Abstract
Background & Aims
Studies are aimed to determine the role of CD36 in intestinal lipid absorption.
Methods
Knockout (KO) and wild-type (WT) lymph fistula mice were used to study fatty acids (FA) and cholesterol uptake, and chylomicron formation and secretion. Uptake of FA and cholesterol was studied by using sucrose polybehenate and fecal dual isotope methods, respectively.
Results
The CD36 KO exhibited significant accumulation of dietary cholesterol in the intestinal lumen at the end of 6-hour lipid infusion and significant reduction of dietary cholesterol transport into the lymph. Fecal dual isotope studies, however, did not show any significant difference in cholesterol uptake, suggesting that given sufficient time, the KO intestine could compensate for the reduced cholesterol uptake observed in the acute lymph fistula studies. Recovery of dietary FA in the intestinal lumen was comparable between WT and KO, consistent with the sucrose polybehenate study. However, the KO mice accumulated more, albeit not significantly, dietary triacylglycerols in the intestine, followed by a significant reduction in lymphatic transport. The ratio of intestinal dietary triacylglycerols to FA was not higher in WT than KO, arguing against impaired lipid esterification. It is rather a deficiency in the formation and secretion of chylomicrons, as supported by the significantly less apolipoprotein B-48 and the smaller, albeit not significantly, lipoprotein particles secreted into the lymph of the KO.
Conclusions
CD36 may play an important role in chylomicron formation and secretion and may also facilitate cholesterol uptake in the proximal intestine.
The transmembrane protein CD36 is expressed in many cell types, including platelets,1 monocytes,2 capillary endothelial cells,3 erythroblasts,4 adipocytes,5 and intestinal,6-8 mammary, and retinal epithelial cells.9 In rat intestinal epithelial cells, CD36 is localized in the apical brush border membranes of mainly the duodenum and jejunum.6,7 Using human intestinal tissues, Lobo et al8 localized CD36 expression along the proximal brush border membranes of the intestinal epithelium.
CD36 binds to a wide range of ligands, such as native and modified lipoproteins,10,11 anionic phospholipids (PL),12 cholesterol,13 and fatty acids (FA).5 Accumulated evidence from studies conducted both in vitro14,15 and in vivo16 supports an important role for CD36 in facilitating FA uptake by adipose and muscle tissues. Its role in intestinal lipid uptake is less clear. In support of such a role are the high levels of CD36 and its expression pattern along the brush border membrane of the proximal intestine, which is typical of proteins implicated in lipid uptake.6,7
A recent study by Drover et al17 showed that CD36 knockout (KO) mice did not exhibit reduced intestinal FA uptake, but had impairments in lymph triacylglcyerol (TG) secretion and in clearance of blood chylomicrons. Whether or not CD36 plays a significant role in intestinal cholesterol uptake and transport in TG-rich lipoproteins in vivo remains unknown. A role in cholesterol uptake has been suggested by the report that CD36 bound cholesterol and that CD36 antibodies inhibited cholesterol uptake by brush border membranes.13
The goal of this study is to further study the role of CD36 in intestinal uptake and transport of cholesterol and TG. Using the well-established conscious lymph fistula model, we explored the effect of CD36 deficiency on formation and secretion of chylomicrons. We also examined whether disruption of the CD36 gene would lead to an overall reduction in intestinal FA and cholesterol uptake by using sucrose polybehenate (SPB)18 and fecal dual-isotope methods, respectively.
Materials and Methods
Materials
Triolein, cholesterol, egg phosphatidylcholine (PC), and sodium taurocholate (NaTC) were purchased from Sigma (St. Louis, MO). The radioactive [9,10-3H(N)] triolein (labels were on the 3 FA molecules, not on the glycerol moiety) and [4-14C] cholesterol were purchased from New England Nuclear (Boston, MA). Silica gel 60 plates were purchased from Fisher Scientific (Pittsburgh, PA) and were activated before use.
Animals
CD36 wild-type (WT) and KO mice were generated as described previously.17 Animals were maintained on regular chow diet under a 12-hour light/12-hour dark cycle at the University of Cincinnati Laboratory Animal Medical Services. Only 4- to 12-month-old male animals were used in our studies.
SPB Method
The SPB method was developed and validated by Jandacek et al.18 It is a noninvasive method for studying the uptake of FA. The animals are fed a diet containing TG and other essential nutrients. The method relies on measuring the ratio of fecal FA versus fecal nonabsorbable fat marker. In this study, the uptake of total dietary FA (mass) was measured against olestra, the nonabsorbable fat marker. Therefore, our SPB study here measured the uptake of overall dietary FA.
Fecal Dual Isotope Method
To compare intestinal cholesterol uptake between the CD36 WT and KO mice (n = 7 in each group), each mouse was first adapted in individual cage for 1 week before study. Each mouse was then gavaged with 200 μL of 20% Liposyn II intralipid (Abbott Laboratories, Chicago, IL) added with 0.35 mg cholesterol, 0.5 μCi [14C] cholesterol, and 5 μCi [3H] sitosterol. Fecal samples from each mouse were collected 24 hours postgavage. Mice were allowed to eat ad libitum (regular chow diet), and food intake was monitored for the period of 48 hours after gavage. Fecal samples (about 200 mg) were saponified by heating them at 80°C for 5 minutes in 4 mL of 0.5 N NaOH in methanol. After allowing the solution to cool, 2 mL of saturated saline and 10 mL of hexane were added, and the solution was mixed and organic layer extracted. The extracted organic layer was allowed to evaporate under a gentle stream of nitrogen. Radioactivity was determined by the scintillation counter after mixing the dried organic layer with 10 mL of scintillation fluid. Cholesterol uptake was determined by calculating the disintegration per minute (dpm) fractions of 14C and 3H from the homogenous gavage solution and the extracted fecal samples:
Lymph and Duodenal Cannulation
Intestinal lymph ducts of anesthetized (ketamine, 80 mg/kg and xylazine, 20 mg/kg) mice were cannulated with polyvinyl chloride (PVC) tubing (inner diameter [ID], 0.20 mm; outside diameter [OD], 0.50 mm) as described by Bollman et al19 with the following modifications. Suture of the lymph cannula was replaced by application of cyanoacrylate glue (Krazy Glue, Itasca, IL); in addition, a PVC tube (ID, 0.5 mm; OD, 0.8 mm) was inserted into the duodenum through a fundal incision of the stomach and secured by a purse-string. Following the surgery, mice were infused with 5% glucose in saline (145 mmol/L NaCl, 4 mmol/L KCl, and 0.28 mol/L glucose) at a rate of 0.3 mL/h. The glucose/saline solution was replaced with the prepared lipid infusate the next morning.
