Abstract
Background
Fructose consumption is rising and its malabsorption causes common gastrointestinal symptoms. Because its absorption capacity is poorly understood, there is no standard method of assessing fructose absorption. We performed a dose response study of fructose absorption in healthy subjects in order to develop a breath test to distinguish normal from abnormal fructose absorption capacity.
Methods
In a double-blind study, 20 healthy subjects received 10% solutions of 15g, 25g and 50g of fructose and 33 % solution of 50g fructose on 4 separate days, at weekly intervals. Breath samples were assessed for hydrogen and methane over five hours, and symptoms were recorded.
Results
No subject tested positive with 15g. Two (10%) tested positive with 25g fructose but were asymptomatic. Sixteen (80 %) tested positive with 50g (10% solution) and 11 (55%) had symptoms. Breath H2 was elevated in 13 (65%), CH4 in 1 (5%) and both in 2 (10%). Twelve (60%) tested positive with 50g (33% solution) and 9 (45%) experienced symptoms. The area under the curve for H2 and CH4 was higher (p< 0.01) with 50g compared to lower doses. There were no gender differences.
Conclusions
Healthy subjects have the capacity to absorb up to 25g fructose whereas many exhibit malabsorption and intolerance with 50g fructose. Hence, we recommend 25g as the dose for testing subjects with suspected fructose malabsorption. Breath samples measured for H2 and CH4 concentration at 30 minute intervals and for 3 hours will detect most subjects with fructose malabsorption.
Keywords: fructose, malabsorption, breath test, intolerance, hydrogen, methane
Introduction
During the last decade, sucrose consumption has increased by at least 25 lbs/yr 1. The source of sweetener has also changed from cane sugar (as sucrose, a disaccharide of glucose and fructose) to corn sweetener (primarily equal molar quantities of the monosaccharides sucrose and fructose). Thus the average consumption of fructose as a monosaccharide has increased dramatically.
Fructose is a six carbon sugar that occurs naturally in fruits such as apples, peaches, prunes etc and honey contains as much as 35 grams/100 grams edible portion2. It is also produced enzymatically from corn as high fructose corn syrup and this form of fructose is commonly used in many food sweeteners, soft drinks, diabetic and diet foods1, 3. Dietary fructose may be ingested as a monosaccharide (eg. High fructose corn syrup) or as a disaccharide (sucrose, eg. table sugar). Sucrose is split by sucrase to produce equal amounts of glucose and fructose and in this form is usually completely absorbed.
Fructose is mostly absorbed in the small intestine through GLUT-5 transporter mediated facilitative diffusion. This is an energy independent process and consequently its absorptive capacity is carrier limited4 Glucose promotes intestinal fructose absorption by solvent drag and passive diffusion2, 5. However, excessive dietary intake of fructose as a monosaccharide can easily overwhelm the absorptive capacity of the small intestine leading to incomplete absorption of fructose (fructose malabsorption). The unabsorbed fructose can serve as an osmotic load and is thereby rapidly propelled into the colon, where its contact with anaerobic flora causes fermentation and production of gas, bloating and diarrhea6 (dietary fructose intolerance).
Breath hydrogen tests have been advocated for the assessment of dietary fructose malabsorption5, 7, 8. In a previous study of patients with unexplained GI symptoms, 134/183 (73%) patients had a positive fructose breath test, indicating fructose malabsorption. We found that 39% of patients exhibited fructose malabsorption when tested with a dose of 25g, and 66% with a dose of 50g of fructose at 10% concentration6, suggesting that fructose malabsorption may be dose related and confirming previous observations5, 7, 8. Furthermore, a higher concentration of fructose was associated with a higher incidence of malabsorption8. Hence, not only the dose but also the concentration of fructose may affect its absorption. In addition, 101/134 (75%) of patients with a positive breath test had their predominant symptom(s) reproduced during the breath test, suggesting dietary fructose intolerance 6.
A recent study of IBS subjects with fructose intolerance and a positive breath test showed that dietary restriction of fructose improved symptoms9. This observation was confirmed by two other recent studies10,11. These studies suggest that the recognition of dietary fructose intolerance may be clinically useful. However, the appropriate dosage and concentration of fructose that should be used in clinical practice to distinguish a normal from an abnormal capacity to absorb dietary fructose, is not clearly known12.
