Abstract
Background
Low intakes of dietary antioxidants may contribute to increases in asthma and allergy.
Objective
We investigated the association of maternal total intakes (foods + supplements) of 10 antioxidant nutrients during pregnancy with wheezing and eczema in 2-y-old children.
Design
Subjects were 1290 mother-child pairs in an ongoing cohort study. Maternal dietary and supplement intakes were assessed by using a validated food-frequency questionnaire administered in the first and second trimesters. Antioxidant nutrient intakes were calculated, and the mean for each nutrient was considered to be the exposure during pregnancy. The outcomes of interest were any wheezing by the child during either the first or second year of life, recurrent wheezing in both years, and eczema in either the first or second year.
Results
No association was observed between maternal total intake of any antioxidant nutrient and eczema. In multivariate logistic regression models, the highest quartile compared with the lowest quartile of maternal total intakes of vitamin E [odds ratio (OR): 0.70; 95% CI: 0.48, 1.03] and zinc (OR: 0.59; 95% CI: 0.41, 0.88) was inversely associated with any wheezing at 2 y of age (P for trend = 0.06 and 0.01 over quartiles of intake for vitamin E and zinc, respectively). Similar results were obtained for recurrent wheezing at 2 y of age with vitamin E (OR: 0.49; 95% CI: 0.27, 0.90) and zinc (OR: 0.49; 95% CI: 0.27, 0.87) (P for trend = 0.05 and 0.06 over quartiles of intake for vitamin E and zinc, respectively).
Conclusion
Our results suggest that higher maternal total intakes of antioxidants during pregnancy may decrease the risks for wheezing illnesses in early childhood.
Keywords: Asthma, diet, antioxidants, eczema, childhood wheezing
INTRODUCTION
Asthma and allergies are important public health problems in industrialized countries. Asthma affects >14 million persons (1) and is the most common chronic disease of childhood in the United States (2). Although the increases in asthma prevalence during past decades may have stabilized in some countries (3–5), recent data from the United States (1, 6) and other countries (7, 8) suggest that the prevalence continues to rise. In addition, striking increases in the prevalence of asthma and allergies are documented between affluent and nonaffluent countries worldwide (9).
No clear reasons are available for the increase in prevalence of asthma and allergies in developed countries, but it is likely that a changing environment and the behaviors associated with a “Westernized” lifestyle contribute to the problem. For example, fewer infections in early childhood may predispose a child to the development of asthma and allergies (10). Other factors implicated in the development of these conditions include family history (11), obesity (12), and exposure to allergens (13), all of which may increase the risk of asthma and allergies, although exposure to endotoxins (14), farm animals (15), and pets (16) may decrease the risk. In addition to these exposures, it was suggested that changes in diet associated with a Western lifestyle—in particular, diets that are deficient in antioxidants—may explain some of these trends (17, 18). It is postulated that lower dietary intakes of antioxidants lead to lesser antioxidant defenses in the lung and greater susceptibility to airway inflammation and asthma (18).
Most published studies that examine associations of antioxidant intake with asthma and asthma-related phenotypes (19) were conducted in adult populations and in populations with established disease. Because most cases of asthma are diagnosed before the age of 6 y (20), it is important to examine exposures in the prenatal (21) and early childhood (22) periods to understand the inception of disease. Maternal diet during pregnancy is a potentially modifiable exposure that has already been shown to influence birth outcomes (23), but it has not been adequately studied with respect to the development of asthma and allergies. Thus, the aim of the current study was to investigate, in a large cohort study with detailed maternal dietary information, the association between maternal intakes of antioxidants during pregnancy with wheezing and eczema in the children in the first 2 y of life.
SUBJECTS AND METHODS
Population and study sample
Study subjects were enrolled from April 22, 1999, to July 31, 2002, in Project Viva, a prospective, observational cohort study of gestational diet, pregnancy outcomes, and offspring health. Recruitment occurred at 8 obstetric offices of Harvard Vanguard Medical Associates, a multispecialty, managed-care group practice in the Boston, MA, area. Recruitment and retention procedures were previously reported (24). The flow of participants through the screening, enrollment, and analysis phases of the current study is shown in Figure 1. Women with singleton pregnancies were eligible for the study if they entered prenatal care within the first 22 wk of gestation, intended to continue their obstetric care at Harvard Vanguard Medical Associates, and were able to answer questionnaires in English. Exclusion criteria included multiple gestation, inability to answer questions in English, plans to move out of the area before delivery, and >22 wk gestation at the initial prenatal clinic appointment.
FIGURE 1.
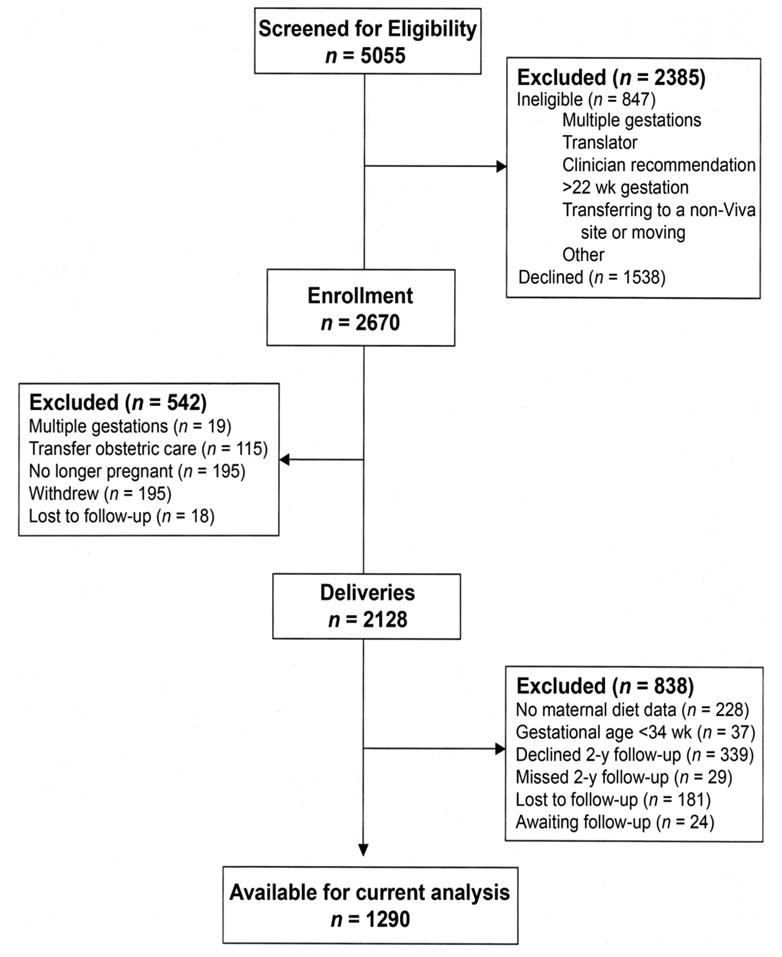
Project Viva participant flow sheet.