Lipid Infusate Preparation
Triolein, [3H] triolein, cholesterol, [14C] cholesterol, and PC were dissolved in chloroform. The chloroform content was evaporated under a steady stream of nitrogen. The chloroform-free lipid mixture was then emulsified with 19 mmol/L NaTC in phosphate-buffered saline (PBS; [(in g) 0.958 Na2HPO4, 2.277 NaH2PO4, 6.8 NaCl, and 0.2982 KCl/L H2O] at pH 6.4.) and sonicated with 1 second on/1 second off pulse until the solution appeared homogenous. The homogeneity of the emulsion was checked by taking samples from the top, the middle, and the bottom of the emulsion and radioactivity determined. We considered the emulsion to be homogenous if the counts did not vary >1%.
The lipid emulsion was infused for 6 hours. The hourly infusate contained 4 μmol triolein with a trace mass of [3H] triolein, 0.78 μmol cholesterol with a trace mass of [14C] cholesterol, 0.78 μmol egg PC, and 5.7 μmol NaTC in PBS.
Collection of Lymph, Intestinal Lumen, and the Small Intestine
Lymph samples were collected hourly. At the end of the 6-hour infusion period, the animals were anesthetized with the ketamine-xylazine mixture, and stomach, small intestine, colon, and luminal content were removed. The small intestine with intact mucosa and muscular layers (unscraped) were then cut into 4 equal segments, and herein referred to as M1–M4 (M1 being the most proximal and M4 being the most distal). We did not scrape the mucosa because it is quite thin in the mouse and, depending on the strength one applies during scraping, there could be considerable variation from experiment to experiment in the amount of mucosal scraping. Tissue samples were homogenized using a Polytron homogenizer, and radioactivity of each sample was measured. In addition, lipid fraction of M1–M4 were immediately extracted using the Folch method20 for further lipid analysis. Lymphatic TG mass was determined using a TG kit from Randox (Crumlin, United Kingdom) as previously described.21
Thin-Layer Chromatography Analysis of M1–M4
Lipids extracted from M1–M4 were separated on silica gel 60 plates using a solvent system of petroleum ether/ethyl ether/glacial acetic acid with 25:5:1 volume ratio. After visualizing the samples and the comigrating reference standards by staining with iodine vapor, samples were scraped into scintillation vials, and 1 mL of absolute alcohol was added to scintillation liquid (Opti Fluor for aqueous samples) for counting of radioactivity.
Lipoprotein Particle Size Analysis
Carbon-coated formvar film on a 400-mesh copper grid (Electron Microscopy Sciences, Hatfield, PA) was floated on a drop of the lymph sample. The grid was dried with filter paper and briefly added with 2% phosphotungstic acid (pH 6.0). For lipid-feeding lymph samples, 5- and 6-hour lymph samples were pooled and diluted with sterile water 1:4 vol:vol before being used for negative staining. Fasting lymph samples (collected 1 hour prior to lipid infusion) were not diluted and were added on grids as described. Standard beads (200 nm) were used for calibration (Duke Scientific Corp, Fremont, CA). The samples were examined and pictures were taken immediately by using the JEOL JEM-1230. The size of the lipoprotein particles was measured by using Adobe Photoshop and software from Reindeer Graphics. An average of 800 particles were sized per lymph sample. A previous pilot study showed that the manual and the digital counting methods agreed closely (data not shown).
Chylomicron Composition
To determine the lipid composition of chylomicron in the lymph, equal aliquots of lymph samples were layered under 0.15 mol/L NaCl and subjected to centrifugation for 30 minutes at 50,000 rpm in MLA-130 rotor in a table top ultracentrifuge (Beckman Instruments, Fullerton, CA). Chylomicrons were removed, and lipid composition was determined as described by using kits from Wako chemicals.
Enzyme-Linked Immunosorbent Assay Analysis of Apolipoproteins
High-binding 96-well plates were precoated overnight at 4°C with 100 μL/well of 1:300 rabbit anti-rat apolipoprotein A-I, A-IV, or B in coating buffer (0.014 mol/L Na2CO3 and 0.035 mol/L NaHCO3, pH 9.6). Each well was then washed 3 times with PBS-T (0.5% Tween-20 in 0.01 mol/L PBS) and blocked with 270 μL of blocking buffer (1% bovine serum albumin in 0.01 mol/L PBS-T) for 2 hours at 4°C. After removing the blocking buffer, 100 μL of either standards or samples were added and incubated overnight at 4°C. Purified rat apolipoprotein A-I, A-IV, or B were used as standards (the linear range of each standard curves were determined in pilot studies). Samples were either from lymph collected 1 hour before lipid infusion (fasting) or from lymph collected during the 3rd–4th hour lipid infusion (feeding; the 3rd hour lymph and 4th hour lymph were pooled). For measuring apolipoprotein A-I, samples were diluted to 1:100; A-IV 1:100; and B 1:400 (sample dilution was determined in pilot studies). Each well was washed 3 times as before and incubated with 100 μL of 1:3000 goat anti-rat apolipoprotein A-I, A-IV, or B in blocking buffer for 2 hours at room temperature. The antibodies were washed 3 times and incubated with 100 μL of 1:500 horseradish peroxidase–linked rabbit anti-goat antibodies in blocking buffer for 1 hour at room temperature. The secondary antibodies were washed as before, and 200 μL of o-phenylenediamine dihydrochloride substrate (Sigma) were added to each well. After 15 minutes, the reaction was stopped by adding 50 μL of 3 mol/L HCl, and the absorbance was read at 490 nm by Synergy HT plate reader (BioTek, Winooski, VT). Samples were measured in triplicate.
Statistical Analysis
The data shown are mean values ± standard errors (SE). To compare groups throughout the 6-hour infusion, 2-way repeated measures analysis of variance (ANOVA) with Tukey as a posttest analysis was used. For comparison of data that have 2 independent variables, 2-way ANOVA was used. A t-test was used for the rest of the data analyses (comparing only 2 groups). Statistical analyses were performed using Sigmastat (SPSS Inc., Chicago, IL), and were considered significant if P < .05.
Results
FA Uptake
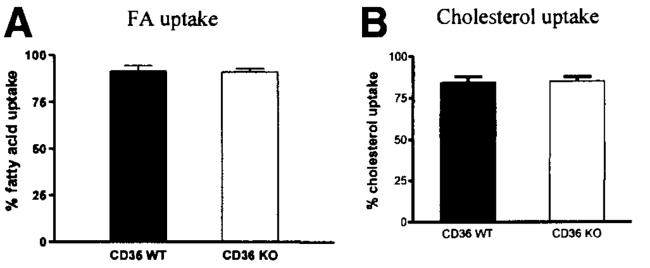
The SPB method allows for the analysis of FA uptake in mice fed ad libitum by determining the content of excreted FA relative to the content of a nonabsorbable marker in their fecal samples. Both CD36 WT (n = 4) and KO (n = 4) mice showed a 91% overall uptake of dietary FA mass (P = .91; Figure 1A), suggesting that ablation of CD36 is not sufficient to reduce overall FA uptake. (Note that because enterocytes do not take up TG but FA from digested TG, we prefer the term FA uptake to TG uptake.)
