Abstract
Chronic hyperglycemia in diabetes induces abnormal nerve pathologies, resulting in diabetic neuropathy (DN). Sensory symptoms of DN can manifest as positive (painful), negative (insensate), or both. Streptozotocin (STZ)-induced diabetic C57Bl/6 mice have reduced cutaneous innervation and display reduced behavioral responses to noxious stimuli, reflecting the insensate aspect of the human syndrome. Current studies were undertaken to determine whether the diabetes-induced deficits in pain responses are reflected by changes in spinal activation in this model of DN. Nocifensive responses of nondiabetic and diabetic mice to formalin injection were measured 1, 3, 5 and 7 weeks post-STZ, and at each time point formalin-induced spinal Fos expression was quantified. Responses of diabetic mice were significantly reduced during the second phase of the formalin test beginning 3 weeks post-STZ, and during Phase 1 beginning 5 weeks post-STZ. Consistent with the behavioral responses, the number of Fos-positive cells in the dorsal horn of diabetic animals was significantly reduced beginning 3 weeks post-STZ and continuing 5 and 7 weeks post-STZ. The deficits at 5 weeks post-STZ were restored by 2-week treatments with insulin or neurotrophins. These results demonstrate that the reduced sensation occurring from progressive peripheral axon loss results in functional deficits in spinal cord activation.
Keywords: Neuropathy, pain behavior, Fos, NGF, GDNF, Insulin
PERSPECTIVE
The reduced expression of the immediate early gene Fos as an indicator of pain transmission supports the diabetes-induced loss of sensation in this Type 1 model of diabetes. This murine model may be better suited to understanding the insensate symptoms of diabetic patients in the absence of chronic pain.
INTRODUCTION
Diabetic neuropathy (DN) is one of the principle chronic complications of both Type 1 and Type 2 diabetes mellitus and currently affects more than half of diabetic patients. In human patients and animal models, DN commonly manifests as a distal symmetric sensory polyneuropathy44,47 characterized by the distal degeneration of peripheral axons.12,18,19,26,29,34 Axonal loss can also be accompanied by segmental demyelination22 and reduced nerve regeneration capacity.27,38 Together these structural changes result in reduced epidermal innervation, decreased nerve conduction velocities, and reduced amplitude of sensory nerve action potentials. However, the exact manner in which the hyperglycemic environment contributes to nerve damage is still unresolved.
In humans, diabetes-induced nerve dysfunction can produce a variable degree of motor and autonomic symptoms, but sensory deficits are the predominant feature of DN. Sensory loss develops in the majority of affected human patients, including both chronic numbness and insensitivity to pain or touch. Painful symptoms (paresthesias, hyperalgesia, tactile allodynia) are only reported in up to 32% of patients with DN, are most likely to present early in the disease progression, and have a slightly higher prevalence in Type 2 diabetes.32,42,43,45
Current animal models of diabetes vary in their presentation of neuropathy symptoms. Streptozotocin (STZ) is commonly used to induce diabetes in rodents because of its specific toxic effects on pancreatic beta cells.28 STZ-treated rats develop mechanical, thermal, and chemogenic hyperalgesia as well as tactile and thermal allodynia.6 These sensory abnormalities are proposed to reflect the painful aspect of human diabetic neuropathy, yet do not model the insensate symptoms suffered by the majority of human patients. In contrast, it's widely known that STZ-treated C57BL/6 mice display reduced sensitivity to mechanical, chemogenic, and, in some cases, thermal stimuli.11,13,49 In addition, STZ-induced diabetic C57BL/6 mice display reduced dermal and epidermal innervation of the hindpaw, as well as abnormalities in the central terminals of primary nociceptive neurons.3,12 Therefore, the STZ-induced diabetic C57BL/6 mouse model may be better suited for exploring abnormal peripheral nerve function associated with insensate symptoms.13
The relationship between an animal's behavior and pain status is inherently subjective, and it is not absolute that rodent nocifensive withdrawal behaviors are directly related to the perceived stimulus intensity. Fos is an immediate-early transcription factor expressed in second order spinal neurons in response to a noxious peripheral stimulus and has been used as a surrogate for peripheral nerve activation. In response to formalin injection into the hindpaw, Fos is expressed in a temporal and spatial pattern consistent with the magnitude of nociceptive input from the hindpaw.1,4,39 To test the hypothesis that diabetes-induced peripheral nerve damage results in suppressed spinal activation, we compared spinal Fos expression in response to formalin injection in nondiabetic and diabetic mice during the progression of neuropathy. In addition, we tested whether insulin or neurotrophin treatments restored Fos expression in a manner consistent with improved behavioral responses. Our results suggest that STZ-induced sensory loss in C57Bl/6 mice reduces Fos expression in the dorsal horn in a manner consistent with peripheral nerve damage and reduced primary afferent input. Moreover, treatments that improve aspects of the neuropathy can similarly improve stimulus-induced Fos expression in the spinal cord.
MATERIALS AND METHODS
Animals
All animal use was in accordance with NIH guidelines and conformed to the principles specified by the University of Kansas Medical Center Animal Care and Use Protocol. In all studies, 8-week-old male C57BL/6 mice (Charles River, Wilmington, MA) were housed 2−4 mice per cage on a 12:12-hour light/dark cycle under pathogen-free conditions with free access to mouse chow and water.