All participants gave written informed consent. Study protocols were approved by the Human Subjects Committees of Harvard Pilgrim Health Care, Brigham and Women’s Hospital, and Beth Israel Deaconess Medical Center.
We obtained information directly from participants and from medical records as detailed previously (24). A trained research assistant met each participant after her initial clinical prenatal visit, at an average of ≈10 wk of gestation, and again at 26–28 wk of gestation. At each visit, an assistant interviewed the participant and gave her a series of questionnaires to complete and mail to the study office. Within 72 h after delivery, an assistant interviewed the mother at the hospital and performed anthropometric measurements on the newborn. Data collected from interviews and questionnaires included demographics (neonate sex, maternal marital status, and race), socioeconomic status (education and household income), and reproductive history (maternal gravidity, prepregnancy height and body mass index, and smoking status). Questionnaires that inquired about infant health, feeding, and the home environment were administered when the child was 6 mo, 1 y, and 2 y of age. Of the 2128 delivered neonates in Project Viva, we excluded 228 participants because of missing first- and second-trimester diet assessment data and an additional 37 participants with a gestation of <34 completed weeks. Mothers of 368 of the remaining 1863 children either did not provide informed consent for child follow-up through age 2 y or missed the 2-y follow-up questionnaire appointment. Of the remaining 1495 children, 181 were lost to follow-up, and 24 have not yet exited the 2-y time window, which left a sample for analysis of 1290 participants.
Dietary assessment
We assessed maternal diet by using semiquantitative food-frequency questionnaires (FFQs) validated for use during pregnancy (25) and used in several of our previous studies of maternal diet and offspring outcomes (26–28). The FFQ used at the first visit reflected intakes in the first trimester; the time referent was “during this pregnancy”—ie, from the date of the last menstrual period until the assessment, at an average of ≈10 wk of gestation. To assess vitamin and supplement intakes during the first trimester, we administered a separate interview that queried dose, duration, and brand or type of multivitamin, prescribed prenatal vitamins, and supplements use by the mother. The FFQ used at the second visit (26–28 wk of gestation) reflected intakes during the second trimester; the time referent was “during the past 3 mo.” The second trimester instrument was the same as that for the first trimester except that we assessed the use of vitamins or supplements as part of the subject-completed FFQ. To calculate intake of nutrients, we used the Harvard nutrient-composition database, which contains food composition values from the US Department of Agriculture, supplemented by other data sources (29). All nutrients were adjusted for total energy intake by using the nutrient residuals method (30). Mean nutrient intakes from the first and second trimesters were used as the exposure in the analyses. If a participant completed only 1 FFQ, then nutrients calculated from that questionnaire were taken as the exposure of interest. Of the 1290 participants, 1135 (88%) completed both FFQs, 96 (7.4%) completed the first-trimester FFQ only, and 59 (4.6%) completed the second-trimester FFQ only.
Definition of 2-y outcome variables
We ascertained the wheezing outcomes from questions from the first- and second-year questionnaires: “Since your child was born/was 12 mo old, has he/she ever had wheezing (or whistling in the chest)?” Any wheeze was defined as a positive response to the question at either the first- or second-year questionnaire. Recurrent wheeze was defined as positive responses to the questions in both the first- and second-year questionnaires. If the child had a positive response to either question but a negative response on the other questionnaire, the child was excluded from the analysis for recurrent wheezing, which made the comparison group for both wheezing outcomes the children who never wheezed in the first 2 y of life. Respiratory infections were defined as a positive response to any of the 3 parts of a question on either the first- or second-year questionnaire: “Since your child was born/was 12 mo old, have you been told by a health care professional (such as a doctor, physician assistant, or nurse practitioner) that your child has (a) bronchiolitis; (b) pneumonia; (c) bronchitis, croup, or any other respiratory infection?” Eczema was defined as a positive response to the question on either the first- or second-year questionnaire: “Have you ever been told by a health care professional (such as a doctor, physician assistant, or nurse practitioner) that your child has eczema?”
Demographic, birth, parental conditions, and other variables
Parental demographic information and health history information were collected by interview and self-administered questionnaires (24). Newborn birth weight data were obtained from hospital records. Multivitamin intake in the first year was determined by the questions on the 6-mo and 1-y questionnaires. At 6 mo, mothers were asked, “Since your baby was born, has your baby taken any multivitamin drops such as Tri-Vit (Tri-Vi-Sol) or Poly-Vi-Sol? Other vitamins or supplements?” At 1 y, mothers were asked, “In the past month, has your child taken any of the following vitamins or supplements? Multivitamin drops such as Tri-Vit (Tri-Vi-Sol) or Poly-Vi-Sol (Yes/No); Other vitamins or supplements?” Duration of breastfeeding was ascertained from detailed questions on the 1-y questionnaire.
Statistical analysis
We examined the associations of each nutrient with each outcome by using bivariate logistic regression models. Nutrients that had significant associations with either wheezing or eczema outcome in the bivariate logistic regression models were then taken forward to multivariate models. To assess the multivariate association between maternal antioxidant intake and the 2-y outcomes, we first performed bivariate analyses to determine the nonnutrient characteristics associated with these outcomes by using logistic regression. Variables that were significantly (P < 0.05) associated with any of the 3 outcomes were included in the respective multivariate model. For the wheezing and the respiratory infections, these variables included birth weight, neonate sex, maternal age, maternal prepregnancy body mass index, breastfeeding duration, the number of children <12 y old in the home, postnatal passive smoke exposure, family income, and maternal and paternal asthma. For eczema, maternal and paternal asthma were replaced with maternal and paternal eczema. In addition, models for the individual nutrients and individual nonnutrient variables were created to test for any potential confounders of the relation between nutrient and outcome. We defined a confounder as a variable that causes >8% change in the estimate when entered into the model. None of the nonnutrient variables caused a change of >8% in the estimate for the antioxidant nutrient in these models; thus, no variable other than those listed earlier was included in the multivariate analyses. Additional multivariate models were created to adjust for maternal intakes of fruit and vegetables (because these are main sources of antioxidants in the diet) and the infant’s intake of multivitamins in the first year (because this is a potential confounder of the maternal diet–infant outcome relation).