Figure 1.
Analysis of FA (A) and cholesterol (B) uptake by the small intestines of CD36 null and WT mice. (A) A minimum of 9 fecal samples from each group were analyzed by the SPB method18 to determine the ratio of fecal FA vs fecal nonabsorbable fat marker. (B) Mice were gavaged with 20% intralipid containing 0.35 mg cholesterol, [14C] cholesterol, and [3H] sitosterol. Fecal samples were collected 24 hours postgavage and analyzed. No statistical significance was found. Values are means ± SE.
Cholesterol Uptake
Using the fecal dual isotope method, we examined if the absence of CD36 caused a change in cholesterol uptake by the intestine. As depicted in Figure 1B, the cholesterol uptake of both WT and KO mice was about 85% (P = .90). These numbers agree well with those obtained by other investigators.22 Food intake of WT and KO mice did not show any significant difference during this study (data not shown).
Both the SPB method and the fecal dual isotope method could not determine the rate and the site of dietary FA and cholesterol uptake and subsequent packaging into chylomicrons for secretion. Also, both methods are influenced by variation in stomach emptying. To more directly determine the uptake and lymphatic transport of TG and cholesterol, we employed the conscious lymph fistula model.
Analysis of Lymphatic Transport of TG and Cholesterol by the Small Intestine
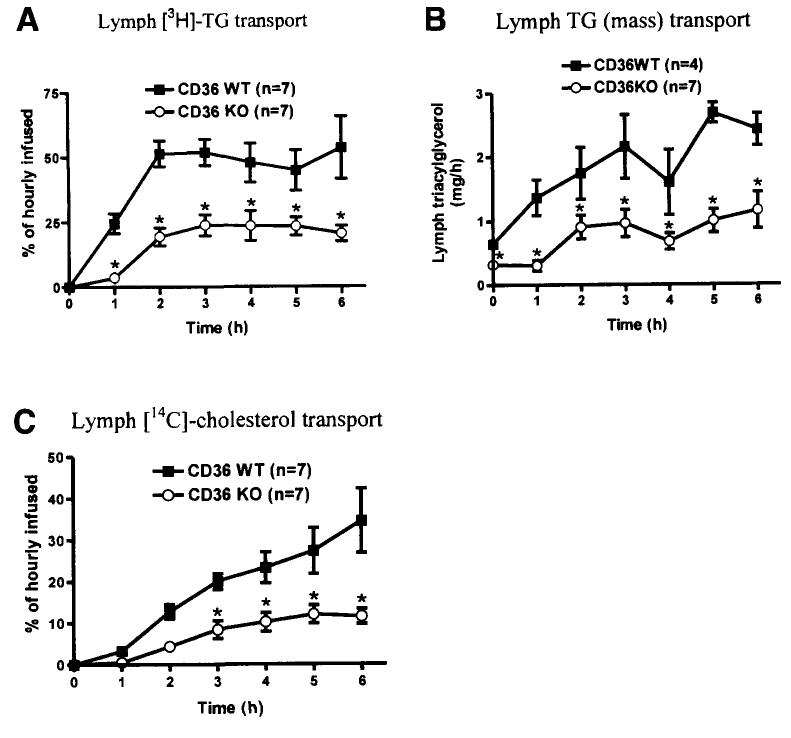
To further determine whether CD36 plays a role in TG and cholesterol transport by the small intestine, we analyzed the lymphatic output of CD36 WT and KO mice. (Note that because FA taken up by the enterocytes needs to be converted to TG for transport into chylomicrons, we prefer using the term TG transport to FA transport.) CD36 KO mice had a significant reduction in lymphatic output of both TG (Figure 2A) and cholesterol (Figure 2C). The reduction in lymphatic radioactive TG output (P < .001) was evident as early as the first hour of infusion (Figure 2A), reaching only 25% of the hourly infused TG as compared to 50% in the WT mice. The total TG mass (Figure 2B) in the lymph of the CD36 KO mice was also lower (P = .003) than that of CD36 WT mice throughout the entire 6-hour study. About 80% of the lymphatic TG mass output was calculated to be derived from the infused (exogenous/dietary) TG in both the CD36 WT and KO mice. CD36 KO mice also showed a reduction (P = .002) in lymphatic transport of the dietary cholesterol, reaching only 10% of the hourly infused load as compared with 30% for WT mice. This decrease was significant from the third hour of infusion onward (Figure 2C).
Figure 2.
(A) [3H]-TG, (B) TG mass, and (C) [14C]-cholesterol transport into the lymph during continuous intraduodenal lipid infusion. Mice were equipped with lymph and duodenal cannulas, and were intraduodenally infused with a lipid emulsion containing labeled TG (the label was on all FA molecules of the TG) and cholesterol for a period of 6 hours. Lymph was collected hourly and analyzed. *P < .05 as determined by Tukey posttest analysis of 2-way repeated measures ANOVA. Values are means ± SE.
Fate of Dietary FA and Cholesterol at the End of 6 Hours of Continuous Infusion
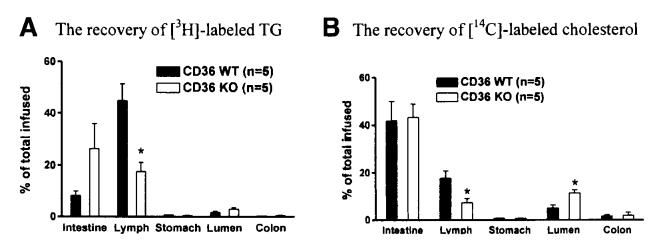
Figure 3A and B show that very little of the dietary FA and cholesterol that were infused were being excreted because only a small amount of radioactivity was recovered from the colon of both groups of animals. Also, there was little if any reflux of infused lipids back to the stomach, judged from the recovery of radioactive counts from the lumen of the stomach from both groups of animals. There was a tendency for luminal [3H] counts to be higher in CD36 KO than in WT mice, but the difference was not statistically significant (P = .1220; Figure 3A, lumen). In contrast, lymphatic transport of TG in CD36 KO mice was significantly decreased relative to that of the WT controls (P = .0069; Figure 3A, lymph), and the CD36 KO mice also had a tendency to accumulate more of the infused radioactive TG in their intestine (P = .105) (Figure 3A, intestine). In a separate acute study, we observed a similar accumulation of labeled TG in the intestine of the CD36 KO when the emulsion was administered by gavage instead of by constant infusion into the duodenum as in our lymph fistula mice (data not shown). Thus, the tendency to accumulate dietary TG in the intestine of the KO was similar irrespective of whether TG was infused at a constant rate into the duodenum or gavaged as a single meal.
