Abstract
Dinitroanilines are of interest as antiprotozoal lead compounds because of their selective activity against the tubulin of these organisms, but concern has been raised due the potentially mutagenic nitro groups. Analogues of N1-phenyl-3,5-dinitro-N4,N4-di-n-butylsulfanilamide (GB-II-150, compound 2b), a selective antimitotic agent against African trypanosomes and Leishmania, have been prepared where the nitro groups are replaced with amino, chloro, cyano, carboxylate, methyl ester, amide, and methyl ketone moieties. Dicyano compound 5 displays IC50 values that are comparable to 2b against purified leishmanial tubulin assembly (6.6 vs. 7.4 μM), Trypanosoma brucei brucei growth in vitro (0.26 vs. 0.18 μM), L. donovani axenic amastigote growth in vitro (4.4 vs. 2.3 μM), and in vitro toxicity against Vero cells (16 vs. 9.7 μM). Computational studies provide a rationale for the antiparasitic order of activity of these analogues and further insight into the role of the substituents at the 3 and 5 positions of the sulfanilamide ring.
Keywords: Leishmania, Trypanosome, Chemotherapy, Tubulin, Dinitroaniline
1. Introduction
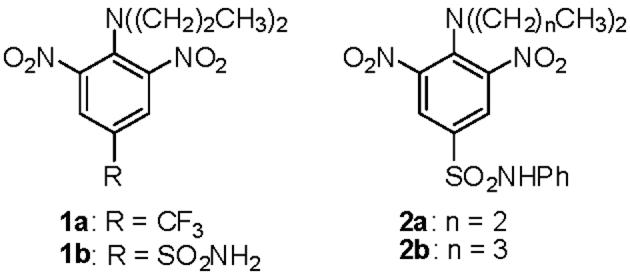
Dinitroaniline herbicides are well known for their activity against protozoan parasites. Chan and Fong first reported the activity of trifluralin (1a) against the pathogenic protozoan Leishmania in 1990 and showed that this compound bound selectively to partially purified leishmanial tubulin compared to rat brain tubulin.1 Another dinitroaniline herbicide, oryzalin (1b), also displayed antimicrotubule activity against Leishmania.2 These compounds were later shown to possess antiparasitic activity against trypanosomes,3, 4 Plasmodium,5, 6 and Toxoplasma.7-9 Given the need for new drug candidates against diseases caused by protozoan parasites,10, 11 the dinitroaniline herbicides are intriguing lead compounds. Replacement of the nitro moieties present in these molecules with other functional groups would be desirable, however, since several nitroaromatic compounds have been shown to be mutagenic.12-14
Our laboratory previously demonstrated that N1-phenyl-3,5-dinitro-N4,N4-di-n-propylsulfanilamide (GB-II-5, compound 2a), N1-phenyl-3,5-dinitro- N4,N4-di-n-butylsulfanilamide (GB-II-150, compound 2b), and other N1-aryl-3,5-dinitroaniline sulfanilamides possess selective antimicrotubule activity against Leishmania and African trypanosomes in vitro and are far superior to 1a and 1b in their potency against these parasites.15-18 Replacement of one of the nitro groups present in 2a with a hydrogen resulted in a profound loss in antiparasitic and antitubulin activity.17 A recent computational study by Mitra and Sept suggested that these nitro groups in 2a serve as hydrogen bond acceptors at a unique binding site occurring on the α-subunit of leishmanial tubulin.19 These authors also predicted that cyano and methyl ketone groups could act as hydrogen bond acceptors if they were substituted for the nitro groups present in the dinitroaniline sulfanilamides. In the present manuscript, we describe the synthesis and biological activity of analogues of 2b where the nitro moieties are replaced by several groups with chemical and physical similarities.
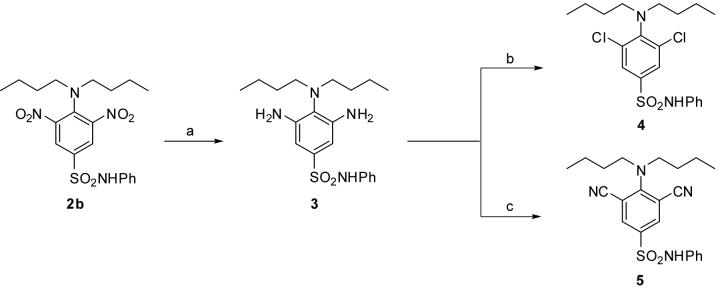
2. Results
2.1. Chemistry
It was proposed that target compounds containing Cl atoms and cyano moieties in place of the nitro groups present in 2b could be introduced through a strategy culminating with a Sandmeyer reaction. Reduction of 2b was carried out using H2 in the presence of 10% Pd-C, which afforded the diamino compound 3 in high yield (Scheme 1). Treatment of 3 with NaNO2 in HCl followed by reaction of the resultant bis-diazonium salt with CuCl afforded the dichloro analogue 4, albeit in low yield (Scheme 1). All efforts to improve the yield of 4 by changing the reaction conditions were unsuccessful. Target compound 5 was also prepared by this strategy, but in extremely low yield (Scheme 1). The diazotization reaction was carried out using NaNO2 and HCl in a mixture of H2O/AcOH containing 3 at 0 °C. This solution was then treated with an excess of CuCN and KCN in a mixture of CHCl3/H2O at 0 °C20 followed by heating to 50 °C. These conditions did not reproducibly provide the desired product. Other related approaches, such as preparing the bis-diazonium salt using either tert-butyl nitrite or tert-butyl nitrite in BF3 etherate in situ, isolating the bis-diazonium salt prior to reaction with cyanide, or adding 18-crown-6 to complex the potassium ion were unsuccessful. The presence of the bulky dibutylamino groups in the substrate may interfere with the Sandmeyer reaction in this case and may be the cause of the poor reaction yield.
Scheme 1.
Reagents and conditions: (a) H2, 10% Pd-C, EtOAc, rt, 98%; (b) (1) NaNO2, conc. HCl, H2O, 0 °C; (2) CuCl, HCl, 0 °C to rt, 23%; (c) (1) NaNO2, conc. HCl, H2O/AcOH, 0 °C; (2) CuCN, KCN, CHCl3/H2O, 0 °C to 50 °C, 12%.