To determine the most parsimonious models, variables that were not significant in the full multivariate models were removed, and the subsequent models were compared with the full models by using likelihood ratio tests. Statistical analyses were performed with the use of SAS statistical software (version 8.2; SAS Institute Inc, Cary, NC).
RESULTS
The characteristics of the participants are presented in Table 1. Three hundred seventy-six (32.4%) children had any wheezing in the first 2 y of life, whereas 136 (13.1%) children had recurrent wheezing. Four hundred ten (31.9%) children had eczema. Fifty-one percent of the children were boys, and 75.6% were white. The mean age of the mothers on entry into the cohort was 32.6 y, 73.2% of the mothers had at least a college education, 62.6% of the mothers lived in households with an annual income of >$70 000, and only 9.5% of the mothers smoked during pregnancy. Of the mothers, 16.4% had asthma and 19.6% had eczema, whereas, of the fathers, 14.5% had asthma and 14.3% had eczema. A comparison of subjects in the analysis and those who were excluded is also presented in Table 1. Because we excluded premature neonates from this current analysis, subjects in the cohort had higher birth weights than did excluded subjects. Furthermore, higher proportions of minority ethnic groups, younger mothers, subjects with shorter duration of breastfeeding, subjects with a low family income, subjects with low educational attainment, and smoking mothers were found in the excluded subjects. No differences in the proportions of maternal asthma and paternal asthma between the 2 groups were observed. For parental eczema, the proportion of maternal and paternal eczema was lower in the group that was excluded from this analysis.
TABLE 1.
Participant characteristics
| Characteristics | In analysis n = 1290 | Excluded subjects1n = 838 | P |
|---|---|---|---|
| Child’s sex | |||
| Male [n (%)] | 658 (51.0) | 438 (52.3) | 0.57 |
| Female [n (%)] | 632 (49.0) | 400 (47.7) | |
| Birthweight (kg) | 3.53 ± 0.512 | 3.35 ± 0.68 | < 0.0001 |
| Race-ethnicity | |||
| White [n (%)] | 975 (75.6) | 424 (52.0) | |
| Black [n (%)] | 134 (10.4) | 214 (26.3) | |
| Hispanic [n (%)] | 69 (5.4) | 85 (10.4) | < 0.0001 |
| Asian [n (%)] | 63 (4.9) | 57 (7.0) | |
| Other [n (%)] | 48 (3.7) | 35 (4.3) | |
| Number of other children in the home younger than 12 y | |||
| >1 [n (%)] | 675 (53.9) | 256 (50.8) | 0.24 |
| Breastfeeding duration | |||
| None [n (%)] | 139 (11.6) | 84 (23.1) | |
| >0 to <4 mo [n (%)] | 269 (22.5) | 152 (41.9) | < 0.0001 |
| 4 to <10 mo [n (%)] | 387 (32.3) | 90 (24.8) | |
| >10 mo [n (%)] | 402 (33.6) | 37 (10.2) | |
| Multivitamin intake in the first year | |||
| Yes [n (%)] | 239 (19.1) | 60 (11.9) | 0.0003 |
| Eczema in the first 2 y of life3 | |||
| Yes [n (%)] | 410 (31.9) | —4 | |
| No [n (%)] | 875 (68.1) | ||
| Any wheezing at 2 y5 | |||
| Yes [n (%)] | 376 (32.4) | —3 | |
| No [n (%)] | 785 (67.6) | ||
| Recurrent wheezing at 2 y4 | |||
| Yes [n (%)] | 136 (13.1) | —3 | |
| No [n (%)] | 902 (86.9) | ||
| Lower respiratory tract infection at 2 y5 | |||
| Yes [n (%)] | 327 (25.7) | —3 | |
| No [n (%)] | 944 (74.3) | ||
| Maternal age (y) | 32.6 ± 4.8 | 30.6 ± 5.6 | < 0.0001 |
| Maternal prepregnancy BMI (kg/m2) | 24.5 ± 5.1 | 25.5 ± 6.2 | < 0.0001 |
| Highest educational level | |||
| High school or some college [n (%)] | 346 (26.8) | 398 (48.8) | |
| College graduate [n (%)] | 491 (38.1) | 252 (30.9) | < 0.0001 |
| Postgraduate degree [n (%)] | 452 (35.1) | 165 (20.3) | |
| Maternal smoking during pregnancy [n (%)] | 122 (9.5) | 133 (15.9) | < 0.0001 |
| Passive smoke exposure in the home (h/w) | 0.26 ± 1.31 | 0.20 ± 1.02 | 0.32 |
| Household income | |||
| ≤$40 000 [n (%)] | 139 (10.8) | 153 (18.3) | |
| >$40 000 to $70 000 [n (%)] | 278 (21.6) | 156 (18.6) | < 0.0001 |
| >$70 000 [n (%)] | 807 (62.6) | 337 (40.2) | |
| Missing | 66 (5.1) | 192 (22.9) | |
| Maternal asthma | |||
| Yes [n (%)] | 211 (16.4) | 137 (16.4) | |
| No [n (%)] | 1079 (83.6) | 701 (83.7) | 0.996 |
| Maternal eczema | |||
| Yes [n (%)] | 253 (19.6) | 116 (13.8) | 0.0006 |
| No [n (%)] | 1037 (80.4) | 722 (86.2) | |
| Paternal asthma | |||
| Yes [n (%)] | 187 (14.5) | 102 (12.2) | 0.13 |
| No [n (%)] | 1103 (85.5) | 736 (87.8) | |
| Paternal eczema | |||
| Yes [n (%)] | 184 (14.3) | 50 (6.0) | < 0.0001 |
| No [n (%)] | 1106 (85.7) | 788 (94.0) |
Numbers do not always add up to 838 because of varied numbers of missing data for each variable.
x̄ ± SD (all such values).
There were not enough numbers with information to make valid comparisons. Most had missing information for these variables.
For the recurrent wheezing variable, children who wheezed in either the first year only or the second year only were excluded from the analyses.
Numbers do not add up to 1290 because of missing data.
The distributions of maternal intakes of nutrients are presented in Table 2. For most of the nutrients, a wide distribution of intake was observed, particularly when supplements were included. Most of the women met the recommended minimum daily requirements for folate (400 ìg/d; 31, 32), zinc (15 mg; 33), vitamin C (85 mg/d; 34), and vitamin E (15 mg/d; 34), when total intakes (foods + supplements) were considered. However, if antioxidant intakes from foods only were considered, >50% of the women would not have met the recommended minimum daily requirements for both folate and zinc.