Figure 3.
Fate of dietary TG (A) and cholesterol (B) at the end of 6-hour continuous infusion. Mice were equipped with lymph and duodenal cannulas, and were intraduodenally infused with a lipid emulsion containing labeled TG (the label was on all FA molecules of the TG) and cholesterol for a period of 6 hours. The recovery of radioactivity in the small intestine, lymph, stomach, intestinal lumen, and colon were determined at the end of the 6 hours by scintillation counter. *P < .05. Values are means ± SE.
As shown in Figure 3B (lumen), significantly higher luminal radioactive cholesterol counts (P = .0073) were recovered in the null mice than the WT controls. As would be expected from the luminal radioactive cholesterol recovery, the CD36 KO had much reduced radioactive cholesterol output into the lymph (P = .0216; Figure 3B, lymph). There was no evidence for more radioactive cholesterol accumulated in the small intestine of the CD36 KO relative to the WT controls (P = .88; Figure 3B, intestine).
Distribution of Radioactive TG and Cholesterol Along the Segments of the Small Intestine
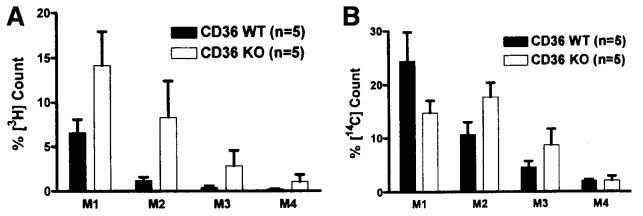
Figure 4A and B show the distribution of dietary TG and cholesterol, respectively, in the 4 equal length segments of the small intestine, M1–M4 (M1 being the most proximal and M4 being the most distal). The distribution of cholesterol in the small intestine of the KO did not show a typical gradual decrease from M1 to M2. The low recovery of both [3H] and [14C] counts in the M4 segments further support our conclusion that the infused materials were either taken up by the small intestine or remained in the lumen but were not excreted. Statistical analysis did not show any significant difference between the WT and KO animals.
Figure 4.
Distribution of dietary TG (A) and cholesterol (B) along the segments of the small intestine. Mice were equipped with lymph and duodenal cannulas, and were intraduodenally infused with a lipid emulsion containing labeled TG (the label was on all FA molecules of the TG) and cholesterol for a period of 6 hours. At the end of the study, small intestines (n = 5) were harvested and divided into 4 equal length segments, from proximal to distal: M1, M2, M3, and M4. The amounts of radioactivity in these segments were determined by scintillation counter. No statistical significance was found between the WT and KO. Values are means ± SE.
Thin-Layer Chromatography Analysis of the Lipid Fraction of the Small Intestine
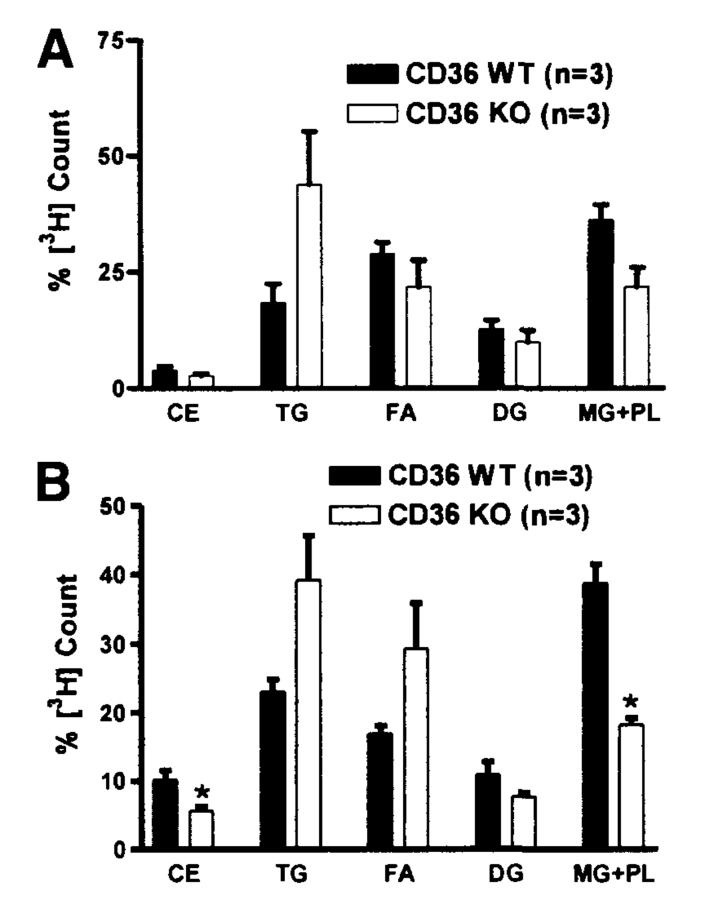
To determine if the efficiency of esterification of FA and cholesterol by the small intestine was responsible for the reduced lymphatic transport, we analyzed the distribution of radioactive counts in the different lipid fractions separated by thin-layer chromatography. Figure 5 shows the relative percentage of [3H] counts in the cholesteryl ester (CE), TG, diacylglycerol (DG), and monoacylglycerol + phospholipids (MG + PL). In the proximal half of the CD36 KO small intestine, about 40%–50% of the [3H] counts were in TG fraction (Figure 5A and B). The differences between WT and KO were only significant in the second segment (M2) of the small intestine; that is, significant decreases in the CE and MG/PL fractions in the small intestine of the KO relative to those of the WT controls (P = .0345 for CE and P = .0019 for MG+PL).
Figure 5.
Thin-layer chromatography analysis of [3H]-labeled lipid fraction of the small intestine. Mice were equipped with lymph and duodenal cannulas, and were intraduodenally infused with a lipid emulsion containing labeled TG (the label was on all FA molecules of the TG) and cholesterol for a period of 6 hours. At the end of the study, the small intestines were divided into 4 equal segments. Only the analysis of the first 2 (most proximal) segments was shown here: (A) M1 and (B) M2. Extracted lipid fraction was separated by thin-layer chromatography into CE, TG, FA, DG, and MG+PL. CE, cholesteryl esters; DG, diacylglycerols; MG+PL, monoacylglycerols and phospholipids. *P < .05. Values are means ± SE.