Experimental Design
In order to assess whether progressive behavioral deficits in diabetic mice represent true hypoalgesia, nondiabetic and diabetic mice were injected with STZ or vehicle on Day 0 and sacrificed 1, 3, 5, or 7 weeks later. Formalin testing was performed on the day of sacrifice for each separate endpoint, and formalin-induced Fos expression was evaluated. Based on these results, further experiments were performed in order to determine whether the concomitant deficits observed in behavioral responses and Fos expression could be restored. At 5 weeks post-STZ, nondiabetic and diabetic mice treated with insulin, neurotrophins, or CSF were tested for formalin responses, sacrificed, and evaluated for Fos expression.
Diabetes Induction
Diabetes was induced by a single intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO; 180 mg/kg body weight) dissolved in 10 mM sodium citrate buffer, pH 4.5.50 Nondiabetic mice were injected with sodium citrate buffer alone. Animal weight and tail vein blood glucose levels using glucose diagnostic reagents (Sigma) were measured 1-week post-STZ and every other week thereafter to assess diabetes. Only STZ-injected mice with blood glucose levels greater than 16.0 mmol/L (288 mg/dL) were included in the diabetic groups; STZ-injected mice with blood glucose levels below that standard were not included in the study.50 Blood collection for the final time point was taken subsequent to formalin testing so that behavioral measurements would not be influenced by the blood draw. Weight and blood glucose levels were compared between nondiabetic and diabetic untreated animals using 2-way analysis of variance (ANOVA) followed by post hoc analysis using the Fisher's PLSD test. Repeated measures (RM) AVOVA was used to analyze weight and blood glucose of treated nondiabetic and diabetic animals pre- and post-treatment.
Behavioral Analysis
The formalin test was performed prior to sacrifice 1, 3, 5 and 7 weeks post-STZ on nondiabetic and diabetic mice by an experienced experimenter blinded to the condition of the mice. After a 1 hr habituation to individual observation chambers, mice were injected subcutaneously with 20 μL of formalin (5% formaldehyde) into the dorsal surface of the right hindpaw using a 1CC insulin syringe and 28-gauge needle. The amount of time devoted to the injected foot (licking and biting) was recorded in two 10-minute windows during the acute (Phase 1; 0−10 min post-injection) and inflammatory (Phase 2; 40−50 min post-injection) phases of the formalin test. Differences in the attentive time spent to the injected foot during each phase were compared between nondiabetic and diabetic mice at each time point using unpaired t-tests.
Fos Immunocytochemistry
The expression of Fos protein was examined in the spinal cords of nondiabetic and diabetic mice. Two hours following formalin injection, when Fos protein is expressed at maximal levels,4 mice were anesthetized with Avertin (1.25% v/v tribromoethanol, Sigma; 2.5% tert-amyl alcohol, Sigma; 200 μL/10 g body weight) and transcardially perfused with ice cold 0.1 M phosphate-buffered saline (PBS; pH 7.4) followed by ice cold 4% paraformaldehyde in PBS. The spinal column and surrounding tissue were removed and post-fixed in the same fixative overnight at 4°C. The lumbar enlargement of the spinal cord was dissected and cryoprotected in 30% sucrose for 24 hours. Spinal segments L4/L5 were frozen in Tissue Tek (Sakura, Torrance, CA) and sectioned on a cryostat at 20 μm. Sections were mounted on Superfrost Plus slides (Fisher Scientific, Chicago, IL) and stored at −20°C.
Following a 5 min thaw at room temperature, slide-mounted tissue was incubated under humidified conditions with 0.5% Triton-X in PBS for 20 min, 10% normal horse serum in PBS for 10 min, and with primary antibodies against c-Fos (rabbit; 1:3000; EMD Biosciences, La Jolla, CA) and NeuN (mouse; 1:1000; Chemicon) diluted in PBS for 16 hr at 4°C. For visualization, the primary antibodies were removed by three washes in PBS, and sections were incubated for 1 hr at 4°C with fluorochrome-conjugated secondary antibodies (chicken anti-rabbit Alexa 488, 1:2000; donkey anti-mouse Alexa 555, 1:2000; Molecular Probes, Eugene, OR). Sections were rinsed and coverslipped before viewing, and sections from all treatment groups and time points were included in each experiment to standardize immunocytochemical processing differences amongst all groups.
Quantification of Fos-positive neurons
The number of dorsal horn spinal neurons ipsilateral to formalin injection containing Fos-like immunofluoresence was counted live under the microscope at 20X by an experimenter blinded to the groups. Fos-positive cells were identified by strong nuclear staining, and only Fos-positive cells colabeled with NeuN, a neuronal marker, were counted. Fos-positive cells were assigned to established dorsal horn laminar regions I/II, III/IV, and V/VI. Counts from the 3 laminar regions were summed to yield the total number of Fos-positive dorsal horn cells for each section. The sum of Fos counts represent the average of labeled dorsal horn nuclei in 10 sections, each separated by at least 60 μm, throughout the length of L4/L5 spinal segments of each cord. Differences in the total number of Fos-positive cells between nondiabetic and diabetic animals at each time point were compared using unpaired t-tests. Differences in the number of Fos-positive cells in laminar regions at each time point were analyzed using 2-way ANOVA followed by post hoc analysis using Fisher's PLSD.