TABLE 2.
Maternal nutrient intakes during pregnancy1
| Nutrient2 | x̄ ± SD | Minimum | 25th Percentile | Median | 75th Percentile | Maximum |
|---|---|---|---|---|---|---|
| Vitamin C (mg/d) | 279.6 ± 192.1 | 47.3 | 207.0 | 254.3 | 313.3 | 3925.6 |
| Vitamin C without supplementation (mg/d) | 174.3 ± 64.8 | 35.0 | 129.1 | 167.3 | 210.8 | 617.5 |
| Vitamin E (mg/d) | 30.7 ± 64.6 | 3.6 | 14.3 | 18.8 | 26.5 | 1202.2 |
| Vitamin E without supplementation (mg/d) | 8.4 ± 6.5 | 2.9 | 5.5 | 6.4 | 8.1 | 67.9 |
| α-Carotene (μg/d) | 878.0 ± 657.7 | 3.9 | 425.8 | 732.0 | 1136.7 | 5221.7 |
| β-Carotene (μg/d) | 4770.3 ± 2293.0 | 323.3 | 3279.7 | 4403.3 | 5875.5 | 36744.5 |
| β-Carotene without supplementation (μg/d) | 3863.5 ± 2036.5 | 325.0 | 2433.3 | 3496.3 | 4925.5 | 16,553.3 |
| β-Cryptoxanthin (μg/d) | 207.3 ± 130.9 | 0.5 | 112.6 | 181.5 | 272.9 | 1207.2 |
| Folate (μg/d) | 1117.6 ± 422.3 | 148.8 | 917.3 | 1157.5 | 1327.4 | 10220.1 |
| Folate without supplementation (μg/d) | 368.4 ± 112.9 | 146.7 | 289.7 | 352.9 | 425.6 | 1196.9 |
| Lutein + zeaxanthin (μg/d) | 2686.8 ± 1724.4 | 133.0 | 1570.5 | 2286.3 | 3317.8 | 14069.6 |
| Lycopene (μg/d) | 7368.8 ± 3979.7 | 489.3 | 4730.2 | 6649.8 | 9273.6 | 33889.0 |
| Copper (mg/d) | 1.6 ± 0.5 | 0.8 | 1.3 | 1.5 | 1.7 | 8.8 |
| Copper without supplementation (mg/d) | 1.4 ± 0.3 | 0.8 | 1.3 | 1.4 | 1.6 | 2.7 |
| Zinc (mg/d) | 30.3 ± 9.3 | 6.8 | 25.2 | 31.5 | 36.0 | 92.0 |
| Zinc without supplementation (mg/d) | 12.4 ± 3.3 | 6.4 | 10.6 | 11.8 | 13.3 | 40.2 |
Nutrients were calculated from food-frequency questionnaires as described in the Methods section. For each participant, the means of the first- and second-trimester nutrients were obtained.
Unless specifically stated, values are for total nutrient intakes (food + supplements).
We did not find any associations between any of the nutrients and eczema in the children at 2 y of age. Neither maternal intakes of fruit and vegetables nor infant multivitamin intake was independently associated with any of the outcomes. However, total intakes of vitamin C, vitamin E, zinc, folic acid, lutein + zeaxanthin, β-carotene, and copper were associated with both wheezing outcomes. The results of the analyses of the wheeze outcomes at 2 y of age are presented in Table 3. For any wheezing, total intakes of vitamin C, vitamin E, zinc, folic acid, lutein + zeaxanthin, β-carotene, and copper were associated with lower risks in univariate models. In a multivariate model adjusted for potential confounders, maternal intakes of zinc in the highest quartile remained protective for any wheezing [odds ratio (OR): 0.59; 95% CI: 0.41, 0.88; P = 0.01], and a trend was observed toward a protective effect for maternal intakes of vitamin E in the highest quartile (OR: 0.70; 95% CI: 0.48, 1.03; P = 0.06). Additional adjustment for multivitamin intake in the first year of life did not change these associations. For recurrent wheezing, similar findings were noted in univariate models. In multivariate models, maternal intakes in the highest quartile of vitamin E (OR: 0.49; 95% CI: 0.27, 0.90) and zinc (OR: 0.49; 95% CI: 0.27, 0.87) remained inversely associated with recurrent wheezing (all P < 0.05 when compared with lowest quartile of intake). Because most of the antioxidants in the diet come from fruit and vegetables, we ran additional models that adjusted for maternal intakes of fruit and vegetable as a way of adjusting for other antioxidants. The results of these additional models did not differ significantly from the models presented in Table 3. Similar results were also obtained when the analysis was stratified by maternal asthma.
TABLE 3.