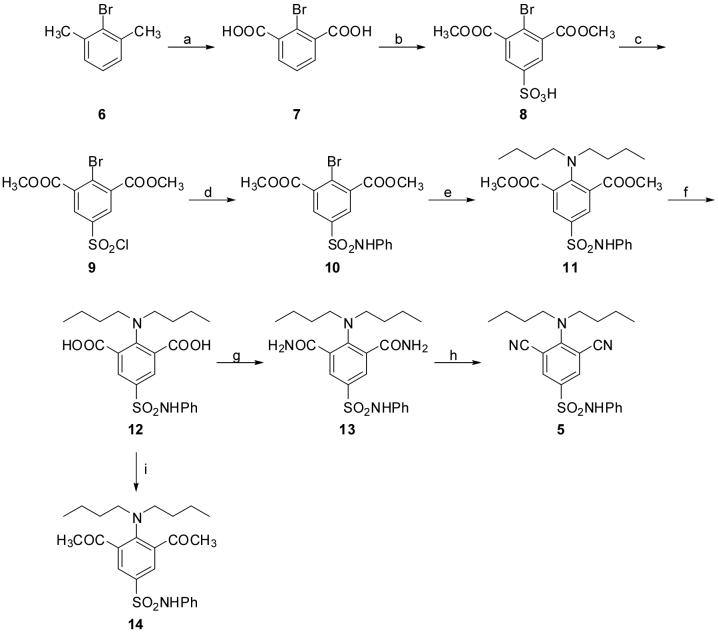
In order to provide a more efficient and reproducible alternative for the preparation of 5 and additional target compounds, another synthetic route was envisioned (see Scheme 2). Permanganate oxidation of commercially available 2-bromo-m-xylene (6) gave 2-bromoisophthalic acid (7),21 which was sulfonylated with fuming sulfuric acid and esterified using methanol/HCl to afford the sulfonic acid 8. Treatment of 8 with POCl3 provided the sulfonyl chloride 9, which was then converted to sulfonamide 10 by reaction with aniline in the presence of pyridine in acetone. An Ullmann reaction22 between sulfonamide 10 and dibutylamine afforded the desired diester analogue 11, which was hydrolyzed to yield diacid 12. The formation of bis-amide 13 was carried out in a one pot reaction between ammonia and the intermediate bis-acid chloride derivative of 12, which in turn was prepared by the reaction of 12 with hexachloroacetone in the presence of PPh3.23 Attempts to synthesize the bis-amide directly from diester 11 were unsuccessful, resulting in quantitative recovery of the starting material. Bis-amide 13 was found to be an excellent precursor for the target dicyano compound 5. Dehydration of 13 using PdCl2 in an acetonitrile/water mixture24 afforded 5 in good and reproducible yield. The synthesis of dicyano compound 5 from bis-amide 13 is an equilibrium process in which the presence of excess acetonitrile favors the formation of 5.
Scheme 2.
Reagents and conditions: (a) KMnO4, H2O, reflux, 66%; (b) (1) fuming H2SO4, 170 °C; (2) HCl, MeOH, reflux, 79% after two steps; (c) POCl3, reflux, 70%; (d) aniline, pyridine, acetone, 0 °C to rt, 66%; (e) dibutylamine, K2CO3, Cu, dioxane, reflux, 79%; (f) 3N NaOH, MeOH, reflux, 88%; (g) (1) CCl3COCCl3, PPh3, THF, 0 °C; (2) NH3/dioxane, rt, 68% after two steps; (h) PdCl2, CH3CN/H2O, 70 °C, 71%; (i) (1) CCl3COCCl3, PPh3, THF, 0 °C; (2) MeMgBr /THF, CuBr, THF, 0 °C, 55% after two steps.
With target compounds 3 − 5 and 11 − 13 in hand, attention was focused on the preparation of bis-ketone 14. Initial attempts to synthesize 14 by treating the bis-acid chloride derivative of 12 with dimethyl malonate in the presence of NaH followed by decarboxylation25 were unsuccessful. Reaction between methyl magnesium bromide and the bis-acid chloride gave compound 14 along with other unidentified side products. The formation of these side products could not be avoided by varying the reaction temperature, time, and the amount of Grignard reagent. We hypothesize that further reaction of the bis-ketone with methyl magnesium bromide was responsible for the occurrence of the side products. Therefore, it was reasoned that a soft nucleophile such as an organocuprate might lead to more efficient production of the diketone. In accord with this expectation, reaction of the acid chloride with a mixture of MeMgBr and CuBr in THF at 0 °C afforded the target bis-ketone 14 in moderate yield (Scheme 2).
2.2. Biological results
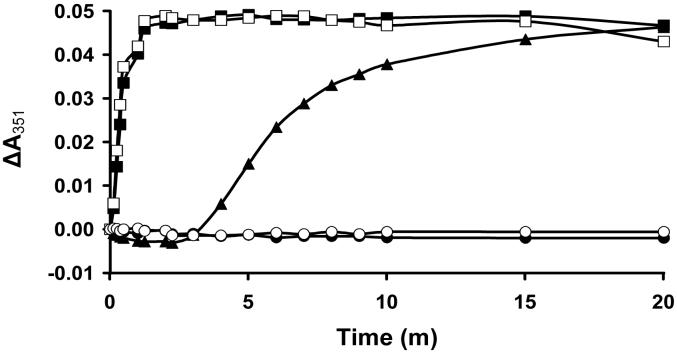
Target compounds 3 – 5 and 11 − 14 were judged against to 2b for their ability to block the assembly of purified tubulin isolated from Leishmania tarentolae (see Table 1). Compound 2b displayed an IC50 value of 7.4 μM, which compares favorably with the previously reported IC50 values for this compound (6.9 μM17 and 5.8 μM26 in the same assay. Of the new compounds, the two most potent inhibitors of leishmanial tubulin assembly were 5 and 4, whose IC50 values of 6.6 μM and 9.1 μM are comparable to and slightly higher than that of 2b. Compounds 3 and 11 − 14 displayed IC50 values > 100 μM according to the previously defined conditions of the assembly assay, which measures the change in absorbance due to tubulin polymerization at 351 nm after 20 min incubation at 30 °C for samples containing the compound of interest compared to controls.17, 18 However, both 3 and 14 caused a pronounced lag in the assembly of leishmanial tubulin at concentrations ≥ 20 μM and thus show a significant effect on the kinetics of polymerization. The effects of 3 and 14 on the kinetics of leishmanial tubulin assembly are dose-dependent, with higher concentrations producing a greater lag in assembly. Similar effects of other antimitotic compounds have been observed previously in our lab16 and by others.27, 28 Leishmanial tubulin assembly was relatively insensitive to 11 − 13 at concentrations up to 100 μM. The effect of 2b, 5, 13, and 14 on parasite tubulin assembly is illustrated in Figure 1. Compounds 2b, 3 – 5, and 14 were also examined for their effects on the assembly of purified porcine brain tubulin (Table 1), with 2b, 4, 5, and 14 displaying little influence on the kinetics or extent of polymerization at the highest concentrations tested. Approximately 50% inhibition of porcine tubulin assembly was observed in assays containing 3 at a concentration of 100 μM.
Table 1.