Here, we tested the following hypothesis; 1) 15g and 25g dose of fructose is more completely absorbed than a 50g dose of fructose and 2) 10% fructose solution (lower osmolarity) is more completely absorbed than a 33% fructose solution (higher osmolarity).In order to test these hypotheses, we performed a randomized, double blind, dose response study of three different doses and two different concentrations of fructose, and assessed its absorption and tolerance in healthy subjects.
Methods
Healthy volunteers with no previous history of gastrointestinal disorders or surgeries, antibiotic use (within 3 months), and who were not taking any medications (except oral contraceptive pills or multi-vitamins) were recruited through a hospital advertisement. They were asked to fill out a health symptom questionnaire and undergo a routine physical examination. Only subjects who were asymptomatic, fulfilled the aforementioned inclusion criteria, and had a normal physical examination were eligible to participate.
Study Protocol
The study required four visits to the motility laboratory at weekly intervals. Subjects were asked to complete a bowel symptom questionnaire in which they recorded their baseline symptoms. One day prior to each visit, subjects were asked to consume a lactose-free and fructose-restricted diet, so as to avoid high baseline values of breath hydrogen or methane from the presence of unabsorbed carbohydrates. No food or drink was allowed for at least 8 hours before the study. Subjects were asked to brush their teeth prior to the test and to refrain from smoking, drinking, sleeping or exercising during the study.
At each visit, a baseline breath sample was obtained. Thereafter, in a random order, on four separate days, one of the following four solutions was administered to each subject; 15g of fructose dissolved in 150 ml of water (10% solution), 25g of fructose in 250 ml (10% solution) of water, 50g of fructose in 500 ml of water (10% solution), or 50g of fructose in 125 ml of water (33% solution). The drink was served at room temperature (approximately 70° F) in a 600 ml opaque coffee mug to camouflage the volume of each solution, and the subjects were asked to drink each solution within 10 minutes. The subject, the research assistant who administered the drink, and the individual who collected and analyzed the breath samples were each blinded to the type of solution. Next, breath samples were collected at 30-minute intervals for 4-6 hours. End expiratory breath samples were collected in a modified (Haldane-Priestley) bag (Quin Tron, Milwaukee, WI). A 20 cc sample of air was withdrawn from the bag and injected into a gas chromatography analyzer (Quinn Torn Microlyzer Self Correcting Model SC, Quin Tron, Milwaukee, WI) for detection of hydrogen (H2) and methane (CH4). Correction factors were used to correct for CO2 and dead space using industry standards. If there was a rise in breath hydrogen or methane, samples were collected until the values returned to baseline or five hours had elapsed. During the study, if the subject experienced any symptoms (Abdominal pain, Cramping, Belching, Bloating, Fullness, Nausea, Diarrhea, Vomiting, and Flatulence) after ingestion of fructose, its severity was documented on a visual analog scale.
Measurements and Analysis
The breath samples were analyzed for hydrogen and methane concentration. Fructose malabsorption was defined as a sustained rise of ≥ 20 ppm of H2 or CH4 or both, over the baseline value or a successive incremental rise of at least 5 ppm over the basal value that was sustained over 3 consecutive breath samples. For example, if a basal breath test sample showed a H2 concentration of 4 ppm, and samples obtained at 2, 2 ½ and 3 hours were 11, 16 and 22 ppm, then the test was designated as an abnormal breath test. By plotting the breath H2 or CH4 values over time, we assessed the profiles for the area under the curve of breath H2 or CH4 for each subject and for each concentration of fructose. The area under the curve (AUC) was not used to define a positive test but was used to provide a semi-quantative assessment of the overall volume of gas produced and an index of fructose malabsorption. We modified our definition of a positive fructose breath test from previously published definitions6 in order to decrease the false positive rate. Repeated measures ANOVA were used to compare the area under the curve data for each concentration. The onset time was defined as the interval between ingestion of fructose and the onset of a sustained rise in breath H2 or CH4. The peak time was defined as the time interval between the ingestion of fructose and the occurrence of peak values of H2 or CH4. A dose response curve was plotted for each subject to assess their ability to absorb fructose.