Associations between maternal antioxidant intakes and child outcomes at 2 y of age1
| Any wheezing in the first 2 y (n = 1161)
|
Recurrent wheezing in the first 2 y (n = 1038)2 |
|||
|---|---|---|---|---|
| Nutrients | Univariate model | Multivariate model3 | Univariate model | Multivariate model4 |
| Vitamin C | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.82 (0.58, 1.15)5 | 0.93 (0.65, 1.35) | 1.02 (0.63, 1.65) | 1.05 (0.62, 1.77) |
| 3 | 0.65 (0.46, 0.93)6 | 0.84 (0.58, 1.22) | 0.85 (0.52, 1.40) | 1.04 (0.60, 1.78) |
| 4 | 0.67 (0.47, 0.94)6 | 0.79 (0.54, 1.15) | 0.56 (0.32, 0.97)6 | 0.75 (0.41, 1.36) |
| P for trend7 | 0.01 | 0.2 | 0.03 | 0.4 |
| Vitamin E | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.71 (0.51, 1.01) | 0.90 (0.62, 1.31) | 0.60 (0.37, 0.98) | 0.79 (0.47, 1.34) |
| 3 | 0.67 (0.48, 0.95)6 | 0.84 (0.58, 1.22) | 0.68 (0.43, 1.10) | 0.90 (0.54, 1.52) |
| 4 | 0.52 (0.36, 0.74)6 | 0.70 (0.48, 1.03) | 0.36 (0.20, 0.62)6 | 0.49 (0.27, 0.90)6 |
| P for trend7 | 0.0004 | 0.06 | 0.0007 | 0.05 |
| Zinc | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.77 (0.55, 1.08) | 0.89 (0.61, 1.29) | 0.56 (0.34, 0.92) | 0.61 (0.35, 1.06) |
| 3 | 0.67 (0.47, 0.94)6 | 0.85 (0.59, 1.24) | 0.73 (0.46, 1.17) | 0.89 (0.53, 1.49) |
| 4 | 0.49 (0.34, 0.70)6 | 0.59 (0.41, 0.88)6 | 0.41 (0.24, 0.69)6 | 0.49 (0.27, 0.87) |
| P for trend7 | < .0001 | 0.01 | 0.004 | 0.06 |
| Lutein + zeaxanthin | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.00 (0.72, 1.41) | 1.20 (0.83, 1.73) | 1.02 (0.63, 1.67) | 1.35 (0.79, 2.32) |
| 3 | 0.85 (0.60, 1.20) | 1.12 (0.77, 1.63) | 0.96 (0.59, 1.56) | 1.59 (0.92, 2.74) |
| 4 | 0.65 (0.45, 0.93)6 | 0.84 (0.56, 1.25) | 0.52 (0.30, 0.90)6 | 0.91 (0.49, 1.67) |
| P for trend7 | 0.01 | 0.4 | 0.03 | 0.9 |
| Folic acid | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.94 (0.67, 1.32) | 1.13 (0.78, 1.64) | 0.77 (0.48, 1.24) | 0.93 (0.55, 1.58) |
| 3 | 0.63 (0.44, 0.89)6 | 0.79 (0.54, 1.16) | 0.58 (0.35, 0.95)6 | 0.75 (0.43, 1.28) |
| 4 | 0.64 (0.45, 0.91)6 | 0.89 (0.60, 1.31) | 0.46 (0.27, 0.78)6 | 0.68 (0.38, 1.22) |
| P for trend7 | 0.002 | 0.2 | 0.002 | 0.1 |
| α-Carotene | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.77 (0.54, 1.09) | 0.87 (0.60, 1.26) | 0.98 (0.60, 1.60) | 1.22 (0.71, 2.09) |
| 3 | 0.82 (0.58, 1.16) | 1.00 (0.69, 1.45) | 0.74 (0.43, 1.25) | 1.01 (0.57, 1.80) |
| 4 | 0.75 (0.53, 1.06) | 0.92 (0.63, 1.34) | 0.88 (0.53, 1.45) | 1.26 (0.72, 2.21) |
| P for trend7 | 0.2 | 0.8 | 0.4 | 0.6 |
| β-Carotene | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.01 (0.71, 1.41) | 1.28 (0.88, 1.88) | 0.85 (0.52, 1.37) | 1.24 (0.72, 2.13) |
| 3 | 0.85 (0.60, 1.20) | 1.21 (0.82, 1.77) | 0.76 (0.46, 1.24) | 1.23 (0.72, 2.13) |
| 4 | 0.71 (0.50, 1.02) | 0.98 (0.66, 1.47) | 0.58 (0.34, 0.97)6 | 1.01 (0.56, 1.81) |
| P for trend7 | 0.04 | 0.9 | 0.04 | 0.9 |
| β-Cryptoxanthin | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.80 (0.57, 1.13) | 0.82 (0.57, 1.18) | 1.36 (0.82, 2.24) | 1.33 (0.78, 2.28) |
| 3 | 0.72 (0.51, 1.02) | 0.81 (0.56, 1.18) | 0.95 (0.56, 1.63) | 0.97 (0.55, 1.72) |
| 4 | 0.92 (0.66, 1.30) | 0.97 (0.67, 1.41) | 1.05 (0.62, 1.78) | 1.28 (0.73, 2.26) |
| P for trend7 | 0.5 | 0.9 | 0.7 | 0.7 |
| Lycopene | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.27 (0.90, 1.80) | 1.50 (1.02, 2.19) | 0.88 (0.53, 1.46) | 1.06 (0.62, 1.83) |
| 3 | 1.26 (0.90, 1.79) | 1.67 (1.14, 2.45) | 1.01 (0.62, 1.64) | 1.40 (0.82, 2.40) |
| 4 | 0.83 (0.58, 1.19) | 0.96 (0.64, 1.42) | 0.70 (0.42, 1.17) | 0.75 (0.42, 1.33) |
| P for trend7 | 0.3 | 0.9 | 0.3 | 0.6 |
| Copper | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.78 (0.55, 1.10) | 0.93 (0.64, 1.35) | 0.63 (0.38, 1.03) | 0.75 (0.43, 1.30) |
| 3 | 0.71 (0.50, 1.01) | 0.91 (0.62, 1.34) | 0.67 (0.41, 1.09) | 1.02 (0.60, 1.75) |
| 4 | 0.69 (0.49, 0.99)6 | 0.90 (0.61, 1.33) | 0.57 (0.34, 0.95)6 | 0.90 (0.51, 1.59) |
| P for trend7 | 0.03 | 0.6 | 0.04 | 1.0 |
Calorie-adjusted nutrients, including supplements, are in quartiles of intake, with the first quartile being the lowest and the fourth quartile being the highest.
One hundred twenty-three children who were reported to wheeze on either the first- or second-year questionnaire only were excluded from the recurrent wheezing analyses.
Adjusted for sex, maternal age, maternal asthma, paternal asthma, family income, passive smoke exposure, breastfeeding, and other children <12 y old in the home.
Adjusted for body weight, sex, maternal age, maternal prepregnancy BMI, maternal asthma, paternal asthma, family income, passive smoke exposure, breastfeeding, and other children <12 y old in the home.
Odds ratio; 95% CI in parentheses (all such values).
P < 0.05 for the comparison with the lowest quartile of intake.
P for trend obtained from trend test (Wald) in logistic regression models.
Maternal intakes of lutein + zeaxanthin in the highest quartile compared with the lowest quartile were inversely associated with the presence of respiratory infections in the 2-y-old children (OR: 0.56; 95% CI: 0.37, 0.85; P = 0.01 for trend across quartiles) in a multivariate model that adjusted for potential confounders. Total maternal intakes of all the other antioxidants—in particular, vitamin C, vitamin E, and zinc—were not associated with respiratory infections in the children.
Because antioxidants come from both foods and supplements (mostly in the form of multivitamin preparations), we examined separately the effects of vitamin E and zinc from foods and those from from supplements (Table 4). Vitamin E intakes from both foods and supplements were inversely associated with both wheezing outcomes, and the contribution of supplements appeared to be more strongly associated with the outcomes. Zinc intakes from both foods only and supplements only were inversely associated with any wheezing with similar effect estimates. Higher zinc intake from supplements only was also inversely associated with recurrent wheezing. The separate effects of antioxidants from foods only and from supplements only did not remain statistically significant in multivariate models.
