath and allowed to interact with tissue receptor sites 5 min before adding the standard peptide. Competitive antagonist activities were evaluated for their ability to shift the deltorphin C (MVD) and dermorphin (GPI) log concentration-response curve to the right; pA2 values were determined using the Schild Plot.39 IC50(nM, mean ± SE) as well as the pA2 were obtained from at least six experiments conducted with fresh tissues.
Fluorescence Spectroscopy
Fluorescence emission spectra were recorded on a Jobin Yvon-Spex FluoroMax-2 spectrofluorometer with 1 nm spectral resolution for excitation and emission. The excitation wavelength was 350 nm. Fluorescence quantum yield (Φ) was determined relative to quinine sulfate (Fluka) in 1 N H2SO4(Φ = 0.546) 30 as a reference. The quantum yield was calculated according to
where the subscripts S and R refer to the sample and reference compounds, respectively, E is the integrated area under the corrected emission spectrum, and A is the absorbance of the solution at the excitation wavelength. Absorbance values were kept below 0.2 to minimize inner filter and self-quenching effects. Since the sample and the reference were in aqueous solution, the correction for the refractive index (nS/nR)2 was considered to be insignificant. To detect the fluorescent signal a Nikon Optiphot microscope (Hoechest filter EX 350 nm, EM 467 nm) was used at 40 × magnification.
Supplementary Material
Acknowledgments
This research was supported in part by the University of Cagliari (PRIN 2004), University of Ferrara, Italy, and the Intramural Research Program of the NIH, and NIEHS. The authors appreciate the professional services of the library staff of the NIEHS. M.E.V. thanks the Human Science Frontier Program Organization for their support with the Career development Award (CDA0032/2005-C). M.E.V. and G.B. sincerely thank Prof. Barbara Imperiali (MIT, Boston, U.S.A.) for her support and useful advice in the development of this project.
References
- (1).Abbreviations. In addition to the IUPAC-IUB Commission on Biochemical Nomenclature. J. Biol. Chem. 1985;260:14–42. this paper uses the following additional symbols and abbreviations: Ac, acetyl; Bid, 1H-benzimidazol-2-yl; Boc, tert- butyloxycarbonyl; DAMGO, [D-Ala2 ,N-Me-Phe4 ,Gly-ol5 ]-enkephalin; DEL C, deltorphin C, (H-Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2); DER, dermorphin (H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2); DMF, N,N-dimethylformamide; DMSO-d6, hexadeuteriodimethyl sulfoxide; Dmt, 2′,6′-dimethyl-L-tyrosine; GPI, guinea-pig ileum; HOBt, 1-hydroxybenzotriazole; HPLC, high performance liquid chromatography; MVD, mouse vas deferens; pA2, negative log of the molar concentration required to double the agonist concentration to achieve the original response; TFA, trifluoroacetic acid; Tic, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid; TIS,.....; TIPP, H.-Tyr-Tic-Phe-Phe-OH.; TLC, thin-layer chromatography; TNBS, ......; WSC = EDC, 1-ethyl-3-[3′-dimethyl)aminopropyl]-carbodiimide hydrochloride; Z, benzyloxycarbonyl; NMM, 4-methylmorpholine; MALDI-TOF, matrix assisted laser desorption ionization time-of-flight. [Google Scholar]
- (2).Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell. Biol. 2002;3:906–18. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
- (3).Jiang P, Guo Z. Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 2004;248:205–229. [Google Scholar]
- (4).Chan P-H, Liu H-B, Chen YW, Chan K-C, Tsang C-W, Tsang C-W, Leung Y-C, Wong KY. J. Am. Chem. Soc. 2004;126:4074–4075. doi: 10.1021/ja038409m. [DOI] [PubMed] [Google Scholar]
- (5).Lippincott-Schwartz J, Patterson GH. Development and Use of Fluorescent Protein Markers in Living Cells. Science. 2003;300:87–91. doi: 10.1126/science.1082520. [DOI] [PubMed] [Google Scholar]
- (6).Heyduk T. Curr. Opin. Biotechnol. 2002;13:292–296. doi: 10.1016/s0958-1669(02)00332-4. [DOI] [PubMed] [Google Scholar]
- (7).Jameson DM, Croney JC, Moens PDJ. [1] Fluorescence: Basic concepts, practical aspects, and some anecdotes. Methods Enzymol. 2003;360:1–43. doi: 10.1016/s0076-6879(03)60105-9. [DOI] [PubMed] [Google Scholar]