Results
Twenty subjects (m/f = 10/10; mean age 31 yrs, range 19- 70 yrs) participated in the study.
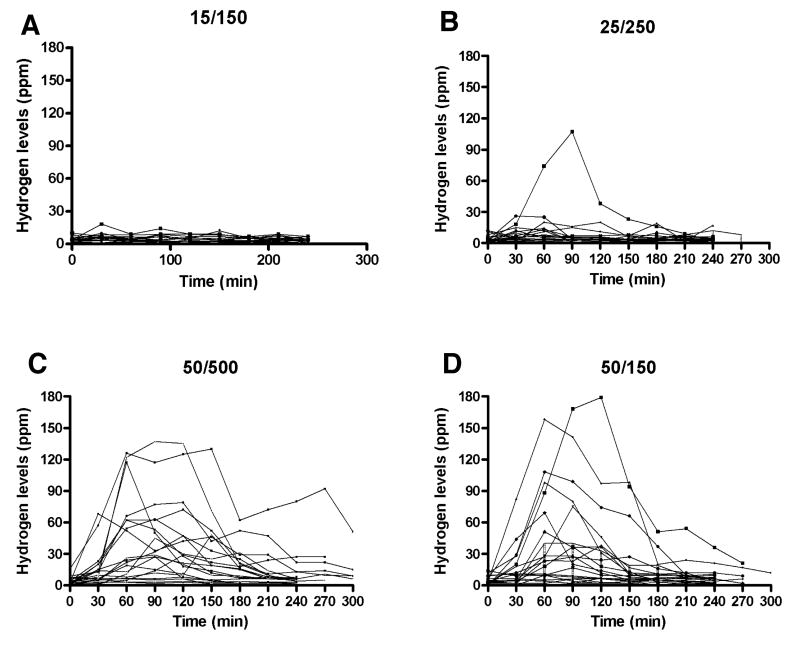
Dose-Related Fructose Absorption (Figure 1)
Figure 1.
Individual profiles of Hydrogen concentration (ppm) in expired air after ingestion of different fructose loads.
Panel A - 15 g fructose in 150 mL water (10% solution)
Panel B - 25 g fructose in 250 mL water (10% solution)
Panel C - 50 g fructose in 500 mL water (10% solution)
Panel D – 50 g fructose in 150 mL water (33% solution)
15g dose
All twenty subjects were able to absorb 15g of fructose without significant elevation of breath hydrogen or methane (Figure IA). None of the subjects reported any symptoms.
25g dose
Eighteen subjects were able to absorb 25g of fructose whereas two subjects (10%) had a positive breath test (Table 1). The peak H2 concentration in these two subjects was 26 and 106 ppm respectively. However, neither subject reported any symptoms. Thus, it appears that most subjects can absorb and tolerate this dose of fructose. The overall area under the curve of breath H2 and CH4 profile was significantly higher (p < 0.05) than that obtained with the 15g dose of fructose (Figure 2). A sub-analysis after excluding the two subjects with a positive test showed that there was no significant difference between the two groups (p > 0.05). None of the subjects with a negative breath test reported symptoms during the test.
Table 1.
Characteristics of the breath H2 and CH4 profiles, and symptoms after ingestion of fructose
| Fructose | 15 g | 25g | 50g (10%) | 50g (33%) | |
|---|---|---|---|---|---|
| % of subjects with a positive breath test | H2 only | 0 | 10 | 65 | 50 |
| CH4 only | 0 | 0 | 5 | 0 | |
| Both | 0 | 0 | 10 | 10 | |
| % of subjects with symptoms during the test | Positive test | 0 | 0 | 69 | 75 |
| Negative test | 0 | 0 | 25 | 25 | |
| Onset time (min)* | N/A | 30 ± 0 | 54 ± 31 | 45 ± 15 | |
| Peak time (min)* | N/A | 90 ± 0 | 81 ± 45 | 74 ± 27 |
mean ± SEM.
Figure 2.