TABLE 4.
Associations of nutrients from foods only and from supplements only with wheezing outcomes at 2 y of age1
| Any wheezing (n = 1161)
|
Recurrent wheezing (n = 1038)
|
|||
|---|---|---|---|---|
| Nutrients | Foods only | Supplements only | Foods only | Supplements only |
| Vitamin E | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.75 (0.53, 1.06)2 | 0.86 (0.61, 1.21) | 0.66 (0.40, 1.07) | 0.83 (0.52, 1.33) |
| 3 | 0.67 (0.47, 0.95)3 | 0.70 (0.50, 0.99)3 | 0.58 (0.35, 0.95)3 | 0.50 (0.30, 0.84)3 |
| 4 | 0.69 (0.49, 0.98)3 | 0.63 (0.45, 0.90)3 | 0.61 (0.37, 1.01) | 0.56 (0.34, 0.93)3 |
| P for trend4 | 0.03 | 0.006 | 0.04 | 0.006 |
| Zinc | ||||
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 0.85 (0.60, 1.19) | 0.98 (0.70, 1.37) | 0.70 (0.43, 1.17) | 0.94 (0.58, 1.50) |
| 3 | 0.60 (0.42, 0.85)3 | 0.67 (0.47, 0.95)3 | 0.65 (0.40, 1.07) | 0.59 (0.35, 0.98)3 |
| 4 | 0.60 (0.42, 0.85)3 | 0.63 (0.45, 0.90)3 | 0.68 (0.42, 1.12) | 0.51 (0.30, 0.87)3 |
| P for trend4 | 0.0008 | 0.002 | 0.1 | 0.004 |
Nutrients are in quartiles of intake, with the first quartile being the lowest and the fourth quartile being the highest.
Odds ratio; 95% CI from univariate logistic regression models in parentheses (all such values).
P < 0.05 for the comparison with the lowest quartile of intake from logistic regression models.
P for trend obtained from trend test (Wald) in logistic regression models.
DISCUSSION
We found that higher maternal intakes of the antioxidants vitamin E and zinc during pregnancy were associated with lower risks of wheezing illnesses in 2-y-old children. These effects were independent of several potential confounders, including maternal asthma status, breastfeeding, and infant multivitamin intake in the first year. Antioxidant nutrients from both foods and supplements contributed to these effects. We did not find any association of maternal antioxidant intake with childhood eczema.
As is true for many longitudinal cohorts, participants from racial minorities and socioeconomically disadvantaged groups have a higher rate of dropout and missing data than do white participants, who are more likely to come from the middle or high socioeconomic class. However, it is not likely that these discrepancies introduced significant bias into our results, because groups that are socioeconomically disadvantaged have lower intakes of antioxidants in their diets (35, 36) and are more likely to have children who wheeze and who develop asthma than are groups with higher socioeconomic status (37).
Many studies have investigated the effects of dietary antioxidants on asthma and asthma-related phenotypes, as reviewed by McKeever and Britton (19). However, most of these studies were conducted in adult populations. Few studies in children have shown a beneficial effect of intakes of either antioxidant vitamins or fruit and vegetables on lung function (38, 39) and wheezing symptoms (38, 40–42). Two previous studies have investigated the effects of prenatal exposure to antioxidants and wheezing in childhood. Martindale et al (43) examined maternal intakes and serum concentrations of antioxidants in a cohort of 1374 mother-infant pairs. They found that higher intakes of vitamin E were associated with lower risks of wheezing by age 2 y. Our findings validate their results for vitamin E intake in pregnancy and also find a protective effect of zinc. Although Martindale et al (43) also found a protective effect of vitamin E against eczema among atopic mothers, we did not find any effects of any antioxidant on eczema, even when stratifying for maternal eczema. The reason for the differences in findings between the current study and that of Martindale et al (43) is unclear. A study by Shaheen et al (44) showed that higher cord blood concentrations of selenium and iron were inversely associated with wheezing in young children. However, the effects were not strong, and differences in measurement of the trace elements in the earlier specimens and the later specimens in that study may have dampened the results.
The mechanisms by which maternal intakes of vitamins E and zinc may reduce the risk of wheezing in young children are presumably the effects of those intakes on the developing immune system (45). Cord blood mononuclear cells from neonates born to mothers with high dietary intakes of vitamin E had lower proliferative responses to antigen stimulation than did cells from neonates born to mothers with lower intakes of vitamin E during pregnancy (46). Vitamin E (47) suppresses interleukin 4 gene expression and protein concentrations in peripheral blood T cells. Subsequent to treatment with both vitamins C and E, human dendritic cells became resistant to phenotypic and functional changes after stimulation with proinflammatory cytokines (48). Zinc is an essential component of DNA-binding proteins with zinc fingers, as are copper and zinc superoxide dismutase and several proteins involved in DNA repair. Thus, zinc plays an important role in transcription factor function, antioxidant defense, and DNA repair (49). Zinc deficiency can lead to decreased Th1 cytokine secretion (45) and promotion of Th2 cytokine responses (44). Although we did not assess immune function in the children, a previous study in our cohort suggests that maternal antioxidant intake during pregnancy is inversely associated with cord blood lymphoproliferative responses to allergens (50), a finding that is similar to earlier results (46).
An alternative nonimmune mechanism by which vitamin E may reduce wheezing in young children is its effect on lung function. Antioxidant supplementation may protect against exposures that may lead to oxidative stress, thus counteracting the effects of these adverse exposures on lung function (51). For zinc, deficiencies during pregnancy increase the risk of fetal growth restriction and low birth weight (52), and these conditions were associated with wheezing and asthma (53). Furthermore, zinc is found in abundance in the airway epithelium, and it acts as an antioxidant and an antiapoptotic agent and participates in antiinflammatory processes (54). In adults, serum zinc has been inversely associated with wheezing symptoms (55). Several studies also reported associations among low hair, serum, or plasma zinc concentrations and established asthma or allergy (56–58). Our study, however, is the first to directly link maternal intake of zinc with wheezing in young children.
Antioxidants from foods and from supplements appeared to contribute to the overall effect of each of the nutrients on the wheezing outcomes. The fact that these separate effects were not significant after control for potential confounders likely implies that it is the total intakes of these nutrients, rather than the individual sources, that are important.