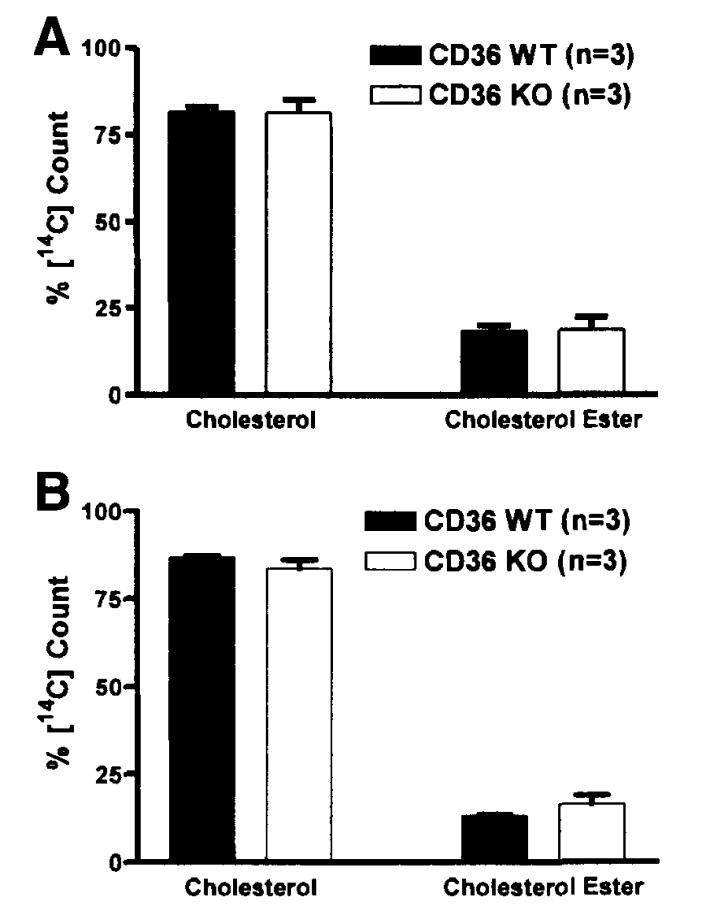
Similarly, the efficiency of cholesterol esterification by the small intestine was compared between WT and KO mice. Figure 6 shows that about 80%–90% of the [14C] counts were recovered in free cholesterol in both CD36 WT and KO mice. No significant differences were observed between the 2 genotypes. There was also no significant difference in FA and cholesterol esterification between WT and KO in the M3 and M4 segments (data not shown).
Figure 6.
Thin-layer chromatography analysis of [14C]-labeled lipid fraction of the small intestine. Mice were equipped with lymph and duodenal cannulas, and were intraduodenally infused with a lipid emulsion containing labeled TG (the label was on all FA molecules of the TG) and cholesterol for a period of 6 hours. At the end of the study, the small intestines were divided into 4 equal segments. Only the analysis of the first 2 (most proximal) segments was shown here: (A) M1 and (B) M2. Extracted lipid fraction was separated by thin-layer chromatography into cholesterol and CE. No statistical significance was found between the WT and KO. Values are means ± SE.
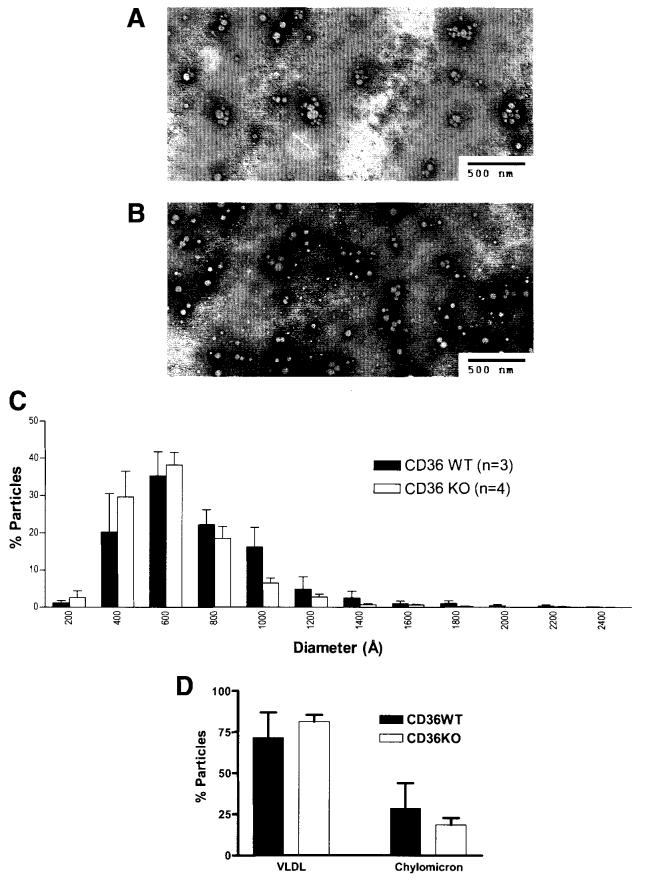
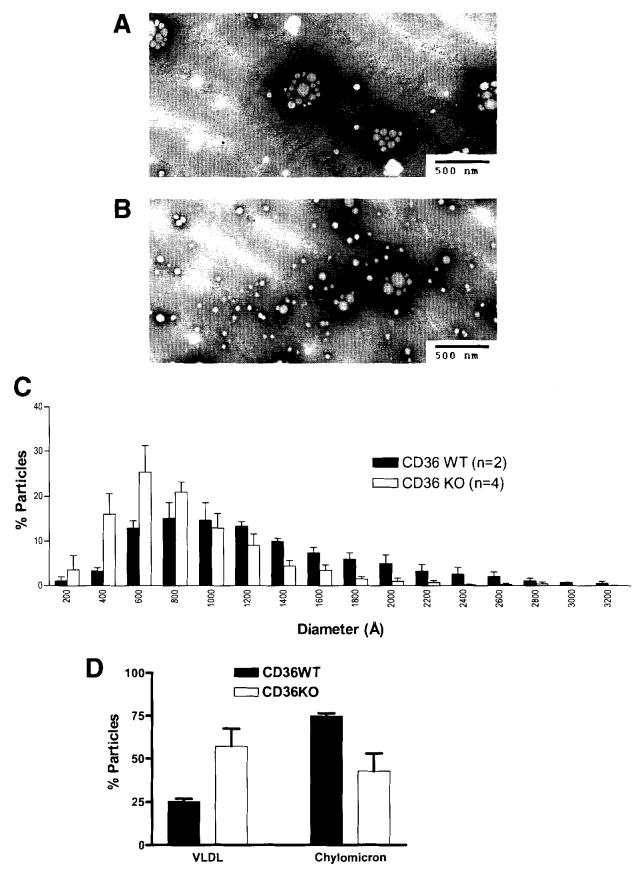
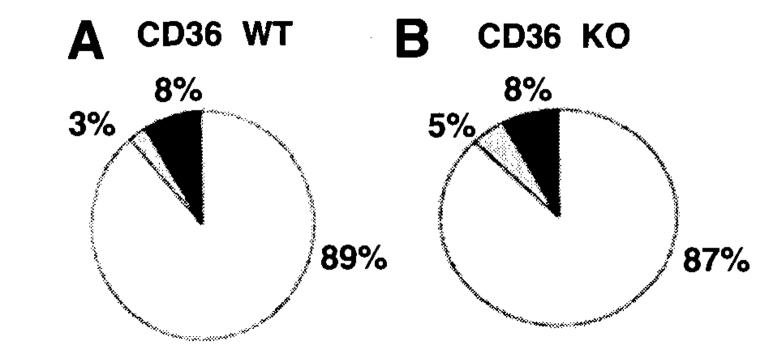
Lipoprotein Particle Size Analysis
Figure 7A and B are electron micrographs showing the fasting lipoprotein particles of CD36 WT and KO mice analyzed by negative staining, respectively. Figure 7C shows the size distribution of the fasting lymph lipoprotein particles (collected 1 hour before lipid infusion) of the WT and CD36 KO mice. There was no statistical difference in the relative number of very-low-density lipoprotein (VLDL) particles between WT and KO mice (P = .506) (Figure 7D). Figure 8A and B depict the images of the particles during lipid feeding stage (during the 5th–6th hour lipid infusion) in WT and KO, respectively. Figure 8C shows the size distribution of the particles. As shown in Figure 8D, only about 42% of the particles secreted by the KO during lipid feeding stage were chylomicrons, compared with about 75% as chylomicrons in the WT animals. This difference, however, was not statistically significant (P = .106; Figure 8B). Nevertheless, the average size of the lipoprotein particles of the CD36 KO mice (841.5 ± 76.1Å) was significantly lower (P = .0325) than that of the CD36 WT mice (1284.0 ± 126.1Å) during the lipid feeding stage.
Figure 7.
Lipoprotein particle size of the lymph of fasted mice. Lipoproteins analyzed were from lymph collected 1 hour prior to lipid infusion (during glucose/saline infusion). Electron micrographs of the lipoprotein particles from CD36 WT (A) and KO (B) mice, and their size distribution (C) and relative VLDL/chylomicron ratio (D) are shown. Particles with diameters of <800 Å are considered VLDL, and ≥800 Å are considered chylomicrons. Standard bars represent 5000 Å (500 nm). No statistical significance was found in the VLDL/chylomicron ratio between the WT and KO. Values are means ± SE.