Biological Activity of Target Compounds (μM)a
| Compound | IC50 vs. L. tarentolae tubulin assembly | IC50 vs. porcine brain tubulin assembly | IC50 vs. L. donovani axenic amastigotesa | IC50 vs. T. b. brucei bloodstream forms | IC50 vs. Vero cells |
|---|---|---|---|---|---|
| 2b | 7.4 ± 0.2 | >40e | 2.3 ± 0.5 | 0.18 ± 0.01 | 9.7 ± 1.1 |
| 3 | >100b | >50 | 12 ± 2 | 4.0 ± 0.1 | 27 ± 4 |
| 4 | 9.1 ± 4.5 | >50e | 11 ± 3 | 1.6 ± 0.1 | 19 ± 1 |
| 5 | 6.6 ± 0.7 | >50e | 4.4 ± 0.9 | 0.26 ± 0.03 | 16 ± 1 |
| 11 | >100 | NT | >50 | >50 | NT |
| 12 | >100 | NT | >50 | >50 | NT |
| 13 | >100 | NT | >50 | >50 | NT |
| 14 | >100b | >100 | 30 ± 7 | 1.6 ± 0.3 | 26 ± 1 |
| pentamidine | NTc | NT | 2.3 ± 0.9 | NT | NT |
| suramin | NT | NT | NT | 0.077 ± 0.001 | NT |
| podophyllotoxind | NT | NT | NT | NT | 0.016 ± 0.006 |
Activities represent the mean ± standard deviation of at least three independent experiments.
Produces a lag in tubulin assembly; see text and Figure 1.
NT: Not tested
Podophyllotoxin is used here solely as a standard for the cytotoxicity assay. Colchicinesite antimicrotubule agents such as podophyllotoxin have little effect on Leishmania tubulin assembly37
Compounds not soluble at higher concentrations
Figure 1.
The assembly of 1.5 mg/mL purified tubulin from Leishmania tarentolae was assessed as described previously26 in the absence (open squares) or presence of 5 (closed circles), 13 (closed squares), or 14 (triangles) at 20 μM or 2b (open circles) at 10 μM. Data shown is from a representative experiment performed on three separate occasions.
Compounds 2b, 3 − 5, and 11 − 14 were docked into the proposed dinitroaniline binding pocket on leishmanial α-tubulin19 using AutoDock29 and Glide.30 These molecules bind similarly as shown in Figure 2 and in a manner consistent with the model proposed by Mitra and Sept.19 Both docking methods show that 4 has the best binding clustering, 2b and 5 follow, and the remaining compounds display worse binding clustering (see Table 2). While there is some variability between the values determined by AutoDock and Glide, the calculated binding energies of 2b, 3 – 5, 11, 13, and 14 are comparable. Interestingly, compound 12, where isosteric replacement of the nitro moieties with carboxylate groups has occurred, shows intermediate binding clustering with AutoDock but displays poor binding clustering with Glide and shows binding energies that are inferior to all of the other target compounds.
Figure 2.
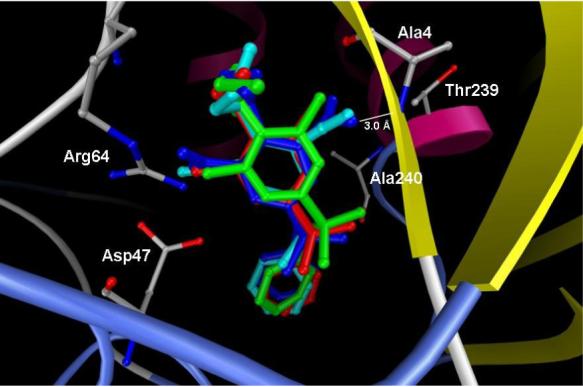
The docked positions of compounds 2b and 3 − 5 are shown as stick models (2b, blue; 3, green; 4, red; 5, cyan), while the tubulin protein is shown as ribbons. Stick models are also shown for Ala4 (which takes part in hydrogen bonding to a nitro group oxygen of compound 2b), Thr239/Ala240 (which participate in electrostatic interactions with the other oxygen atom of this nitro moiety), and Arg64/Asp47 (which form a salt bridge and interact with the other nitro group of 2b through the solvent network). This figure was created with the aid of AutoDock.
Table 2.
In Silico Binding Energies and Binding Clustering of Target Compounds
| Compound | Percent binding clustering (AutoDock) | Binding energy (AutoDock, in kcal/mol) | Percent binding clustering (Glide) | Binding energy (Glide, in kcal/mol) |
|---|---|---|---|---|
| 2b | 93 | −11.0 | 44 | −7.9 |
| 3 | 4 | −9.8 | 3 | −8.9 |
| 4 | 96 | −10.2 | 76 | −9.9 |
| 5 | 92 | −10.7 | 44 | −8.5 |
| 11 | 2 | −11.2 | 2 | −7.6 |
| 12 | 33 | −7.0 | 2 | −3.2 |
| 13 | 2 | −10.3 | 2 | −8.7 |
| 14 | 2 | −10.6 | 2 | −7.5 |
The activities of the target compounds against Leishmania donovani axenic amastigotes, Trypanosoma brucei brucei bloodstream forms, and African Green Monkey kidney (Vero) cells are shown in Table 1. Consistent with previous reports, 2b possesses potent in vitro activity against these organisms and displays excellent selectivity against African trypanosomes compared to the mammalian cell line.17, 18 Compound 5 was only 1.4-fold less active than 2b against T. b. brucei and 2-fold less active than this compound against L. donovani while showing 1.6-fold lower toxicity against Vero cells. Compounds 4 and 14 were 6-fold less potent than 5 against T. b. brucei, while 3 displayed 15-fold lower activity than 5 against this parasite. In terms of antileishmanial activity, 3 and 4 were nearly 3-fold less active than 5 against L. donovani, while 14 was 7-fold less potent in this assay. Compounds 11 − 13 failed to display measurable activity against either Leishmania or African trypanosomes at the highest concentrations tested.
3. Discussion
Nitro groups have thus far proven essential for the antiparasitic antimitotic activity of dinitroaniline sulfonamide analogues. Mitra and Sept's binding site model for dinitroaniline sulfonamide 2a on leishmanial α-tubulin permitted formulation of the first hypothesis regarding the role of these nitro moieties in tubulin binding.19 According to this model, one of the nitro groups makes several direct interactions with the protein. Electrostatic interactions are observed between one of the oxygen atoms in this moiety and partially positively charged structural motif consisting of Thr239 and Ala240, while the other oxygen atom of this group accepts a hydrogen bond from the amide NH of Ala4 (see Figure 2). The second nitro group does not interact directly with α-tubulin; instead, a solvent network connects this moiety to the salt bridge between Asp47 and Arg64. Through docking experiments, Mitra and Sept also showed that an analogue of 2a bearing cyano groups in place of the nitro moieties displays in silico binding affinity to leishmanial tubulin similar to 2a, while an analogue possessing acetyl groups in these positions binds more strongly than 2a to the parasite protein. In this work, we have prepared a series of analogues of 2b, which is slightly superior to 2a in vitro antiparasitic activity and differs in structure from 2a only in the presence of an additional methylene group in the N4 dialkyl chains.17 Replacement of the nitro moieties of 2b with carboxylate groups (12) represents an isosteric substitution. Substitution of the nitro groups with methyl ester (11), amide (13) and acetyl (14) groups were envisioned as nonclassical bioisosteric replacements.31 Nitrile, methyl ester, amide, and acetyl functional groups present in compounds 5, 11, 13, and 14, respectively, have similar lipophilic and electronic properties as compared to the nitro group.32 Evaluation of 3 – 5 and 11 − 14 for their effects on purified leishmanial tubulin assembly now permits refinement of the hypotheses advanced by Mitra and Sept and provides greater insight into the role of these groups in tubulin-ligand interactions.