Tobacco smoke is a main source of environmental antioxidants. Exposure to environmental tobacco smoke was associated with wheezing in our analysis, and we adjusted for this exposure. However, there were too few homes with this exposure (n = 92) to enable us to investigate any effect modification. When we repeated the analyses on the homes without exposure to smoking, the results did not change.
Because much of the wheezing that occurs in early life is due to respiratory infections (specifically, viral respiratory infections), we also investigated the effects of maternal antioxidant intakes on diagnosed childhood respiratory infections. Although we saw a protective effect of maternal intakes in the highest quartile of lutein + zeaxanthin, no association was observed between the other antioxidants—in particular vitamin C, vitamin E, or zinc—and respiratory infections. Thus, it is likely that the effects of these antioxidants on wheezing were not due to prevention of respiratory infections in the children. We recognize, however, that we did not have rigorous ascertainment of respiratory infections.
We recognize several other limitations in our study. First, we asked about children’s wheezing symptoms only once in the first year and once in the second year. Thus, we are unable to assess frequency of wheezing in each year with confidence because of potential recall error. However, it is likely that participants who responded affirmatively to the wheezing questions had children who had clinically significant wheezing illnesses. Because we found consistent associations between maternal antioxidant intake and both wheezing outcomes gives us confidence that we captured the clinically important wheezing symptoms. We used wheezing symptoms as our outcome for this analysis because a diagnosis of asthma in early childhood is difficult. Although we recognize that not all wheezing illnesses in early childhood equate to asthma, children who experience recurrent wheezing episodes are more likely to develop asthma than are children with transient wheezing episodes (59, 60). Further follow-up of this cohort with the planned collection of markers of atopy will allow us to make more definite conclusions about the association between maternal antioxidant intake and the development of asthma and allergies in those mothers’ children.
For maternal dietary assessment, we did not have serum markers of individual nutrients. However, the FFQ that we used for this study has been validated in pregnancy (25). Furthermore, we assessed maternal diet at 2 points in the pregnancy, and the use of multiple assessments of diet was shown to dampen the effects of measurement error and variation because of changes in diet over a specified time (61). Furthermore, we believe that the 2 assessments provide a better representation of diet throughout the pregnancy. The other strength of our study is that we gathered detailed information about breastfeeding and multivitamin intake in infancy, and we adjusted for the effects of these exposures.
In summary, we found that higher maternal intakes of antioxidant nutrients during pregnancy—in particular, vitamin E and zinc—are associated with lower risks of wheezing illnesses in 2-y-old children. Continued follow-up of our cohort will help determine whether these effects will translate to a lower risk of asthma as the children grow older.
Acknowledgments
MWG designed the overall study; AAL, SLR-S, JWR-E, DRG, CAC, and STW were responsible for the study design; AAL, NPL, KGT, JWR-E, DRG, CAC, and MWG were responsible for data analysis; AAL wrote the manuscript draft, and AAL, SLR-S, NPL, KGT, JWR-E, DRG, CAC, and STW provided critical review of the manuscript; SLR-S provided the programming for the analysis; AAL and CAC provided the definition of the variables; and MWG was responsible for overseeing the data collection. None of the authors had a conflict of interest related to the data in the manuscript.
Footnotes
Supported by grants HL61907, HL64925, HD34568, AI35786, and HL68041 from the National Institutes of Health.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 2.Lara M, Rosenbaum S, Rachelefsky G, et al. Improving childhood asthma outcomes in the United States: a blueprint for policy action. Pediatrics. 2002;109:919–30. doi: 10.1542/peds.109.5.919. [DOI] [PubMed] [Google Scholar]
- 3.Braun-Fahrlander C, Gassner M, Grize L, et al. No further increase in asthma, hay fever and atopic sensitisation in adolescents living in Switzerland. Eur Respir J. 2004;23:407–13. doi: 10.1183/09031936.04.00074004. [DOI] [PubMed] [Google Scholar]
- 4.Ronchetti R, Villa MP, Barreto M, et al. Is the increase in childhood asthma coming to an end? Findings from three surveys of schoolchildren in Rome, Italy. Eur Respir J. 2001;17:881–6. doi: 10.1183/09031936.01.17508810. [DOI] [PubMed] [Google Scholar]
- 5.Verlato G, Corsico A, Villani S, et al. Is the prevalence of adult asthma and allergic rhinitis still increasing? Results of an Italian study. J Allergy Clin Immunol. 2003;111:1232–8. doi: 10.1067/mai.2003.1484. [DOI] [PubMed] [Google Scholar]
- 6.Carter ER, Debley JS, Redding GJ. Changes in asthma prevalence and impact on health and function in Seattle middle-school children: 1995 vs 2003. Ann Allergy Asthma Immunol. 2005;94:634–9. doi: 10.1016/S1081-1206(10)61320-8. [DOI] [PubMed] [Google Scholar]
- 7.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non-farming environments. A nationwide study over three decades. Clin Exp Allergy. 2004;34:38–43. doi: 10.1111/j.1365-2222.2004.01841.x. [DOI] [PubMed] [Google Scholar]
- 8.Latvala J, von Hertzen L, Lindholm H, Haahtela T. Trends in prevalence of asthma and allergy in Finnish young men: nationwide study, 1966–2003. BMJ. 2005;330:1186–7. doi: 10.1136/bmj.38448.603924.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 10.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176–81. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- 12.Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax. 2001;56(suppl):64–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Gold DR, Burge H, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheezing in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–36. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163:322–8. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 15.Braun-Fahrlander C, Gassner M, Grize L, et al. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clin Exp Allergy. 1999;29:28–34. doi: 10.1046/j.1365-2222.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 16.Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol. 2001;108:509–15. doi: 10.1067/mai.2001.117797. [DOI] [PubMed] [Google Scholar]
- 17.Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171–4. doi: 10.1136/thx.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–17. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 19.McKeever TM, Britton J. Diet and asthma. Am J Respir Crit Care Med. 2004;170:725–9. doi: 10.1164/rccm.200405-611PP. [DOI] [PubMed] [Google Scholar]
- 20.Yunginger JW, Reed CE, O’Connell EJ, Melton LJ, III, O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146:888–94. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 21.Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. J Allergy Clin Immunol. 2000;105:S493–8. doi: 10.1016/s0091-6749(00)90049-6. [DOI] [PubMed] [Google Scholar]
- 22.von Mutius E. Pediatric origins of adult lung disease. Thorax. 2001;56:153–7. doi: 10.1136/thorax.56.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowles ER. Prenatal nutrition and birth outcomes. J Obstet Gynecol Neonatal Nurs. 2004;33:809–22. doi: 10.1177/0884217504270599. [DOI] [PubMed] [Google Scholar]
- 24.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–5. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 25.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–62. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n–3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–83. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh SY, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Lipshultz SE, Gillman MW. Maternal protein intake is not associated with infant blood pressure. Int J Epidemiol. 2005;34:378–84. doi: 10.1093/ije/dyh373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillman MW, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Lipshultz SE. Maternal calcium intake and offspring blood pressure. Circulation. 2004;110:1990–5. doi: 10.1161/01.CIR.0000143199.93495.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Agriculture ARS. USDA national nutrient database for standard reference, release 14. Washington, DC: US Department of Agriculture; 2001. [Google Scholar]
- 30.Willett WC. Implications of total energy intake for epidemiologic studies of breast and large-bowel cancer. Am J Clin Nutr. 1987;45:354–60. doi: 10.1093/ajcn/45.1.354. [DOI] [PubMed] [Google Scholar]
- 31.Proceedings of the 1992 International Symposium on Public Health Surveillance. Atlanta, Georgia, April 22–24, 1992. MMWR Morb Mortal Wkly Rep. 1992;41(suppl):1–218. [PubMed] [Google Scholar]
- 32.ACOG Committee on Practice Bulletins. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 44, July 2003. (Replaces Committee Opinion Number 252, March 2001) Obstet Gynecol. 2003;102:203–13. [PubMed] [Google Scholar]
- 33.Institute of Medicine, Food and Nutrition Board. Committee on Nutritional Status During Pregnancy, Part II: dietary intake and nutrient supplements. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 34.Institute of Medicine, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of DRIs, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press, 2000.