- (8).Chen C-A, Yeh R-H, Yan X, Lawrence DS. Biosensors of protein kinase action: from in vitro assays to living cells. Biochim. Biophys. Acta. 2004;1697:39–51. doi: 10.1016/j.bbapap.2003.11.012. [DOI] [PubMed] [Google Scholar]
- (9).Valeur B. Molecular Fluorescence: Principles and Applications. Wiley-VCH; Weinheim, Germany, New York: 2002. [Google Scholar]
- (10).Weber G, Farris FJ. Synthesis and spectral properties of a hydrophobic fluorescent probe 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979;18:3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- (11).(a) Saroja G, Soujanya T, Ramachandran B, Samanta A. 4-Aminophthalimide derivatives as environment-sensitive probes. J. Fluoresc. 1998;8:405–410. [Google Scholar]; (b) Vázquez ME, Rothman DM, Imperiali B. A new environment-sensitive fluorescent amino acid for Fmoc-based solid phase peptide synthesis. Org. Biomol. Chem. 2004;2:1965–1966. doi: 10.1039/b408001g. [DOI] [PubMed] [Google Scholar]
- (12).Grabchev I, Chovelon J-M, Qian X. A copolymer of 4-N,N-dimethylaminoethylene-N-allyl-1,8-naphthalimide with methylmethacrylate as a selective fluorescent chemosensor in homogeneous systems for metal cations. J. Photochem. Photobiol. A. 2003;158:37–43. [Google Scholar]
- (13).(a) Cohen BE, McAnaney TB, Park ES, Jan YN, Boxer SG, Jan LY. Probing Protein Electrostatics With a Synthetic Fluorescent Amino. Acid. Science. 2002;296:1700–1703. doi: 10.1126/science.1069346. [DOI] [PubMed] [Google Scholar]; (b) Vázquez ME, Nitz M, Stehn J, Yaffe MB, Imperiali B. Fluorescent caged phosphoserine Peptides as Probes to Investigate Phosphorylation-Dependent Protein Associations. J. Am. Chem. Soc. 2003;125:10150–10151. doi: 10.1021/ja0351847. [DOI] [PubMed] [Google Scholar]
- (14).Chen H, Chung NN, Lemieux C, Zelent B, Vanderkooi JM, Gryczynski I, Wilkes BC, Schiller PW. [Aladan3]TIPP: a fluorescent δ-opioid antagonist with high δ-receptor binding affinity and δ selectivity. Biopolymers (Peptide Science) 2005;80:325–331. doi: 10.1002/bip.20200. [DOI] [PubMed] [Google Scholar]
- (15).Hazzum E, Chang KJ, Shecter Y, Wilkinson S, Cautrecasas P. Fluorescent and photoaffinity enkephalin derivatives: preparation and interaction with opiate receptors. Biochem. Biophys. Res. Commun. 1979;88:841–846. doi: 10.1016/0006-291x(79)91485-2. [DOI] [PubMed] [Google Scholar]
- (16).Mihara H, Lee S, Shimohigashi Y, Aoyagi H, Kato T, Izumiya N, Costa T. μ and δ Opioid receptor probes: fluorescent enkephalins with high receptor affinity and specificity. FEBS Lett. 1985;193:35–38. doi: 10.1016/0014-5793(85)80074-0. [DOI] [PubMed] [Google Scholar]
- (17).Berezowska I, Chung NN, Lemieux C, Zelent B, Szeto HH, Schiller PW. Highly potent fluorescent analogues of the opioid peptide [Dmt1 ]DALDA. Peptides. 2003;24:1195–1200. doi: 10.1016/j.peptides.2003.07.004. [DOI] [PubMed] [Google Scholar]
- (18).Berezowska I, Lemieux C, Chung NN, Zelent B, Schiller PW. Dansylated analogues of the opioid peptide [Dmt1]DALDA: in vitro activity profiles and fluorescence parameters. Acta Biochim. Pol. 2004;51:107–113. [PubMed] [Google Scholar]
- (19).Goldstein A, Nestor JJ, Naidu A, Newman SR. “DAKLI”: A multipurpose ligand with high affinity and selectivity for dynorphin (κopioid) binding sites. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7375–7379. doi: 10.1073/pnas.85.19.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kshirsagar T, Nakano AH:, Law P-Y, Elde R, Portoghese PS. NTI4F: a non-peptide fluorescent probe selective for functional delta opioid receptors. Neurosci. Lett. 1998;249:83–86. doi: 10.1016/s0304-3940(98)00379-6. [DOI] [PubMed] [Google Scholar]
- (21).Kumar V, Murray TF, Aldrich JV. Extended TIP(P) Analogues as precursors for labeled δ-opioid receptor ligands. J. Med. Chem. 2000;43:5050–5054. doi: 10.1021/jm000362h. [DOI] [PubMed] [Google Scholar]