In accord with previous expectations,19 dicyano compound 5 displays activity in the leishmanial tubulin assembly assay comparable to that of dinitro lead 2b. Dichloro compound 4 shows potency similar to 5 in this assay, while 3 and 14 display weaker activity and 11 − 13 are essentially inactive. Refinements to the binding site model19 through the present docking studies provide a rationale for these observations. Compounds 2b, 4, and 5, the most effective inhibitors of leishmanial tubulin assembly described in this work, also exhibited the best AutoDock and Glide scores of the group. At the positions where nitro groups occur in 2b, each of these compounds possesses functional groups that can serve as a hydrogen bond acceptor. Figure 2 clearly shows the proposed hydrogen bond formed between a nitro group oxygen atom of 2b and the amide group of Ala4. Similarly, a Cl atom of 4 and particularly a cyano group of 5 can also serve as hydrogen bond acceptors as pictured in Figure 2. While a hydrogen bond acceptor is required where one of the nitro moieties are located in 2b, close inspection of the binding site model suggests that activity should be maintained when a different functional group is placed at the other position. Although the second nitro group of 2b is proposed to interact with the solvent network as mentioned earlier, placement of a bulky hydrophobic group at this subpocket consisting of Trp21, Phe24, Met36, Ile42 and His61 may also permit favorable interactions at the binding site. Individual molecular dynamics simulations will be required to determine whether the salt bridge between Asp47 and Arg64 interacts with the newer analogues through the solvent network.
Inactive compounds 11 − 13 show poor scores in molecular docking studies. Steric considerations are likely to be responsible in part for the lack of activity of diester 11, while amide bond resonance limits the availability of the lone pair electrons present on the amino group of 13. Although the substitution of nitro moieties with carboxylate groups in 12 is an isosteric replacement, the nitro moieties lack a net charge while the carboxylate groups are expected to possess a negative charge at physiological pH. The difference in net charge between 2b and 12 is likely to be responsible for the lack of activity of the latter molecule. Compounds 3 and 14 also display poor AutoDock and Glide scores but have measurable effects on leishmanial tubulin assembly (albeit weaker than 2b, 4, and 5). As an arylamine, 3 is potentially carcinogenic,33 but this compound was prepared as a necessary intermediate in the synthesis of 4 and was tested because of the contribution that could be made to the structure-activity relationship. The van der Waals radius of the nitrogen atom (1.55 Å) is 0.2 Å smaller than that of chlorine,34 suggesting that the lone pair electrons of the amino group of 3 may not be positioned ideally to serve as a hydrogen bond acceptor. In addition, the amino group is most often a hydrogen bond acceptor rather than a donor. One of the oxygen atoms of the nitro group in 2b is proposed to provide electrostatic interactions with Thr239 and Ala240 at the binding site, which would not be possible for the methyl group of diketone 14.19
Data shown in Table 1 and Figure 1 provide support for the hypothesis that the disruption of microtubule assembly plays a critical role in the antikinetoplastid action of the target molecules. The rank order of these compounds for activity against leishmanial tubulin assembly (2b, 5, 4 > 3, 14 > 11 − 13) is very similar to the rank order for activity against L. donovani axenic amastigotes in vitro (2b > 5 > 3, 4 > 14 > 11 − 13) and T. b. brucei in vitro (2b, 5 > 4, 14 > 3 > 11 − 13). Minor variation between antiparasitic potency and activity against leishmanial tubulin could be due to the ability of the compounds to reach their cellular target or, in the case of antitrypanosomal activity, to subtle differences between the ligand susceptibility of leishmanial and trypanosomal tubulin (the amino acid identity between L. tarentolae and T. brucei tubulin is 94%).26 Given the potent antitrypanosomal activity and selectivity of compounds 2b and 5, further investigation of the effects of these molecules on purified trypanosomal tubulin and the trypanosomal cell cycle is warranted.
In addition to the presence of nitro groups, another barrier to the development of 2a and 2b as antikinetoplastid drug candidates is metabolic instability. Neither compound showed significant activity against murine African trypanosomiasis when administered intraperitoneally at 4 × 20 mg kg−1 day−1.17 Rapid and extensive metabolism of 2b was later observed, primarily through N4-alkane oxidation, providing a rationale for the lack of activity of these dinitroaniline sulfanilamides.35 While the metabolic stability of 5 remains to be investigated, this molecule retains the N4-dibutyl chain that was the site of extensive metabolism in 2b. However, a recent SAR investigation revealed that a range of hydrophobic substituents was permitted at the N4 position in terms of antiparasitic and antitubulin activity,18 and the tubulin binding site model for 2a and its analogues19 indicates that sufficient space is available for the design of molecules containing substituents at N4 that may be less susceptible to metabolism. The ability to substitute the cyano groups for the potentially mutagenic nitro moiety while retaining activity provides further flexibility in the design of antimitotic antiparasitic molecules. Given the current knowledge of the SAR for this group of molecules and the availability of a binding site model for the generation of new target compounds, the design of new structural classes of antimitotic antikinetoplastid agents with increased metabolic stability may now be possible.
4. Conclusion
Replacement of the nitro groups present in antimitotic antikinetoplastid molecule 2b with cyano moieties provides a compound (5) which displays potent and selective activity against African trypanosomes and Leishmania. Activity is also observed when other groups are placed at these positions, although such molecules are less potent than the corresponding nitro- and cyano-containing compounds. This knowledge, together with refinement of the tubulin binding site model for such ligands, represents a point of departure from the dinitroaniline scaffold in the design of selective antitubulin antiparasitics and provides renewed hope for the development of tubulin-targeted drugs against African trypanosomes, Leishmania, and perhaps other parasitic protozoans as well.
5. Experimental Section
5.1. General Methods
All reagents and solvents were from the Sigma-Aldrich Corporation (St. Louis, MO) unless otherwise indicated. Nuclear magnetic resonance spectra were obtained at 250 or 300 MHz for 1H and 62.5 or 75 MHz for 13C using instruments from Bruker. Thin layer chromatography was conducted on precoated TLC plates from E. Merck (Darmstadt, Germany). HPLC analysis was carried out using a System Gold Model 127 pump equipped with a Model 166 UV detector (Beckman, Fullerton, CA) and a 10 × 250 mm Polaris C18-A column (Varian, Palo Alto, CA). Melting points were recorded on a Thomas-Hoover capillary melting point apparatus and are uncorrected.