Insulin Administration
Three weeks after diabetes induction, slow-release insulin pellets (13 +/− 2 mg each; 0.1 U/day/implant; LinShin Canada, Inc., Scarborough, Ontario, Canada) were implanted subcutaneously in the dorsal skin of diabetic mice (2 implants for the first 20 g body weight; 1 implant for each additional fraction of 5 g body weight) and remained for 2 weeks. After one week an additional pellet was added if blood glucose levels were not below 16 mmol/L. Weight, blood glucose levels, and symptoms of hyperglycemia were monitored as previously detailed. Sham pellets were not administered. At 5 weeks post-STZ, behavioral analysis, sacrifice, and Fos immunocytochemistry were performed and quantified as described above. Differences in the attentive time spent to the injected foot during each phase of the formalin test were compared between nondiabetic, untreated diabetic, and insulin-treated diabetic mice using 1-way ANOVA followed by post hoc analysis using Fisher's PLSD. Differences in the total number of Fos-positive cells were compared using 1-way ANOVA followed by Fisher's PLSD. Differences in the number of Fos-positive cells in laminar regions were analyzed using 2-way ANOVA followed by Fisher's PLSD.
Neurotrophin Administration
Beginning 3 weeks after diabetes induction, diabetic and nondiabetic mice received daily intrathecal injections (50 μL; 1 μg neurotrophin) of NGF or GDNF (Chemicon International, Inc.) for 2 weeks. Growth factors were delivered intrathecally to improve delivery to the DRG, avoiding problems related to the insufficient retrograde transport of trophic factors that is known to occur in diabetic animals.3,12 Individual trophic factors were dissolved in artificial cerebrospinal fluid (CSF) at a concentration of 20nM. Control mice received intrathecal injections of CSF alone as a control for the intrathecal injection paradigm. Intrathecal injections were delivered between the L6 and the S1 vertebrae using a 28-gauge insulin syringe.21 The physiological conditions of all CSF-, NGF- and GDNF-treated mice were monitored as previously detailed. At 5 weeks post-STZ, behavioral analysis, sacrifice, and Fos immunocytochemistry were performed and quantified as described above. Differences in the attentive time spent to the injected foot during each phase of the formalin test were compared between CSF-, NGF- and GDNF-treated diabetic and nondiabetic mice using 1-way ANOVA followed by post hoc analysis using the Fisher's PLSD test. Differences in the total number of Fos-positive cells were compared using 1-way ANOVA followed by Fisher's PLSD. Differences in the number of Fos-positive cells in laminar regions were analyzed using 2-way ANOVA followed by Fisher's PLSD.
RESULTS
STZ-Induced Diabetes
STZ-injected mice demonstrated symptoms typical of STZ-induced diabetes including polydipsia, polyuria, and weight loss. By 1 week post-STZ, over 90% of STZ-injected mice had significantly higher blood glucose levels compared to vehicle-injected mice; this difference was maintained throughout the study (Table 1). Nondiabetic mice increased in weight by 30.5% over the course of the study, while diabetic mice lost 14.0% of their weight in the first week following STZ injection and failed to gain weight in the remaining weeks (Table 1). Despite these differences, nondiabetic and diabetic mice were generally similar in their locomotor/grooming activity during the observation period after formalin injection, suggesting any reductions in the sensitivity to formalin were not simply due to poor health or lethargy in diabetic mice.
Table 1.
Weight and blood glucose levels measured at weeks 0, 1, 3, 5 and 7 post-STZ of untreated nondiabetic and diabetic mice that underwent both formalin testing and evaluation for Fos expression. Data for each week includes values for all animals tested on that day from all time point groups. Weight is in grams; glucose levels are mmol/L. NM, not measured. Data represented as means +/− standard error of mean.
| Week 0 | Week 1 | Week 3 | Week 5 | Week 7 | |
|---|---|---|---|---|---|
| Nondiabetic: | |||||
| Weight | 20.5 +/− 0.95 | 23.2 +/− .0.31 | 24.4 +/− 0.73 | 25.4 +/− 0.51 | 27.8 +/− 0.91 |
| N = 21 | N = 20 | N = 16 | N = 11 | N = 6 | |
| Glucose | NM | 7.9 +/− 0.34 | 8.7 +/− 0.91 | 7.5 +/− 0.36 | 7.4 +/− 1.80 |
| N = 20 | N = 16 | N = 11 | N = 5 | ||
| Diabetic: | |||||
| Weight | 22.2 +/− 0.29 | 19.1* +/− 0.48 | 19.2* +/− 0.42 | 19.3* +/− 0.90 | 20.6# +/− 1.75 |
| N = 20 | N = 20 | N = 15 | N = 10 | N = 4 | |
| Glucose | NM | 25.8* +/− 0.82 | 25.4* +/− 0.77 | 21.3* +/− 0.82 | 20.7# +/− 1.33 |
| N = 20 | N = 15 | N = 10 | N = 4 |
P < 0.0001 vs nondiabetic mice,
P < 0.05 vs nondiabetic mice.