Hydrogen and methane concentration (AUC in mm3) after ingestion of different doses of fructose (mean ± SEM)
50g dose (10%)
Four subjects were able to absorb 50g of fructose whereas sixteen subjects (80%) exhibited a positive breath test (Table 1). The mean area under the curve of breath H2 and CH4 profile was significantly higher (p = 0.006) than that obtained with either the 15g or 25g dose of fructose, but was not different to that of the 33% fructose solution (Figure 2). Also, 69% of subjects with a positive breath test reported symptoms during the test; gas (30%), belching (15%), abdominal pain (15%), diarrhea (15%), and bloating (10%). Additionally, 25% of subjects with a negative breath test also reported similar symptoms (Table 1).
50g dose (33%)
Eight subjects (40%) were able to absorb 50g of fructose at this higher concentration whereas twelve subjects (60%) had a positive breath test (Table 1). The mean area under the curve of breath H2 and CH4 profile was significantly higher (p < 0.05) when compared to those following ingestion of either 15g or 25g fructose. Seventy five percent of subjects with a positive test experienced symptoms during the test; gas (30%), bloating (15%), belching (15%), abdominal pain (10%), headache (10%), and diarrhea (5%). Also, 25% of subjects with a negative breath test reported symptoms.
Characteristics of Breath H2 and CH4 Responses
Six subjects (30%) had methanogenic flora13, of which four subjects exhibited fructose malabsorption. Following fructose malabsorption, a rise in breath H2 or CH4 always occurred within 3 hours. For a positive breath test, the onset time for a rise in breath H2 or CH4 ranged between 30- 54 minutes(Table 1). The time interval between the ingestion of fructose and a peak H2 or CH4 concentration ranged between 74 - 90 minutes (Table 1). Both the onset time and the time for reaching peak concentration were not significantly (p > 0.05) different between the four doses, although there was a trend towards a faster onset and a higher peak for the higher doses and for the 33% concentration (Table 1). The mean baseline H2 values were similar across study days: 3.4, 3.8, 4.5 and 4 ppm respectively for the 15g, 25g, 50g (10%) and 50g (33%) doses. The mean baseline CH4 values were also similar: 4.5, 3.9, 4.9 and 5.3 ppm respectively.
There was no difference (p > 0.05) between male and female subjects in either the incidence of fructose malabsorption or the prevalence of symptoms during the test. For example, following a 50g (10%) dose, 9/10 (90%) males and 7/10 (70%) females had a positive breath test (p > 0.05), and 6/10 (60%) males and 5/10 (50%) females reported symptoms during the breath test.
Adverse Effects
Two subjects developed significant bloating, discomfort and pain with a 50g dose that subsided after a few hours. Several others experienced transient symptoms as summarized above.
Discussion
The capacity of the human small intestine to absorb fructose is unclear. Some studies have suggested that healthy humans malabsorb fructose at doses as low as 5g5, although others have found malabsorption at doses of 37.5 g or higher8. Consequently, the assessment of dietary fructose malabsorption has remained problematic2. Our objectives were to examine fructose absorption in healthy subjects and to facilitate the development of a breath test that can be used in patients with suspected dietary fructose malabsorption to distinguish a normal from an abnormal capacity to absorb dietary fructose
We found that our healthy subjects were able to completely absorb a 15g dose of fructose. This finding differs from that of Rumessen et al14 who reported that one subject (10%) malabsorbed after ingestion of either 5g or 10g of fructose and four subjects (40%) malabsorbed after receiving a 20 g dose. Unlike their study, we used strict criteria for selecting our healthy subjects and restricted their dietary intake of carbohydrate on the day before the study, in order to prevent high basal values of hydrogen or methane that could influence test interpretation. Secondly, we performed a randomized, double blind study, at weekly intervals, in equal number of men and women and measured both H2 and CH4, whereas the previous study was single blinded, not always randomized, the studies were repeated after two days, and there was male predominance. Thirdly, the observation that healthy subjects malabsorb doses of fructose as low as 5-15 g, most likely stems from repeated studies in a single subject who, although healthy, may have unrecognized fructose malabsorption.
Following ingestion of 25 g fructose, 2 of our subjects showed evidence for fructose malabsorption. In one subject there was a significant increase in breath hydrogen with a peak H2 concentration of 106 ppm. The second subject showed a modest rise with a peak H2 concentration of 26 ppm. However, both subjects were asymptomatic. These findings are consistent with those of others who found either no malabsorption 8 or 19% malabsorption with a 25g dose7. Also, similar to our study, none of the subjects in these previous studies were symptomatic.