5.2. N1-Phenyl-3,5-diamino-N4,N4-di-n-butylsulfanilamide (3).
To a degassed solution of 2b (1 g, 2.22 mmol) in EtOAc (44 mL), 10 wt% Pd on activated carbon (133 mg) was added and stirred at rt under an atmosphere of H2 for one day. The reaction mixture was then filtered through celite and evaporated to afford 3 (850 mg, 98%) as a colorless solid, mp = 85−86 °C. 1H NMR (CDCl3) δ 0.88 (t, J = 7.1 Hz, 6H), 1.19−1.44 (m, 8H), 2.95 (t, J = 7.7 Hz, 4H), 4.01 (bs, 4H), 6.51 (s, 2H), 6.61 (bs, 1H), 7.06−7.31 (m, 5H); 13C NMR (CDCl3) δ 14.01, 20.48, 31.81, 52.68, 104.24, 121.61, 125.09, 125.15, 129.15, 136.85, 136.88, 146.75; HRMS (ESI) calcd for C20H30N4NaO2S (M+Na)+ 413.1987, measured (M+Na)+ 413.1994. Anal. (C20H30N4O2S) calcd C, 61.51; H, 7.74, N, 14.35; found C, 61.53; H, 7.77; N, 14.15.
5.3. N1-Phenyl-3,5-dichloro-N4,N4-di-n-butylsulfanilamide (4).
To a suspension of diamine 3 (200 mg, 0.51 mmol) in a mixture of conc. HCl (1.5 mL) and H2O (0.5 mL), an ice cold solution of NaNO2 in H2O (1 mL) was added very slowly over 15 min at 0 °C. To this an ice-cold solution of CuCl (126 mg, 1.26 mmole) in HCl (1 mL) was added dropwise with stirring. The reaction mixture was stirred for 3 h while warming to rt, then was neutralized with 6N NH4OH. This mixture was extracted with dichloromethane and washed successively with water, brine and dried. The crude product was then purified by column chromatography using hexanes/EtOAc (10:1) to afford 4 (51 mg, 23%) as a colorless solid, mp = 90−91 °C. 1H NMR (CDCl3) δ 0.86 (t, J = 7.2 Hz, 6H), 1.16−1.42 (m, 8H), 3.17 (t, J = 7.2 Hz, 4H), 6.62 (s, 1H), 7.09−7.34 (m, 5H), 7.65 (d, J = 0.9 Hz, 2H); 13C NMR (CDCl3) δ 13.93, 20.16, 30.79, 52.22, 122.00, 125.99, 127.94, 129.52, 134.82, 135.74, 135.83, 149.69. HRMS (ESI) calcd for C20H26Cl2N2O2S (M+Na)+ 451.0990, measured (M+Na)+ 451.0970. Anal. (C20H26Cl2N2O2S) calcd C, 55.94; H, 6.10, N, 6.52; found C, 56.11; H, 6.17; N, 6.48.
5.4. 2-Bromoisophthalic acid (7).21
To a suspension of 2-bromo-m-xylene 6 (13.9 g, 75 mmol) in water (260 mL), KMnO4 (18 g, 113 mmol) was added and refluxed overnight. Another portion of KMnO4 (18 g, 113 mmol) was added and again refluxed for 24h. A third portion of KMnO4 (18 g, 113 mmol) was added and refluxed for an additional day. The reaction mixture was filtered while hot through celite and washed with hot water, then the combined aqueous phase was evaporated to about 75 mL and neutralized with 20% HCl. The reaction mixture was then kept at 0 °C overnight and the crystals formed were filtered and dried to afford 7 (12.1 g, 66%) as a white solid, mp = 209−210 °C (literature 218 °C36). 1H NMR (DMSO-d6) δ 7.50−7.71 (m, 3H). 13.61 (s, 2H).
5.5. 4-Bromo-3,5-bis(methoxycarbonyl)benzenesulfonic acid (8).
2-Bromoisophthalic acid 7 (5.0 g, 20.4 mmol) was added to fuming sulfuric acid (26−29%, 12 mL) at rt and heated to 170 °C for 4h. The reaction mixture was cooled to rt, added to ice and neutralized with sodium bicarbonate. The crystals formed were filtered, co-evaporated with toluene and dried. This crude material was then suspended in MeOH (150 mL) and conc. HCl (6 mL) was added and refluxed for 24 h. Solvent was removed under reduced pressure, then the residue was co-evaporated with toluene (3×) and dried under vacuum to afford 8 (5.7 g, 79% after two steps), mp = 139−140 °C. 1H NMR (DMSO- d6) δ 3.88(s, 6H), 7.94 (s, 2H); HRMS (ESI) calcd for C10H9BrO7S (M)+ 351.9252, measured (M)+ 351.9255.
5.6. Dimethyl 2-bromo-5-(chlorosulfonyl)isophthalate (9).
A suspension of 8 (2.08 g, 5.89 mmol) in POCl3 (6.3 mL, from Acros Organics, Morris Plains, NJ) was refluxed for 24 h. This mixture was then cooled to rt, added to ice water and stirred for 20 min. The crystals formed were filtered, washed with ice water and dried to afford 9 (1.52 g, 70%) as colorless solid, mp = 98−99 °C. 1H NMR (CDCl3) δ 4.03 (s, 6H), 8.35 (s, 2H); 13C NMR (CDCl3) δ 53.51, 127.68, 129.93, 137.06, 142.98, 164.64.
5.7. Dimethyl 2-bromo-5-(phenylsulfamoyl)isophthalate (10).
To a solution of 9 (3.0 g, 8.07 mmol) in acetone (60 mL), pyridine (616 mg, 7.79 mmol, from Mallinckrodt Chemicals, Phillipsburg, NJ) was added at rt, and the solution was cooled to 0 °C. Aniline (725 mg, 7.79 mmol) was then added and the solution was stirred at 0 °C and allowed to warm to rt overnight. Solvent was evaporated and the remaining mixture was extracted using EtOAc. The organic layer was washed with sat. NH4Cl, dried over anhydrous Na2SO4, and evaporated. Purification by silica gel flash column chromatography using hexane/ethyl acetate 5:1 to 4:1 afforded 10 (2.29 g, 69%), mp = 106−107 °C. 1H NMR (CDCl3) δ 3.95 (s, 6H), 6.68 (s, 1H), 7.07−7.34 (m, 5H), 8.04 (s, 2H); 13C NMR (CDCl3) δ 53.22, 122.58, 124.76, 126.45, 129.67, 130.49, 135.32, 136.16, 138.58, 165.35.