Diabetes-Induced Deficits in Formalin-Induced Pain Behavior
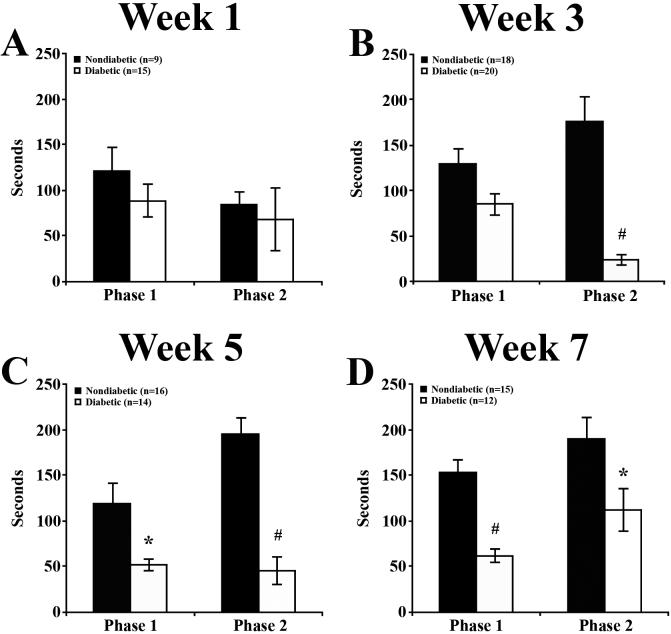
To determine the progression of diabetes-induced sensory deficits, the responsiveness to formalin injection was assessed in groups of nondiabetic and diabetic mice 1, 3, 5 and 7 weeks following STZ injection (Figure 1). In nondiabetic mice, formalin injection resulted in a typical biphasic response including both an acute (Phase 1) and an inflammatory (Phase 2) phase.36 In comparison, diabetic mice gradually developed diminished responses to formalin injection during the 7 weeks following STZ-injection compared to nondiabetic mice. At 1 week post-STZ (Figure 1A), the amount of time devoted to the injected foot did not differ between nondiabetic and diabetic mice. At week 3 post-STZ (Figure 1B), diabetic mice displayed a significantly reduced Phase 2 response, responding 86.6% less than nondiabetic mice, the most severe reduction of the study. By week 5 (Figure 1C), responses during both Phase 1 and Phase 2 were significantly reduced (by 56.6% and 76.8%, respectively) in diabetic compared to nondiabetic mice. This pattern was maintained 7 weeks post-STZ (Figure 1D) when responses by diabetic mice were significantly reduced during Phase 1 and Phase 2 by 59.6% and 40.9%, respectively.
Figure 1. The behavioral response to formalin is impaired in diabetic mice.
The behavioral responses of nondiabetic and diabetic mice to formalin injection recorded in 10 min. windows during Phase 1 and Phase 2 of the formalin test prior to sacrifice at 1 (A), 3 (B), 5 (C), and 7 (D) weeks following STZ injection. (A) One week post-STZ, the amount of time devoted to the injected foot did not differ between nondiabetic and diabetic mice. (B) At 3 weeks post-STZ, diabetic mice devoted significantly less time to the injected foot during Phase 2. (C) Diabetic mice displayed significantly reduced behavioral responses during both Phase 1 and 2 at 5 weeks post-STZ. (D) This pattern was maintained during both Phase 1 and 2 at 7 weeks post-STZ. Data plotted as means +/− standard error of mean. * P < 0.05 vs nondiabetic mice, # P < 0.0001 vs nondiabetic mice.
Diabetes-Induced Reductions in Spinal Fos Expression
To determine whether the diabetes-induced deficits in pain behaviors were reflected in changes in spinal activation, mice were sacrificed following the formalin test, and the expression of Fos protein was evaluated in tissue sections of L4-L5 lumbar segments of spinal cords from both nondiabetic and diabetic mice 1, 3, 5 and 7 weeks following STZ-injection. In sections from both groups of animals, Fos immunoreactivity was concentrated in the nuclei of small neurons in the superficial and neck regions of the dorsal horn ipsilateral to the formalin injection (Figure 2A, B). Few Fos-positive neurons were observed in the contralateral dorsal horn. In spinal cord sections from diabetic animals (Figure 2D, F, H, J), the number of Fos-positive neurons was visibly decreased by week 5 (Figure 2H) compared to nondiabetic animals (Figure 2G), an effect more pronounced by week 7 (Figure 2I, J.)
Figure 2. Formalin-induced expression of Fos in the mouse lumbar spinal cord.