- (22).Chang A-C, Chao CC, Takemori AE, Gekker G, Hu S, Peterson PK, Portoghese PS. Arylacetamide-derived fluorescent probes: synthesis, biological evaluation, and direct fluorescent labeling of δ opioid receptors in mouse microglial cells. J. Med. Chem. 1996;39:1729–1735. doi: 10.1021/jm950813b. [DOI] [PubMed] [Google Scholar]
- (23).Balboni G, Salvadori S, Dal Piaz A, Bortolotti F, Argazzi R, Negri L, Lattanzi R, Bryant SD, Jnsmaa Y, Lazarus LH. Highly selective fluorescent analogue of the potent δ-opioid receptor antagonist Dmt-Tic. J. Med. Chem. 2004;47:6541–6546. doi: 10.1021/jm040128h. [DOI] [PubMed] [Google Scholar]
- (24).Vázquez ME, Blanco JB, Imperiali B. Photophysics and biological applications of the environment-sensitive fluorophore 6-N,N-dimethylamino-2.3-naphthalimide. J. Am. Chem. Soc. 2005;127:1300–1306. doi: 10.1021/ja0449168. [DOI] [PubMed] [Google Scholar]
- (25).Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- (26).Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Direct influence of C-terminally substituted amino acids in the Dmt-Tic pharmacophore on δ-opioid receptor selectivity and antagonism. J. Med. Chem. 2004;47:4066–4071. doi: 10.1021/jm040033f. [DOI] [PubMed] [Google Scholar]
- (27).Salvadori S, Marastoni M, Balboni G, Borea P, Tomatis R. Opioid peptides: synthesis and biological properties of dermorphin related hexapeptides. Eur. J. Med. Chem. 1990;25:171–177. [Google Scholar]
- (28).Oya M, Takahashi T. The steric hindrance of the stepwise reaction of N-carboxy alpha amino acid anhydride with the alpha amino acid ester. Bull. Chem. Soc. Japan. 1981;54:2705–2707. [Google Scholar]
- (29).Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol. Pharmacol. 2000;58:1570–1580. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- (30).Meech SR, Phillips D. Photophysics of some common fluorescence standards. J. Photochem. 1983;23:193–217. [Google Scholar]
- (31).Salvadori S, Guerrini R, Balboni G, Bianchi C, Bryant SD, Copper PS, Lazarus LH. Further studies on the Dmt-Tic pharmacophore: Hydrophobic substituents at the C-terminus endow δ antagonists to manifest μ agonism or μ antagonism. J. Med. Chem. 1999;42:5010–5019. doi: 10.1021/jm990165m. [DOI] [PubMed] [Google Scholar]
- (32).Hancock WS, Battersby JE. A new micro-test for the detection of incomplete coupling reactions in solid-phase peptide synthesis using 2,4,6-trinitrobenzenesulphonic acid. Analytical Biochemistry. 1976;71:260–264. doi: 10.1016/0003-2697(76)90034-8. [DOI] [PubMed] [Google Scholar]
- (33).Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J. Med. Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- (34).Lazarus LH, Salvadori S, Santagada V, Tomatis R, Wilson WE. Function of negative charge in the “address domain” of deltorphins. J. Med. Chem. 1991;34:1350–1359. doi: 10.1021/jm00108a017. [DOI] [PubMed] [Google Scholar]
- (35).Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. Synthesis and opioid activity of N,N-dimethyl-Dmt-Tic-NH-CH(R)-R’ analogues: acquisition of potent δ antagonism. Bioorg. Med. Chem. 2003;11:5435–5441. doi: 10.1016/j.bmc.2003.09.039. [DOI] [PubMed] [Google Scholar]
- (36).Lazarus LH, Wilson WE, De Castiglione R, Guglietta A. Dermorphin gene sequence peptide with high affinity and selectivity for δ-opioid receptors. J. Biol. Chem. 1989;264:3047–3050. [PubMed] [Google Scholar]
- (37).Cheng Y-C, Prusoff WH. Relationships between the inhibition constant (Ki) and the concentration of inhibition which cause 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- (38).Melchiorri P, Negri L, Falconieri-Erspamer G, Severini C, Corsi R, Soaje M, Erspamer V, Barra D. Structure-activity relationships of the δ-opioid-selective agonists, deltorphins. Eur. J. Pharmacol. 1991;195:201–207. doi: 10.1016/0014-2999(91)90536-y. [DOI] [PubMed] [Google Scholar]
- (39).Tallarida RJ, Murray RB. Manual of Pharmacological Calculation. 2nd ed. Springer-Verlag; New York: 1986. [Google Scholar]
- (40).Kosterlitz HW, Watt AJ. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone) Br. J. Pharmacol. 1968;33:266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.