5.8. Dimethyl 2-(di-n-butylamino)-5-(phenylsulfamoyl)isophthalate (11).
To a solution of 10 (2.26 g, 5.27 mmol) in dioxane (40 mL), K2CO3 (870 mg, 6.3 mmol) was added followed by Cu (50 mg, 0.79 mmol) and dibutylamine (2.07 g, 16.0 mmol). The reaction mixture was refluxed overnight. Solvent was evaporated, then the residue was extracted with EtOAc, and washed with sat. NH4Cl followed by brine and dried using anhydrous MgSO4. Purification by silica gel flash column chromatography (hexane/ethyl acetate 10:1) afforded 11 (1.99 g, 79%) as a yellow viscous liquid. 1H NMR (CDCl3) 0.86 (t, J = 7.3 Hz, 6H), 1.19−1.31 (m, 4H), 1.47−1.57 (m, 4H), 3.04 (t, J = 7.4 Hz, 4H), 3.87 (s, 6H), 6.76 (s, 1H), 7.08−7.30 (m, 5H), 8.01 (s, 2H); δ ; 13C NMR (CDCl3) δ 13.81, 19.95, 29.63, 52.58, 52.86, 121.82, 125.54, 126.23, 128.36, 129.41, 133.25, 136.25, 153.36, 167.25; HRMS (ESI) calcd for C24H33N2O6S (M+H)+ 477.2059, measured (M+H)+ 477.2061. Anal. (C24H32N2O6S) calcd C, 60.48; H, 6.77, N, 5.88; found C, 60.65; H, 7.02; N, 5.50.
5.9. 2-(Di-n-butylamino)-5-(phenylsulfamoyl)isophthalic acid (12).
To a solution of 11 (518 mg, 1.09 mmol) in MeOH (30 mL), 3N NaOH (5 mL) was added at rt and the reaction mixture was refluxed for 2 days. Solvent was evaporated; the residue was dissolved in water (5 mL) and neutralized with 2N HCl. The crystals formed were filtered and dried to afford 12 (430 mg, 88%) as a colorless solid, mp = 237−238 °C. 1H NMR (DMSO- d6) δ 0.77 (t, J = 7.2 Hz, 6H), 1.12−1.24 (m, 4H), 1.33−1.42 (m, 4H), 3.10−3.15 (m, 4H), 7.03−7.27 (m, 5H), 7.99 (s, 2H), 10.28 (s, 1H), 14.19 (bs, 2H); 13C NMR (DMSO- d6) δ 14.07, 19.88, 29.50, 53.28, 120.93, 124.95, 129.35, 129.69, 131.65, 132.36, 137.81, 150.72, 168.05; HRMS (ESI) calcd for C22H28N2NaO6S (M+Na)+ 471.1566, measured (M+Na)+ 471.1560. Anal. (C22H28N2O6S) calcd C, 58.91; H, 6.29, N, 6.25; found C, 58.31; H, 6.09; N, 5.99.
5.10. 2-(Di-n-butylamino)-5-(phenylsulfamoyl)isophthalamide (13).
To a suspension of the diacid 12 (50 mg, 0.11 mmol) and triphenylphosphine (58 mg, 0.22 mmol, from Acros Organics, Morris Plains, NJ) in THF (3 mL), hexachloroacetone (30 μL, 0.20 mmol) was added at 0 °C and stirred for 1h. Then, 0.5M NH3 in dioxane (1.32 mL, 0.67 mmol) was added, the reaction mixture was allowed to warm to rt and was stirred for 1h. Solvent was evaporated and water was added. The product was then extracted with EtOAc, washed with brine and dried over anhydrous Na2SO4. Evaporation of the solvent followed by column chromatography using hexane/EtOAc 3:1 to 2:1 afforded 13 (33.7 mg, 68%) as a colorless solid, mp = 161−162 °C. HPLC (MeOH/H2O 65/35, 1.0 mL/min, 254 nm) >98%, tR = 4.09 min. 1H NMR (acetone-d6) δ 0.84 (t, J = 7.4 Hz, 6H), 1.19−1.32 (m, 4H), 1.45−1.56 (m, 4H), 3.17−3.22 (m, 4H), 6.89 (bs, 2H), 7.07−7.10 (m, 1H), 7.21−7.29 (m, 4H), 7.89 (bs, 2H), 8.01 (s, 2H), 9.02 (s, 1H); 13C NMR (acetone-d6) 14.27, 21.18, 31.05, 54.47, 121.71, 125.50, 130.15, 130.87, 134.44, 135.32, 138.75, 152.20, 169.61. HRMS (ESI) calcd for C22H30N4O4S (M+H)+ 447.2066, measured (M+H)+ 447.2046.
5.11. N1-Phenyl-3,5-diacetyl-N4,N4-di-n-butylsulfanilamide (14).
To a solution of diacid 12 (50 mg, 0.11 mmol) and triphenylphosphine (58 mg, 0.22 mmol) in dry THF (3 mL), hexachloroacetone (30 μL, 0.20 mmol) was added at 0 °C. The clear yellow reaction mixture was stirred at 0 °C for 1.5 h to give the corresponding diacyl chloride, which was used as such for next reaction. To a suspension of CuBr (157 mg, 1.11 mmol) in THF (2 mL), methyl magnesium bromide (370 μL, 3.0 M in THF, 1.11 mmol) was added at 0 °C and stirred at room temperature for 1 h, which gave a light yellow green suspension. The reaction was cooled once again to 0 °C, and the solution of the diacyl chloride derivative of 12 in THF (3 mL) was transferred by cannula. The resulting reaction mixture was stirred 0 °C for 40 min. Water (6 mL) was added, phases were separated and the aqueous phase was extracted with EtOAc (3 × 5 mL). The combined organic layers were washed with aq. NH4Cl (10 mL, 10% w/v), dried over anhydrous Na2SO4, and concentrated. Silica gel flash column chromatography of the crude material [hexane/EtOAc (8:1 to 4:1)] gave compound 14 (26.7 mg, 55%) as light yellow solid, mp = 99−100 °C. HPLC (MeOH/H2O 80/20, 1.0 mL/min, 254 nm), >98%, tR = 5.24 min.1H NMR (CDCl3) δ 0.85 (t, J = 7.2 Hz, 6H), 1.14−1.26 (m, 4H), 1.41−1.53 (m, 4H), 2.46 (s, 6H), 2.94 (t, J = 7.8 Hz, 4H), 6.50 (s, 1H), 7.07−7.28 (m, 5H), 7.72 (s, 2H); 13C NMR (CDCl3) δ 13.76, 20.11, 29.03, 29.58, 54.22, 122.17, 125.71, 129.36, 129.54, 130.30, 135.83, 136.34, 151.13, 200.95; HRMS (ESI) calcd for C24H32N2NaO4S (M+Na)+ 467.1980, measured (M+Na)+ 467.1984.