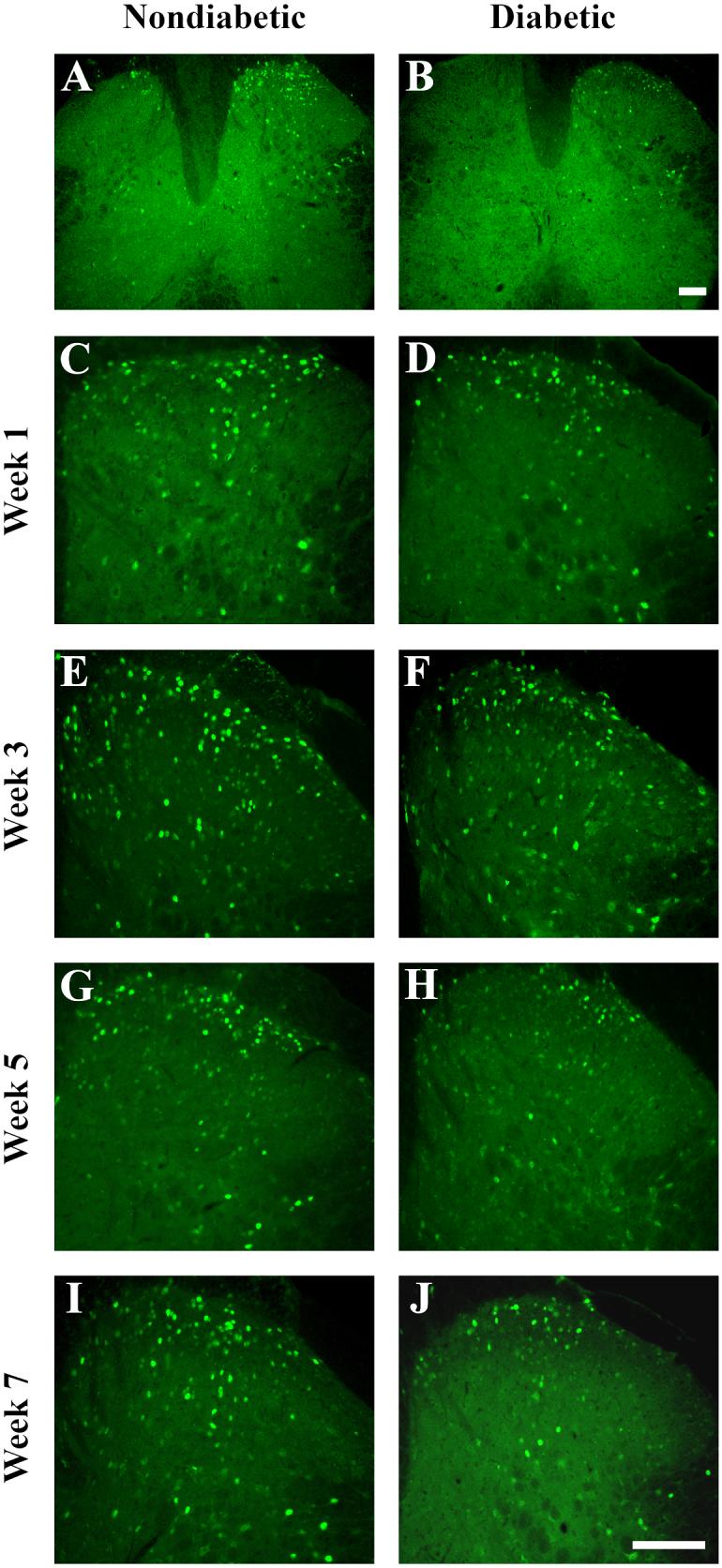
Representative images at low magnification (A, B) and high magnification (C-J) of Fos-positive neurons in the lumbar spinal cord after formalin injection. A, B) Formalin-induced Fos expression was apparent predominantly on the ipsilateral side to the formalin injection in both nondiabetic and diabetic mice, albeit in fewer neurons in diabetic mice. Images were taken from mice 7 weeks post-STZ. C-J) Comparisons of Fos-positive neurons were made between nondiabetic (left side) and diabetic (right side) mice. Fos expression appeared relatively normal in diabetic animals at weeks 1 (C, D) and 3 (E, F) post-STZ. However, Fos expression was visibly decreased in diabetic mice at 5 (G, H) and 7 (I, J) weeks. Scale bar, 100 μm for each image.
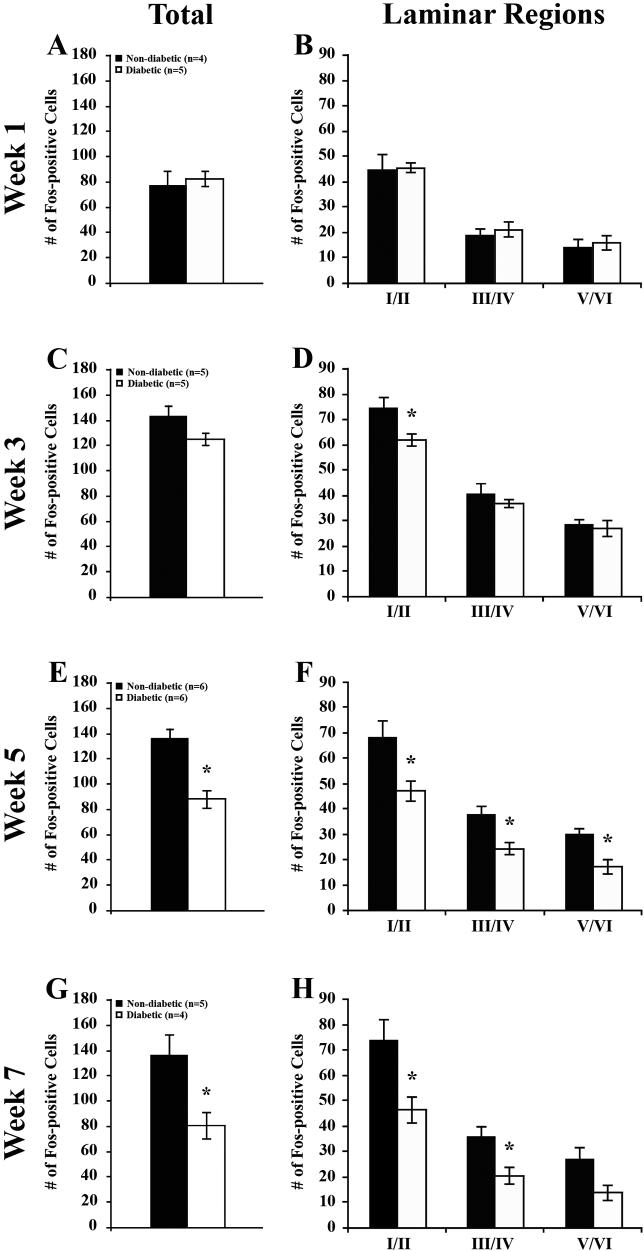
In nondiabetic mice, the total number of Fos-positive neurons per 20 μm section in the dorsal horn averaged 143.1 +/− 8.65, 136.1 +/− 7.89 and 136.1 +/− 17.04 at 3, 5 and 7 weeks post-STZ, respectively (Figure 2E, G, I; 3C, E, G). At each time point, laminae I/II contained the greatest percentage of Fos-positive neurons, containing a little over half the total number of Fos-positive neurons per dorsal horn section (Figure 2; 3B-E). Laminae V/VI represented the lowest contribution to the total, never containing more than 30.0 +/− 2.03 Fos-positive neurons per section.