Figure 8.
Lipoprotein particle size of lymph of lipid fed mice. Lipoproteins analyzed were from lymph collected during the 5th–6th hour lipid infusion. Electron micrographs of the lipoprotein particles from CD36 WT (A) and KO (B) mice, and their size distribution (C) and relative VLDL/chylomicron ratio (D) are shown. Particles with diameters of <800 Å are VLDL, and ≥800 Å are chylomicrons. Standard bars are 5000 Å (500 nm). No statistical significance was found in the VLDL/chylomicron ratio between the WT and KO. Values are means ± SE.
Chylomicron Composition
Figure 9 depicts the chylomicron lipid composition. The chylomicrons secreted contain mostly TG (about 88%) with small amounts of PL (about 8%) and cholesterol (about 4%).
Figure 9.
Lipid composition of chylomicrons from lipid fed mice. Chylomicrons were isolated from lymph collected during 6 hours of lipid infusion. Each chylomicron lipid, TG (white), total cholesterol (gray), and PL (black) is expressed as a percentage of the total content. No statistical significance was found.
Apolipoprotein Secretion by the Small Intestine
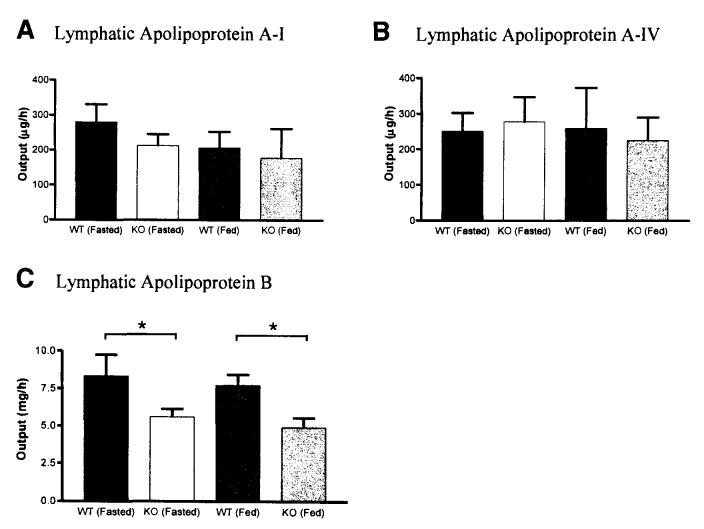
By using enzyme-linked immunosorbent assay (ELISA), the amounts of lymphatic apolipoprotein A-I, A-IV, and B were determined (Figure 10A–C). The only significant difference observed was the apolipoprotein B secretion between the WT and KO animals. The KO mice secreted significantly less apolipoprotein B compared with the WT mice during both fasting (glucose/saline infusion) and lipid feeding (3rd–4th hour lipid infusion) stages (2-way ANOVA, P = .009).
Figure 10.
Apolipoprotein secretion into lymph during fasting and lipid feeding. Fasting samples were from lymph collected 1 hour prior to lipid infusion (during glucose/saline infusion); lipid-feeding samples were from lymph collected during the 3rd–4th hour of lipid infusion. Amounts of lymphatic apolipoprotein A-I (A), A-IV (B), and B (C) were determined by ELISA. See Methods for details. *P = .009 for the genotype factor (2-way ANOVA). Values are means ± SE.
Discussion
The scavenger receptor CD36 is expressed in the brush border membranes of the proximal small intestine of both rodents6,7 and humans.8 CD36 binds to a wide variety of ligands, including lipoproteins,10,11 anionic PL,12 and long-chain FA.5 Its high expression levels and defined localization in the small intestine together with its high affinity for lipids suggest a role for CD36 in lipid absorption. In this study, using the CD36-deficient mouse model equipped with lymph and intraduodenal cannulas, we directly examined the role of CD36 in uptake and lymphatic transport of both TG and cholesterol.