In contrast, when a 50g dose of fructose was administered, the majority of subjects exhibited malabsorption. In these subjects there was significant elevation of breath H2 or CH4 concentration. Furthermore, approximately 50 % of subjects reported mild to moderate belching, bloating or diarrhea. These observations emphasize that a 50g dose is inappropriate and un- physiological for the evaluation of subjects with suspected fructose malabsorption.
Thus, although the absorption capacity is variable in healthy subjects, a dose of 25g fructose may represent a critical threshold for bolus ingestion of this sugar, above which significant malabsorption can occur. This finding supports our hypothesis. Interestingly, about 31% of subjects who received the 50g dose of fructose at a concentration of 33% and 25% of subjects who received the 10% concentration did not report any symptoms, despite the evidence for malabsorption. Thus, the breath test appears to be quite sensitive for detecting fructose malabsorption, and by itself may overestimate the true prevalence of dietary fructose intolerance. Hence, an assessment of symptoms, both during and immediately after the test together with a positive breath test provides the best evidence for a diagnosis of dietary fructose intolerance. Likewise, symptoms alone after ingestion of fructose may not accurately predict fructose malabsorption, because 10-20 % of healthy subjects experienced mild gastrointestinal symptoms without any evidence for fructose malabsorption. Also, subjects who have a normal fructose breath with a 25g dose may still have dietary fructose intolerance, if they ingest a higher amount (>25g) of fructose in their diet. This limitation of the breath test should be considered in the evaluation of these patients. A prospectively maintained food diary along with dietary consultation can be very useful in the clinical evaluation of these patients.
Approximately 30 % of our study population had a methanogenic flora, and four subjects had elevated methane levels after ingestion of fructose. In 2 subjects (10%), there was an exclusive rise of methane only suggesting that any hydrogen produced was shunted into the methanogenic pathway13. Thus, when compared to another study8, the higher yield of a positive breath test in our study may in part be due to the assessment of methane production. Consequently, an assessment of both will facilitate a more accurate detection of fructose malabsorption and reduce the incidence of false negative tests.
A previous study reported that fructose malabsorption increased from 37.5% to 71.4% when the concentration of ingested fructose increased from 10% to 20%8. However, at a similar dose (50g), and at a higher concentration of fructose (33% solution), we were unable to identify a concentration-dependent malabsorption of fructose, which negated our hypothesis. Thus, our study suggests that fructose malabsorption is more likely to depend on the amount of fructose consumed rather than the concentration of fructose. It is also possible that the healthy upper gut is capable of handling the higher osmotic concentration or that fructose absorption is independent of its concentration gradient in the lumen15.
We collected breath samples for a total duration of five hours. Reviewing the profiles for the area under the curve, it became apparent that the rise in H2 or CH4 typically occurred within 60 minutes, and the mean time for reaching a peak H2 or CH4 concentration was 77 minutes (range = 30-180 min). Hence, an abnormal breath test was usually apparent within 180 minutes. Consequently, by analyzing breath samples that are obtained for a total duration of three hours, and at 30-minute intervals, it should be possible to detect most subjects with fructose malabsorption.
One other possibility for an abnormal fructose breath test is small bowel bacterial overgrowth. This is unlikely because we examined asymptomatic healthy individuals. Also, a previous study showed that after ingestion of fructose along with a radioisotope meal, both symptoms and a rise in breath H2 always coincided with the arrival of isotope in the caecum16. Thus, in our study, a positive breath test most likely resulted from fructose malabsorption.
Our study confirms previous observations that the incidence of gastrointestinal symptoms after ingestion of fructose is quite variable in healthy subjects5-8. Thus, symptoms or a breath test alone is unlikely to accurately detect dietary fructose malabsorption. However, the genesis of symptoms and the threshold for reporting symptoms may vary across individuals, and can depend on various factors including health status, patient expectation, genetic predisposition, and environmental factors, individual variation in sensory thresholds, the rate of gas production, the shunting of gas into alternate pathways, as well as its absorption, consumption and transport10, 14, 17-20.