Figure 3. Quantification of formalin-induced Fos expression in diabetic mice.
A, C, E, G) Quantification of total Fos expression in the dorsal horn of nondiabetic and diabetic mice. Comparisons between nondiabetic and diabetic mice revealed a significant decrease in Fos-positive neurons at weeks 5 and 7.
B, D, F, H) Quantification of Fos expression within laminar regions. B) One week post-STZ, an analysis of Fos-positive neurons within specific laminar regions revealed no significant differences between nondiabetic and diabetic animals. C) In contrast, analysis at 3 weeks post-STZ revealed a significant reduction in Fos-positive neurons within laminae I/II in diabetic versus nondiabetic mice. D) More severe reductions in Fos-positive neurons in diabetic mice were evident at 5 (D) and 7 (E) weeks post-STZ in all laminae, with the exception of laminae V/VI at 7 weeks. Data plotted as means +/− standard error of mean. * P < 0.05 vs nondiabetic mice.
At 1 week post-STZ, there were no significant differences between nondiabetic and diabetic mice in the number of Fos-positive cells found in any laminar group (Figure 3B) or in total (Figure 2C, D; 3A). At 3 weeks post-STZ, although there was no significant difference in the total number of Fos-positive cells (Figure 2E, F; 3C), there were significantly fewer Fos-positive cells in laminae I/II (Figure 3D) of spinal cords from diabetic compared to nondiabetic mice. There were no differences found in the other laminar regions. By 5 weeks post-STZ, the decline in the total number of dorsal horn Fos-positive cells per section reached statistical significance (Figure 2G, H; 3E), at which time diabetic mice had 35.5% fewer than nondiabetic mice. All laminar regions appeared to contribute to this reduction, with significant losses in laminae I/II, III/IV, and V/VI (Figure 3F). By week 7, there was a significant 40.7% reduction in the total number of Fos-positive cells (Figure 2I, J, 3G), due primarily to reductions in laminae I/II and III/IV (Figure 3H).
Insulin Effects on Diabetes-Induced Deficits in Pain Behavior and Fos Expression
To evaluate the ability of insulin to restore the diabetes-induced deficits in pain behavior and Fos expression, diabetic mice 3 weeks post-STZ received insulin from slow-release pellets for 2 weeks. At the end of the 2-week insulin treatment, insulin-treated animals had increased in body weight from 19.5 +/− 2.26 g at the start of treatment to 23.3 +/− 0.84 g (P = 0.0987) and significantly improved over untreated diabetic mice (P < 0.05). Similarly, the blood glucose levels of insulin-treated diabetic animals decreased from 22.8 +/− 1.71 mmol/L to 17.3 +/− 3.43 mmol/L at the time of sacrifice. Though this level was not significantly different from untreated diabetic animals (21.3 +/− 0.82 mmol/L at 5 weeks post-STZ; P = 0.0902), the insulin-treated animals improved in overall appearance and general health, and it is possible that the insulin pellets were nearly depleted by the end of the study and thus failed to maintain euglycemia throughout the entire 14-day treatment.
To determine whether insulin treatment could restore the sensory deficits induced by diabetes, the behavioral response to formalin injection of insulin-treated diabetic mice 5 weeks post-STZ was compared with that of the nondiabetic and untreated diabetic mice at the same time point. Insulin-treated mice did not devote significantly more attentive time to the injected foot during Phase 1 of the formalin test. However, insulin restored the behavioral responses during Phase 2 to that of nondiabetic mice (P = 0.5392), significantly increasing the time devoted to the injected foot over untreated diabetic mice (Phase 2, P < 0.0001; Figure 4A). Similarly, insulin restored formalin-induced spinal Fos expression in diabetic mice, significantly increasing the number of Fos-positive dorsal horn neurons per section compared to untreated diabetic mice, both in total (P < 0.05, Figure 4B) and in laminae I/II (P < 0.05, Figure 4C). It is important to note that the nondiabetic and untreated diabetic animals did not receive sham pellets, creating a potential confound.
Figure 4. Insulin increases formalin-induced pain responses and spinal Fos expression.