Previous work by Goudriaan et al23 and Drover et al17 suggests that CD36 does not function in uptake of dietary FA. We reexamined this by using the SPB method, which measures the mass percentage of the fecal FA excretion (combined from several different types of FA) to the nonabsorbable lipid marker. No deficiency in overall uptake of FA mass could be detected in CD36-deficient mice. We also examined if a particular type of FA could be less efficiently taken up by CD36 KO mice. We compared linoleic as well as linolenic uptake between WT and KO mice, and found that the uptake of these 2 FA was not significantly different between WT and KO mice (unpublished data). This is also supported by the similar recovery of [3H] counts (the [3H] was labeled on the oleic acids of the triolein) from the intestinal lumen of both WT and KO in our studies.
Although CD36 has been well-characterized as mediating the uptake of free FA in adipose tissue and muscle, in the intestine its deletion does not appear to cause an overall reduction in the uptake of FA (after hydrolysis of TG to MG and FA). Our findings are consistent with those of Drover et al.17 As a result of the high FA concentrations that occur in the intestine during a lipid meal, it is possible that passive transport of FA may mediate most of the uptake in this tissue, as discussed recently.24 However, the present data do not rule out the possibility that other FA transporters could compensate for FA uptake in the absence of CD36. Such FA transporters include FATP425 and FABPpm.26 To truly address the issue, we have to delete these genes and then determine the effect on the uptake of FA by the small intestine. In essence, we cannot rule out completely the possibility that CD36 mediates uptake of FA.
Although uptake of FA by the small intestine does not seem to depend on CD36, the formation and secretion of chylomicrons appear to be, as supported by several lines of evidence. First, lymphatic TG transport was significantly reduced in the CD36 KO mice for both the exogenous (infused/dietary) TG and endogenous TG (originating from the FA derived from biliary PL). Second, the recovery of dietary TG in the intestine was considerably higher, albeit not statistically significant, in the CD36 KO than in the WT mice. Third, CD36 KO mice secreted significantly less apolipoprotein B and had a tendency to secrete less chylomicrons. All of these data suggest that CD36 plays an important role in chylomicron formation and secretion.
Compared to the WT animals, the CD36 KO mice had a tendency to have higher (but not statistically significant) dietary TG in their intestine, which localized mostly in the proximal intestine. This is in line with normal CD36 expression being higher in proximal intestine than the distal small intestine.6,7 The high dietary TG in the lumen of the KO was not caused by a defect in the FA esterification because the ratio of dietary TG to FA was not higher in WT than KO intestine. As discussed, it is more likely to be a deficiency in the formation and secretion of chylomicrons.
The intestine of the CD36 KO mice secreted less apolipoprotein B. Assuming that there is only 1 apolipoprotein B in a lipoprotein particle,27,28 our data suggest that there were fewer lipoproteins being secreted in the KO mice. Our data also seem to indicate that CD36 interferes with the formation of chylomicrons but not of VLDL particles, as suggested by the considerably fewer, albeit not significant, chylomicron particles being secreted in the KO relative to the WT controls (the average particle sizes are significantly different, however, between WT and KO). Although chylomicrons are empirically defined as TG-rich lipoprotein particles ≥800 Å in diameter in contrast to <800 Å in diameter for VLDL, Tso et al29 proposed that secretion of both particles involve 2 different pathways. The data from CD36 KO mice support this concept. Interestingly, our studies did not show a postprandial increase in apolipoprotein A-IV secretion, a phenomenon well-observed in adult rats.30 Our studies of other types of mice and baby rats also did not show any postprandial increase in apolipoprotein A-IV secretion (data not shown), suggesting to us that this postprandial increase in apolipoprotein A-IV secretion may be absent in small animals. This interesting observation will be validated in future experiments.
A recent study reported that CD36 was found in the Golgi apparatus of the adipocytes.15 This finding opens up the possibility that CD36 may also act intracellularly, such as mediating the targeting of primordial lipoprotein particles from the endoplasmic reticulum to the Golgi compartment. This could explain why CD36 KO animals had a defect in chylomicron secretion into the lymph. It is also possible that the MTP-mediated lipidation of prechylomicrons to form mature chylomicrons is regulated by CD36. More studies are certainly required to help us understand the molecular mechanism of how CD36 mediates chylomicron formation and secretion.
Our studies, however, do not agree well with those of Goudriaan et al.23,31 Using an inhibitor of intravascular lipolysis, Triton WR1339, they showed that CD36 KO and WT mice were comparable in the plasma recovery of infused TG and FA. We cannot explain the discrepancy between their findings and ours, but it should be noted that their plasma TG recovery was only about 15% of the total infused by the end of 4 hours as compared with our lymphatic recoveries of about 44% of the total infused for WT and 18% for the KO animals. The lymph fistula model provides us with a direct measurement of the secretion of chylomicrons and VLDL by the small intestine and our experimental model is not complicated by factors such as stomach emptying. In contrast, in the WR1339 study, stomach emptying could potentially be a complicating factor. In addition, we do not know if WR1339 affects the formation and secretion of intestinal chylomicrons and VLDL, which could potentially explain the low plasma recovery in the study reported by Goudriaan et al.23
Although our lymph cannulation studies showed a reduced cholesterol uptake by the CD36 KO mice, our fecal dual isotope data did not show a difference. We are not surprised by the apparent different answers yielded by the 2 approaches for the following reasons. First, our lymph cannulation studies are acute studies (only lasted for 6 hours). These type of acute studies would allow investigators to examine the digestion, uptake, and packaging of the dietary TG into chylomicrons by the small intestine (probably the proximal small intestine) within a narrow time frame. For example, a slower uptake process would be detected in an acute study, but may not be detected in a more chronic study, such as in a fecal dual isotope study. Also, the chronic study may involve more length of the gut, and the gut certainly has more time to take up the luminal FA and cholesterol. Second, in our lymph cannulation studies we continuously infused cholesterol into the intestinal lumen, bypassing the stomach. In contrast, the fecal dual isotope method is prone to alteration in stomach emptying and/or intestinal motility to regulate the flow of the emulsion bolus. All of these factors may affect cholesterol uptake. Therefore, we conclude that CD36 may still mediate cholesterol uptake, but its absence can certainly be compensated by many factors; these factors include slower gastric emptying and the presence of other cholesterol transporters, especially the ones expressed throughout the more distal part of the intestine, such as Niemann-Pick C1 Like 1.32
There was no difference in the amount of dietary cholesterol accumulated in the CD36 KO versus the WT intestines. Esterification of cholesterol in the small intestine was also similar between the CD36 KO and the WT animals. As would be expected from the reduced cholesterol uptake, the CD36 KO mice transported significantly less cholesterol into the lymph.