5.12. N1-Phenyl-3,5-dicyano-N4,N4-di-n-butylsulfanilamide (5).
The title compound was synthesized with slight modification of the literature procedure.24 To a solution of bis-amide 13 (65 mg, 0.146 mmol) in a mixture of acetonitrile and water (2:1 ratio, 15 mL), PdCl2 (12.5 mg, 0.073 mmol) was added. The resulting yellow solution was stirred at 70 °C for 24 h. Acetonitrile was removed in vacuo and the residue was extracted with CH2Cl2 (3 × 10 mL) and dried over anhydrous Na2SO4. Silica gel flash column chromatography [hexane/EtOAc (10:1)] of the crude material gave compound 5 (42.5 mg, 71%) as white solid, mp = 145−146 °C. 1H NMR (CDCl3) δ 0.91 (t, J = 7.2 Hz, 6H), 1.23−1.39 (m, 4H), 1.55−1.65 (m, 4H), 3.62 (m, 4H), 6.64 (s, 1H), 7.09−7.37 (m, 5H), 7.99 (m, 2H); 13C NMR (CDCl3) δ 13.64, 19.69, 30.09, 52.77, 107.56, 116.15, 122.15, 126.51, 129.76, 130.80, 135.33, 138.11, 158.89; HRMS (ESI) calcd for C22H26N4NaO2S (M+Na)+ 433.1674, measured (M+Na)+ 433.1666. Anal. (C22H26N4O2S) calcd C, 64.36; H, 6.38, N, 13.65; found C, 64.00; H, 6.40; N, 13.13.
5.13. Susceptibility Testing of Parasites and Vero Cells.
The susceptibility of Leishmania donovani axenic amastigote-like parasites,16, 37 Trypanosoma brucei brucei bloodstream forms,38 and Vero cells38 to growth inhibition by compounds of interest was assayed as described previously.
5.14. Tubulin Assembly Assays.
Tubulin from Leishmania tarentolae26 and from pig brain17 was isolated as outlined earlier. Assembly reactions, used to assess the effect of compounds on tubulin polymerization, were carried out as described previously17, 26 except that the final concentration of porcine brain tubulin was 2 mg/mL rather than 1.5 mg/mL in order to achieve better reproducibility in the assembly of control samples.
5.15. AutoDock.
AutoDock version 4.0.029 was used for docking simulations. The Lamarckian genetic algorithm (LGA) was selected for ligand conformational searching because it has enhanced performance relative to simulated annealing or the simple genetic algorithm. For the ligands, all hydrogens were added, Gasteiger charges39 were assigned, then non-polar hydrogens were merged. 80 × 80 × 70 3-D affinity grids centered on the 2b binding site with 0.375 Å spacing were calculated for each of the following atom types: a) protein: A (aromatic C), C, HD, N, NA, OA, SA; b) ligand: C, A, OA, HD, S, N, NA, e (electrostatic) and d (desolvation) using Autogrid4. The ligand's translation, rotation and internal torsions are defined as its state variables and each gene represents a state variable. LGA adds local minimization to the genetic algorithm, enabling modification of the gene population. The docking parameters were as follows: trials of 100 dockings, population size of 150, random starting position and conformation, translation step ranges of 2.0 Å, rotation step ranges of 50°, elitism of 1, mutation rate of 0.02, crossover rate of 0.8, local search rate of 0.06, and 50 million energy evaluations. Final docked conformations were clustered using a tolerance of 1.5 Å root-mean-square deviations (RMSD).
5.16. Glide.30
Ligand Preparation: The molecules were built in Maestro Suite and were minimized using the OPLS-2005 forcefield. A maximum of 32 stereoisomers were allowed to generate per ligand. Possible ionization states were assigned at a target pH of 7.0 ± 2.0. Target Preparation: The homology modeled structure of Leishmania tubulin19 was prepared for docking using the protein preparation utility provided by Schrodinger LLC. This process optimizes the protonation states and tautomers of His residues as well as the position of hydroxyl and thiol hydrogens. The structure was then subjected to Impact minimization with a cut off RMSD of 0.3Å. Partial atomic charges were assigned according to the OPLS-2005 force field. Molecular Docking: The model was submitted to grid map calculations using a box of 12Å cube centered on the bound ligand 2b. The consequent docking has been conducted using the Glide program, with the default settings using the previously prepared ligands. The most stable Glide pose, one per ligand, was retained.
Acknowledgements.
This work was funded by NIH Grant AI061021 (to K.A.W.). We would also like to thank Dr. David Sept for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data. 1H and 13C NMR spectra for 13 and 14.
References and notes
- 1.Chan M, Fong D. Inhibition of Leishmanias but not host macrophages by the antitubulin herbicide trifluralin. Science. 1990;249:924–926. doi: 10.1126/science.2392684. [DOI] [PubMed] [Google Scholar]
- 2.Chan M, Triemer RE, Fong D. Effect of the anti-microtubule drug oryzalin on growth and differentiation of the parasitic protozoan Leishmania mexicana. Differentiation. 1991;46:15–21. doi: 10.1111/j.1432-0436.1991.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan M, Grogl M, Chen C-C, Bienen EJ, Fong D. Herbicides to curb human infections: In vitro and in vivo effects of trifluralin on the trypanosomatid protozoans. Proc. Natl. Acad. Sci. USA. 1993;90:5657–5661. doi: 10.1073/pnas.90.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traub-Cseko Y, Ramalho-Ortigao J, Dantas A, de Castro S, Barbosa H, Downing K. Dinitroaniline herbicides against protozoan parasites: the case of Trypanosoma cruzi. Trends Parasitol. 2001;17:136–141. doi: 10.1016/s1471-4922(00)01834-1. [DOI] [PubMed] [Google Scholar]
- 5.Nath J, Schneider I. Anti-malarial effects of the anti-tubulin herbicide trifluralin: studies in Plasmodium falciparum. Clin. Res. 1992;40:331A. [Google Scholar]
- 6.Dow G, Armson A, Boddy M, Itenge T, McCarthy D, Parkin J, Thompson R, Reynoldson J. Plasmodium: assessment of the antimalarial potential of trifluralin and related compounds using a rat model of malaria, Rattus norvegicus. Exp. Parasitol. 2002;100:155–160. doi: 10.1016/s0014-4894(02)00016-4. [DOI] [PubMed] [Google Scholar]