- 35.Nebeling LC, Forman MR, Graubard BI, Snyder RA. The impact of lifestyle characteristics on carotenoid intake in the United States: the 1987 National Health Interview Survey. Am J Public Health. 1997;87:268–71. doi: 10.2105/ajph.87.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watt RG, Dykes J, Sheiham A. Socio-economic determinants of selected dietary indicators in British pre-school children. Public Health Nutr. 2001;4:1229–33. doi: 10.1079/phn2001202. [DOI] [PubMed] [Google Scholar]
- 37.Litonjua AA, Carey VJ, Weiss ST, Gold DR. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatr Pulmonol. 1999;28:394–401. doi: 10.1002/(sici)1099-0496(199912)28:6<394::aid-ppul2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Cook DG, Carey IM, Whincup PH, et al. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax. 1997;52:628–33. doi: 10.1136/thx.52.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilliland FD, Berhane KT, Li YF, Gauderman WJ, McConnell R, Peters J. Children’s lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol. 2003;158:576–84. doi: 10.1093/aje/kwg181. [DOI] [PubMed] [Google Scholar]
- 40.Farchi S, Forastiere F, Agabiti N, et al. Dietary factors associated with wheezing and allergic rhinitis in children. Eur Respir J. 2003;22:772–80. doi: 10.1183/09031936.03.00006703. [DOI] [PubMed] [Google Scholar]
- 41.Forastiere F, Pistelli R, Sestini P, et al. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment) Thorax. 2000;55:283–8. doi: 10.1136/thorax.55.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nja F, Nystad W, Lodrup Carlsen KC, Hetlevik O, Carlsen KH. Effects of early intake of fruit or vegetables in relation to later asthma and allergic sensitization in school-age children. Acta Paediatr. 2005;94:147–54. doi: 10.1111/j.1651-2227.2005.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 43.Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–8. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 44.Shaheen SO, Newson RB, Henderson AJ, Emmett PM, Sherriff A, Cooke M. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J. 2004;24:292–7. doi: 10.1183/09031936.04.00117803. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–28. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 46.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal responses to allergens. Clin Exp Allergy. 2002;32:43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 47.Li-Weber M, Giaisi M, Treiber MK, Krammer PH. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401–8. doi: 10.1002/1521-4141(200209)32:9<2401::AID-IMMU2401>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 48.Tan PH, Sagoo P, Chan C, et al. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol. 2005;174:7633–44. doi: 10.4049/jimmunol.174.12.7633. [DOI] [PubMed] [Google Scholar]
- 49.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–8. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Litonjua AA, Tantisira KG, Finn PW, et al. Maternal antioxidant intake during pregnancy and cord blood lymphoproliferative responses. Am J Respir Crit Care Med. 2004;169:A501. abstr. [Google Scholar]
- 51.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–9. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 52.King J. Determinants of maternal zinc status during pregnancy. Am J Clin Nutr. 2000;71(suppl):1334S–43S. doi: 10.1093/ajcn/71.5.1334s. [DOI] [PubMed] [Google Scholar]
- 53.Lewis S, Richards D, Bynner J, Butler N, Britton J. Prospective study of risk factors for early and persistent wheezing in childhood. Eur Respir J. 1995;8:349–56. doi: 10.1183/09031936.95.08030349. [DOI] [PubMed] [Google Scholar]
- 54.Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE. Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review Pharmacol Ther. 2005;105:127–49. doi: 10.1016/j.pharmthera.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz J, Weiss ST. Dietary factors and their relation to respiratory symptoms. The second National Health and Nutrition Examination Survey. Am J Epidemiol. 1990;132:67–76. doi: 10.1093/oxfordjournals.aje.a115644. [DOI] [PubMed] [Google Scholar]
- 56.Di Toro R, Galdo Capotorti G, Gialanella G, Miraglia del Giudice M, Moro R, Perrone L. Zinc and copper status of allergic children. Acta Paediatr Scand. 1987;76:612–7. doi: 10.1111/j.1651-2227.1987.tb10530.x. [DOI] [PubMed] [Google Scholar]
- 57.Kadrabova J, Mad’aric A, Podivinsky F, Gazdik F, Ginter F. Plasma zinc, copper and copper/zinc ratio in intrinsic asthma. J Trace Elem Med Biol. 1996;10:50–3. doi: 10.1016/s0946-672x(96)80008-3. [DOI] [PubMed] [Google Scholar]
- 58.Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Garg ML. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids. 2000;35:967–74. doi: 10.1007/s11745-000-0607-x. [DOI] [PubMed] [Google Scholar]
- 59.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 60.Ly NP, Gold DR, Weiss ST, Celedon JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117:e1132–8. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 61.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–9. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]