It is important to point out that the lymphatic vessel that we cannulated collects lymph transported from both proximal and distal intestine. However, the nonlymphatic route exists for the intestine, and the relative amount of such transport could be inferred from the unaccounted fraction of the lipid infused at the end of lymph fistula studies (% nonlymphatic = 100% − total % that could be recovered from lymph, lumen, intestine, stomach, and colon; Figure 3). This nonlymphatic transport, which probably reflects mostly the portal transport,33 was not significantly different between the WT and KO mice (data not shown).
In conclusion, our data suggest that CD36 plays an important role in chylomicron formation and secretion. CD36 may also play a role in cholesterol uptake, but its absence does not seem to be sufficient to cause an overall reduction in intestinal cholesterol uptake.
Acknowledgments
The authors thank the University of Cincinnati Mouse Metabolic Phenotype Center DK-59630 for providing many of the phenotypic tests relevant to the study.
Abbreviations used in this paper
- CE
cholesterol ester
- DG
diacylglycerol
- FA
fatty acid
- MG
monoacylglycerol
- NaTC
sodium taurocholate
- PC
phosphatidylcholine
- PL
phospholipid
- PVC
polyvinyl chloride
- SE
standard error
- SPB
sucrose polybehenate
- TG
triacylglcyerol
- VLDL
very-low-density lipoprotein
Footnotes
Supported by the National Institute of Health (grants DK-56910, and DK-56863 to P Tso and DK60022 and DK33301 to N.A.), a predoctoral fellowship award from the American Heart Association, Ohio Valley Affiliate, and the National Health Research institute scholarship for cardiovascular diseases (A.M.N.).
References
- 1.Phillips DR, Agin PP. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977;252:2121–2126. [PubMed] [Google Scholar]
- 2.Talle MA, Rao PE, Westberg E, Allegar N, Makowski M, Mittler RS, Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983;78:83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 3.Knowles DM, Tolidjian B, Marboe C, D'Agati V, Grimes M, Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984;132:2170–2173. [PubMed] [Google Scholar]
- 4.Edelman P, Vinci G, Villeval JL, Vainchenker W, Henri A, Miglierina R, Rouger P, Reviron J, Breton-Gorius J, Sureau C. A monoclonal antibody against an erythrocyte ontogenic antigen identifies fetal and adult erythroid progenitors. Blood. 1986;67:56–63. [PubMed] [Google Scholar]
- 5.Abumrad NA, el Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 6.Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP) Eur J Biochem. 1996;238:368–373. doi: 10.1111/j.1432-1033.1996.0368z.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Yang Y, Braunstein E, Georgeson KE, Harmon CM. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am J Physiol Endocrinol Metab. 2001;281:E916–E923. doi: 10.1152/ajpendo.2001.281.5.E916. [DOI] [PubMed] [Google Scholar]
- 8.Lobo MV, Huerta L, Ruiz-Velasco N, Teixeiro E, de la Cueva P, Celdran A, Martin-Hidalgo A, Vega MA, Bragado R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem. 2001;49:1253–1260. doi: 10.1177/002215540104901007. [DOI] [PubMed] [Google Scholar]
- 9.Greenwalt DE, Watt KW, So OY, Jiwani N, PAS IV. an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV) Biochemistry. 1990;29:7054–7059. doi: 10.1021/bi00482a015. [DOI] [PubMed] [Google Scholar]
- 10.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 11.Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res. 1998;39:777–788. [PubMed] [Google Scholar]
- 12.Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 13.Werder M, Han CH, Wehrli E, Bimmler D, Schulthess G, Hauser H. Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine. Biochemistry. 2001;40:11643–11650. doi: 10.1021/bi0109820. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahimi A, Sfeir Z, Magharaie H, Amri EZ, Grimaldi P, Abumrad NA. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc Natl Acad Sci U S A. 1996;93:2646–2651. doi: 10.1073/pnas.93.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16:24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Febbraio M, Guy E, Coburn C, Knapp FF, Jr, Beets AL, Abumrad NA, Silverstein RL. The impact of overexpression and deficiency of fatty acid translocase (FAT)/CD36. Mol Cell Biochem. 2002;239:193–197. [PubMed] [Google Scholar]
- 17.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med. 1949;33:1349–1352. [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Nauli AM, Zheng S, Yang Q, Li R, Jandacek R, Tso P. Intestinal alkaline phosphatase release is not associated with chylomicron formation. Am J Physiol Gastrointest Liver Physiol. 2003;284:G583–G587. doi: 10.1152/ajpgi.00482.2002. [DOI] [PubMed] [Google Scholar]
- 22.Howles PN, Carter CP, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem. 1996;271:7196–7202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 23.Goudriaan JR, Dahlmans VE, Febbraio M, Teusink B, Romijn JA, Havekes LM, Voshol PJ. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol Cell Biochem. 2002;239:199–202. [PubMed] [Google Scholar]
- 24.Tso P, Nauli A, Lo CM. Enterocyte fatty acid uptake and intestinal fatty acid-binding protein. Biochem Soc Trans. 2004;32:75–78. doi: 10.1042/bst0320075. [DOI] [PubMed] [Google Scholar]
- 25.Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, Daniels T, Stricker-Krongrad A, Lodish HF, Stahl A. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem. 2003;278:49512–49516. doi: 10.1074/jbc.M309759200. [DOI] [PubMed] [Google Scholar]
- 26.Stremmel W, Lotz G, Strohmeyer G, Berk PD. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985;75:1068–1076. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albers JJ, Kennedy H, Marcovina SM. Evidence that Lp[a] contains one molecule of apo[a] and one molecule of apoB: evaluation of amino acid analysis data. J Lipid Res. 1996;37:192–196. [PubMed] [Google Scholar]
- 28.Hayashi H, Fujimoto K, Cardelli JA, Nutting DF, Bergstedt S, Tso P. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am J Physiol. 1990;259:G709–G719. doi: 10.1152/ajpgi.1990.259.5.G709. [DOI] [PubMed] [Google Scholar]
- 29.Tso P, Drake DS, Black DD, Sabesin SM. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am J Physiol. 1984;247:G599–G610. doi: 10.1152/ajpgi.1984.247.6.G599. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–G1006. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- 31.Goudriaan JR, den Boer MA, Rensen PC, Febbraio M, Kuipers F, Romijn JA, Havekes LM, Voshol PJ. CD36 deficiency in mice impairs lipoprotein lipase-mediated triglyceride clearance. J Lipid Res. 2005;46:2175–2181. doi: 10.1194/jlr.M500112-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, lyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 33.Mansbach CM, II, Dowell RF, Pritchett D. Portal transport of absorbed lipids in rats. Am J Physiol. 1991;261:G530–G538. doi: 10.1152/ajpgi.1991.261.3.G530. [DOI] [PubMed] [Google Scholar]