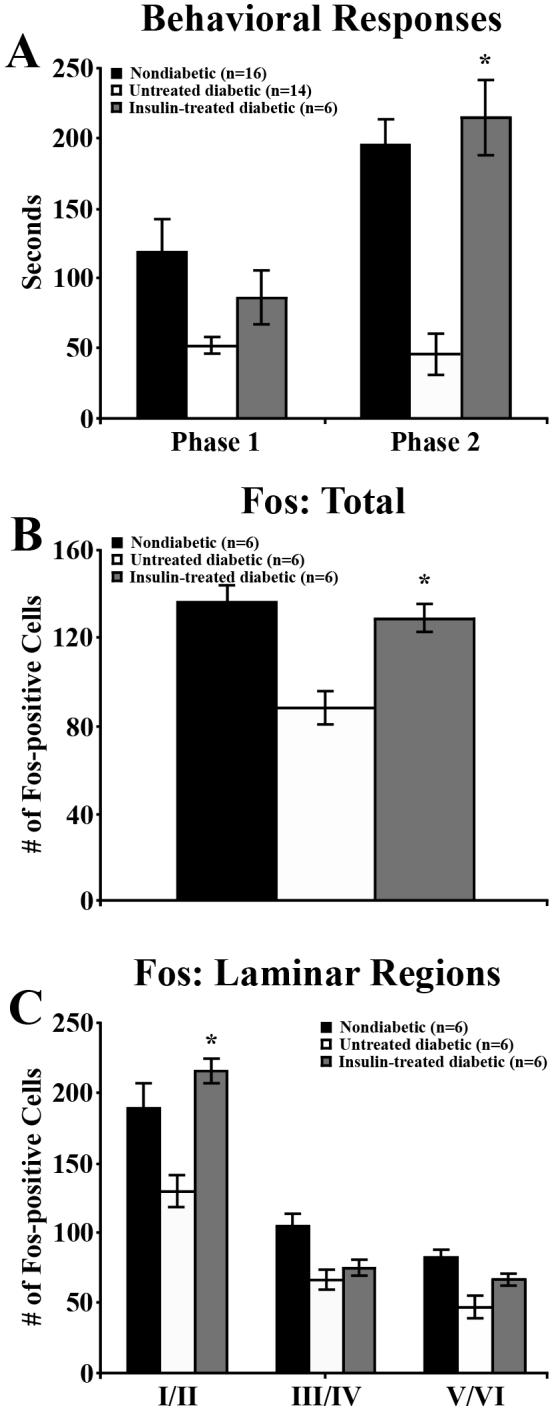
The behavioral responses (A) and Fos expression (B, C) of nondiabetic, untreated diabetic, and insulin-treated diabetic mice following formalin injection at 5 weeks post-STZ. Nondiabetic and untreated diabetic groups are the same mice used in Figure 3 E, F. A) During Phase 1, behavioral responses of insulin-treated diabetic animals were not significantly higher than in untreated diabetic animals. During Phase 2 of the formalin test, insulin-treated diabetic mice devoted significantly more time to the injected foot than untreated diabetic mice and did not perform significantly different from nondiabetic mice. B) Insulin-treated diabetic mice also had significantly greater total numbers of Fos-positive dorsal horn neurons per spinal cord section than untreated diabetic mice and were not significantly different from nondiabetic mice (P = 0.4901). C) Insulin significantly increased the number of Fos-positive neurons per section in laminae I/II compared to untreated diabetic mice. Data plotted as means +/− standard error of mean. * P < 0.05 vs untreated diabetic mice, # P < 0.0001 vs untreated diabetic mice.
Neurotrophin Effects on Diabetes-Induced Deficits in Pain Behavior and Fos Expression
To evaluate the ability of neurotrophins to restore the diabetes-induced deficits in pain behavior and Fos expression, diabetic mice received daily intrathecal injections of NGF, GDNF, or vehicle (CSF) 3 weeks post-STZ for a duration of 2 weeks. CSF-treated diabetic mice decreased in weight from 21.5 +/− 0.65 g at the time of STZ-injection to 18.5 +/− 1.66 g at 5 weeks post-STZ (Table 2). At the time of sacrifice blood glucose levels were 26.3 +/− 0.53 mmol/L. Diabetic mice receiving intrathecal treatment with either NGF or GDNF for 2 weeks underwent decreases in weight and continued hyperglycemia similar to CSF-diabetic mice. Statistical analysis showed there was not a significant effect of treatment on weight (P = 0.8902) or glucose (P = 0.9449) over time, suggesting NGF and GDNF intrathecal treatments had no effect on these parameters (Table 2).
Table 2.
Weight and blood glucose levels of diabetic and nondiabetic mice receiving 2-week intrathecal treatment with CSF, NGF, or GDNF starting 3 weeks post-STZ. Weight is in grams; glucose levels are mmol/L. Data represented as means +/− standard error of mean. Statistical analysis did not reveal an effect of treatment on weight or blood glucose.