- 7.Stokkermans T, Schwartzman J, Keenan K, Morrissette N, Tilney L, Roos D. Inhibition of Toxoplasma gondii replicaton by dinitroaniline herbicides. Exp. Parasitol. 1996;84:355–370. doi: 10.1006/expr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 8.Morrissette N, Murray J, Roos D. Subpellicular microtubules associate with an intramembranous particle lattice in the protozoan parasite Toxoplasma gondii. J. Cell Sci. 1997;110:35–42. doi: 10.1242/jcs.110.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Morrissette N, Mitra A, Sept D, Sibley L. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol. Biol. Cell. 2004;15:1960–1968. doi: 10.1091/mbc.E03-07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- 11.Renslo A, McKerrow J. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 12.Berman J. Structure-function analysis of antimicrotubule dinitroanilines against promastigotes of the parasitic protozoan Leishmania mexicana. Antimicrob. Agents Chemother. 1994;38:1692. doi: 10.1128/aac.38.7.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aβmann N, Emmrich M, Kampf G, Kaiser M. Genotoxic activity of important nitrobenzenes and nitroanilines in the Ames test and their structure-activity relationship. Mutat. Res. 1997;395:139–144. doi: 10.1016/s1383-5718(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 14.Poli P, de Mello M, Buschini A, Mortara R, de Albuquerque C, da Silva S, Rossi C, Zucchi T. Cytotoxic and genotoxic effects of megazol, an anti-Chagas' disease drug, assessed by different short-term tests. Biochem. Pharmacol. 2002;64:1617–1627. doi: 10.1016/s0006-2952(02)01390-4. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya G, Salem M, Werbovetz K. Antileishmanial dinitroaniline sulfonamides with activity against parasite tubulin. Bioorg. Med. Chem. Lett. 2002;12:2395–2398. doi: 10.1016/s0960-894x(02)00465-1. [DOI] [PubMed] [Google Scholar]
- 16.Werbovetz K, Sackett D, Delfin D, Bhattacharya G, Salem M, Obrzut T, Rattendi D, Bacchi C. Selective antimicrotubule activity of N1-phenyl-3,5-dinitro-N4,N4-di-n-propylsulfanilamide (GB-II-5) against kinetoplastid parasites. Mol. Pharm. 2003;64:1325–1333. doi: 10.1124/mol.64.6.1325. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya G, Herman J, Delfin D, Salem M, Barszcz T, Mollet M, Riccio G, Brun R, Werbovetz K. Synthesis and antitubulin activity of N1- and N4-substituted 3,5-dinitro sulfanilamides against African trypanosomes and Leishmania. J. Med. Chem. 2004;47:1823–1832. doi: 10.1021/jm0304461. [DOI] [PubMed] [Google Scholar]
- 18.George T, Johnsamuel J, Delfín D, Yakovich A, Mukherjee M, Phelps M, Dalton J, Sackett D, Kaiser M, Brun R, Werbovetz K. Antikinetoplastid antimitotic activity and metabolic stability of dinitroaniline sulfonamides and benzamides. Bioorg. Med. Chem. 2006;14:5699–5710. doi: 10.1016/j.bmc.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Mitra A, Sept D. Binding and interaction of dinitroanilines with apicomplexan and kinetoplastid α-tubulin. J. Med. Chem. 2006;49:5226–5231. doi: 10.1021/jm060472+. [DOI] [PubMed] [Google Scholar]
- 20.Thomasco L, Gadwood R, Weaver E, Ochoada J, Ford C, Zurenko G, Hamel J, Stapert D, Moerman J, Schaadt R, Yagi B. The synthesis and antibacterial activity of 1,3,4-thiadiazole phenyl oxazolidinone analogues. Bioorg. Med. Chem. Lett. 2003;13:4193–4196. doi: 10.1016/j.bmcl.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Padwa A, Krumpe K, Kassir J. Rhodium carbenoid mediated cyclizations of o-alkynyl-substituted α-diazoacetophenones. J. Org. Chem. 1992;57:4940–4948. [Google Scholar]
- 22.Beletskaya I, Cheprakov A. Copper in cross-coupling reactions - the post-Ullmann chemistry. Coord. Chem. Rev. 2004;248:2337–2364. [Google Scholar]
- 23.Villeneuve G, Chan T. A rapid, mild and acid-free procedure for the preparation of acyl chlorides including formyl chloride. Tet. Lett. 1997;38:6489–6492. [Google Scholar]
- 24.Maffioli S, Marzorati E, Marazzi A. Mild and reversible dehydration of primary amides with PdCl2 in aqueous acetonitrile. Org. Lett. 2005;7:5237–5239. doi: 10.1021/ol052100l. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Guo Y, Frey R, Ji Z, Curtin M, Ahmed A, Albert D, Arnold L, Arries S, Barlozzari T, Bauch J, Bouska J, Bousquet P, Cunha G, Glaser K, Guo J, Li J, Marcotte P, Marsh K, Moskey M, Pease L, Stewart K, Stoll V, Tapang P, Wishart N, Davidsen S, Michaelides M. Thienopyrimidine ureas as novel and potent multitargeted receptor tyrosine kinase inhibitors. J. Med. Chem. 2005;48:6066–6083. doi: 10.1021/jm050458h. [DOI] [PubMed] [Google Scholar]
- 26.Yakovich A, Ragone F, Alfonzo J, Sackett D, Werbovetz K. Leishmania tarentolae: Purification and characterization of tubulin and its suitability for antileishmanial drug screening. Exp. Parasitol. 2006;114:289–296. doi: 10.1016/j.exppara.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batra J, Powers L, Hess F, Hamel E. Derivatives of 5,6-diphenylpyridazin-3-one: Synthetic antimitotic agents which interact with plant and mammalian tubulin at a new drug-binding site. Cancer Res. 1986;46:1889–1893. [PubMed] [Google Scholar]
- 28.Bai R, Cichacz Z, Herald C, Pettit G, Hamel E. Spongistatin 1, a highly cytotoxic, sponge-derived, marine natural product that inhibits mitosis, microtubule assembly, and the binding of vinblastine to tubulin. Mol. Pharmacol. 1993;44:757–766. [PubMed] [Google Scholar]
- 29.Huey R, Morris G, Olson A, Goodsell D. A semiemperical free energy force field with charge-based desolvation. J. Comput. Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- 30.Friesner R, Banks J, Murphy R, Halgren T, Klicic J, Mainz D, Repasky M, Knoll E, Shelley M, Perry J, Shaw D, Francis P, Shenkin P. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 31.Patani G, LaVoie E. Bioisosterism: a rational approach in drug design. Chem. Rev. 1996;96:3147–3176. doi: 10.1021/cr950066q. [DOI] [PubMed] [Google Scholar]
- 32.Silverman R. The organic chemistry of drug design and drug action. Second Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- 33.Windmill K, McKinnon R, Zhu X, Gaedigk A, Grant D, McManus M. The role of xenobiotic metabolizing enzymes in arylamine toxicity and carcinogenesis: Functional and localization studies. Mutat. Res. 1997;376:153–160. doi: 10.1016/s0027-5107(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 34.Bondi A. van der Waals volumes and radii. J. Phys. Chem. 1964;68:441–451. [Google Scholar]
- 35.Wu D, George T, Hurh E, Werbovetz K, Dalton J. Pre-systemic metabolism prevents in vivo antikinetoplastid activity of N1, N4-substituted 3,5-dinitro sulfanilamide, GB-II-150. Life Sci. 2006;79:1081–1093. doi: 10.1016/j.lfs.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Coulson E. Tar hydrocarbons. I. Reduction products of pyrene. J. Chem. Soc. 1937:1298–1305. [Google Scholar]
- 37.Werbovetz K, Brendle J, Sackett D. Purification, characterization, and drug susceptibility of tubulin from Leishmania. Mol. Biochem. Parasitol. 1999;98:53–65. doi: 10.1016/s0166-6851(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 38.Salem M, Werbovetz K. Antiprotozoal compounds from Psorothamnus polydenius. J. Nat. Prod. 2005;68:108–111. doi: 10.1021/np049682k. [DOI] [PubMed] [Google Scholar]
- 39.Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity - a rapid access to atomic charges. Tetrahedron. 1980;36:3219–3228. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data. 1H and 13C NMR spectra for 13 and 14.