In summary, our study revealed that healthy subjects completely absorbed a dose of 15g fructose. About 10% of subjects malabsorbed a dose of 25g, but they were asymptomatic. Therefore, 25g fructose can distinguish a normal from an abnormal capacity to absorb fructose and a positive breath test with this dose suggests an abnormally low capacity to absorb dietary fructose. Thus, 25g of fructose is the appropriate dose for testing subjects with suspected dietary fructose malabsorption. Breath samples obtained at 30-minute intervals, and for a duration of three hours, and analyzed for both hydrogen and methane concentration will detect most individuals with dietary fructose malabsorption.
Acknowledgments
This research was supported in part by NIH grant RO1 DK 57100-05 and grant RR00059 from the General Clinical Research Centers Program, National Center for Research Resources. The authors acknowledge the excellent technical assistance of Ms. Joan Kemp, Mrs. Michelle Jackson and secretarial assistance of Mrs. Heidi Vekeman. We also sincerely appreciate the expert critique and assistance of Robert Summers, M.D. with the preparation of this manuscript. Portions of this work were presented at the Digestive Disease Week 2003, May 17-22, Orlando, Florida and published as an abstract in Gastroenterology 2003; 124: 312.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agriculture USDo. Agriculture Fact Book. 1998 Volume http://www.usda.gov/news/pubs/fbook98/afb98.pdf.
- 2.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol. 2004;99:2046–50. doi: 10.1111/j.1572-0241.2004.40266.x. [DOI] [PubMed] [Google Scholar]
- 3.Rumessen JJ. Fructose and related food carbohydrates. Sources, intake, absorption, and clinical implications. Scand J Gastroenterol. 1992;27:819–28. doi: 10.3109/00365529209000148. [DOI] [PubMed] [Google Scholar]
- 4.Riby JE, Fujisawa T, Kretchmer N. Fructose absorption. Am J Clin Nutr. 1993;58:748S–753S. doi: 10.1093/ajcn/58.5.748S. [DOI] [PubMed] [Google Scholar]
- 5.Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27:1161–8. doi: 10.1136/gut.27.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YK, Johlin FC, Jr, Summers RW, Jackson M, Rao SS. Fructose intolerance: an under-recognized problem. Am J Gastroenterol. 2003;98:1348–53. doi: 10.1111/j.1572-0241.2003.07476.x. [DOI] [PubMed] [Google Scholar]
- 7.Truswell AS, Seach JM, Thorburn AW. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr. 1988;48:1424–30. doi: 10.1093/ajcn/48.6.1424. [DOI] [PubMed] [Google Scholar]
- 8.Ravich WJ, Bayless TM, Thomas M. Fructose: incomplete intestinal absorption in humans. Gastroenterology. 1983;84:26–9. [PubMed] [Google Scholar]
- 9.Choi YK, Rao SSC, et al. Fructose Intolerance in IBS and Utility of Fructose Restricted Diet. Journal of Clinical Gastroentrology. 2007 doi: 10.1097/MCG.0b013e31802cbc2f. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 10.Johlin FC, Jr, Panther M, Kraft N. Dietary fructose intolerance: diet modification can impact self-rated health and symptom control. Nutr Clin Care. 2004;7:92–7. [PubMed] [Google Scholar]
- 11.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–9. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Simren M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1–4. doi: 10.1056/NEJM199507063330101. [DOI] [PubMed] [Google Scholar]
- 14.Rumessen JJ, Gudmand-Hoyer E. Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology. 1988;95:694–700. doi: 10.1016/s0016-5085(88)80016-7. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Schedl HP, Summers RM, Lambert GP, Chang RT, Xia T, Gisolfi CV. Fructose transport mechanisms in humans. Gastroenterology. 1997;113:1171–9. doi: 10.1053/gast.1997.v113.pm9322512. [DOI] [PubMed] [Google Scholar]
- 16.Summers RW, J F. Fructose intolerance is due to rapid orocecal transit and not small bowel overgrowth. Gastroenterology. 2001;120:A1369. [Google Scholar]
- 17.Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313–27. doi: 10.1016/s0025-7125(05)70288-1. [DOI] [PubMed] [Google Scholar]
- 18.Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–24. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 19.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–93. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 20.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–9. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]