| Pre-Treatment | Post-Treatment | |||
|---|---|---|---|---|
| Diabetic | ||||
| CSF | Weight | 19.0 +/− 0.91 | 18.5 +/− 1.66 | |
| Glucose | 22.6 +/− 0.93 | 26.3 +/− 0.56 | ||
| NGF | Weight | 19.3 +/− 0.42 | 20.3 +/− 0.97 | |
| Glucose | 24.8 +/− 0.88 | 26.2 +/− 0.61 | ||
| GDNF | Weight | 20.0 +/− 1.00 | 19.3 +/− 1.20 | |
| Glucose | 23.6 +/− 0.94 | 26.8 +/− 0.39 | ||
| Nondiabetic | ||||
| CSF | Weight | 22.5 +/− 0.65 | 24.5 +/− 0.50 | |
| Glucose | 8.2 +/− 0.16 | 8.5 +/− 0.70 | ||
| NGF | Weight | 22.8 +/− 0.48 | 23.8 +/− 0.48 | |
| Glucose | 8.5 +/− 0.57 | 6.5 +/− 1.29 | ||
| GDNF | Weight | 21.5 +/− 0.65 | 24.3 +/− 0.25 | |
| Glucose | 8.1 +/− 0.48 | 6.7 +/− 1.35 |
To evaluate the ability of neurotrophins to restore diabetes-induced behavioral and functional deficits, formalin responses and Fos expression were evaluated in 5-week post-STZ diabetic mice treated with CSF, NGF, or GDNF. Although not significant, the time devoted to the injected foot during Phase 1 and Phase 2 of the formalin test in NGF-treated animals was elevated (Figure 5A). GDNF failed to increase the time devoted to the injected foot during Phase I, but did produce a non-significant increase in Phase 2 (Figure 5A). It should be pointed out that the number of animals treated was only 4−7, which was lower than the N's of formalin experiments in Figure 1. Importantly, however, both NGF and GDNF treatments increased the total number of Fos-positive cells expressed in diabetic spinal cords over CSF-treated diabetic mice (NGF, P < 0.05; GDNF, P = 0.0602; Figure 5B). In NGF-treated mice, Fos expression was increased in laminae I/II (P < 0.05), III/IV (P < 0.05), and V/VI (P < 0.05); in GDNF-treated mice, laminae III/IV (P = 0.0555) and V/VI (P < 0.05; Figure 5C).
Figure 5. NGF and GDNF increase formalin-induced spinal Fos expression.
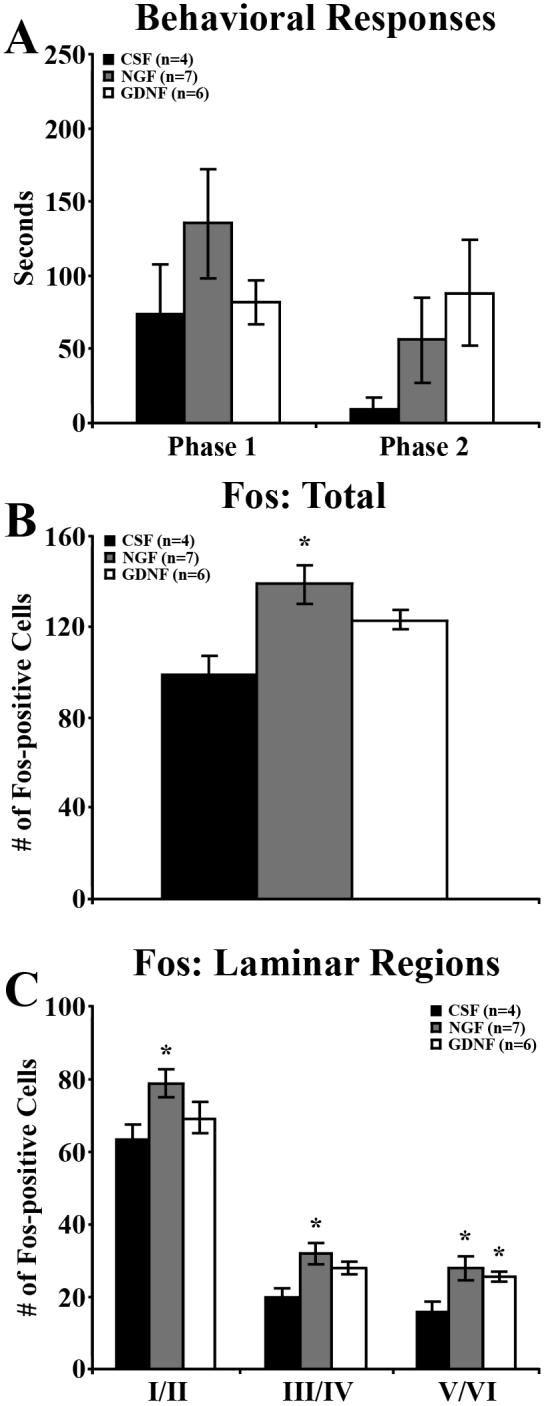
The behavioral responses (A) and Fos expression (B, C) of diabetic mice receiving 2 weeks of daily intrathecal injections of vehicle (CSF), NGF, or GDNF. A) At 5 weeks post-STZ, statistical analysis did not show an effect of treatment group on the Phase 1 or 2 behavioral responses over time. B) Both NGF and GDNF treatment increased the overall number of Fos-positive neurons per section compared to CSF-treated mice. C) NGF treatment significantly increased Fos-positive neurons within all laminae, while GDNF treatment increased Fos-positive neurons within laminae III/IV and V/VI. Data plotted as means +/− standard error of mean. * P < 0.05 vs CSF-injected diabetic mice.
Neurotrophin Effects on the Pain Behavior and Fos Expression of Nondiabetic Mice
In addition to neurotrophin treatment in diabetic mice, we also treated nondiabetic mice to 2 weeks of daily intrathecal injections with CSF, NGF, or GDNF (Figure 6). Statistical analysis did not reveal a significant effect of treatment on behavioral responses to the formalin test during Phase 1 (P = 0.9528) or Phase 2 (P = 0.3924; Figure 6A). Similarly, neurotrophin treatment did not alter total Fos expression (P = 0.8542; Figure 6B) or Fos expression in laminar groups (P = 0.6640; Figure 6C).
Figure 6. NGF and GDNF did not alter formalin responses or formalin-induced spinal Fos expression in nondiabetic mice.
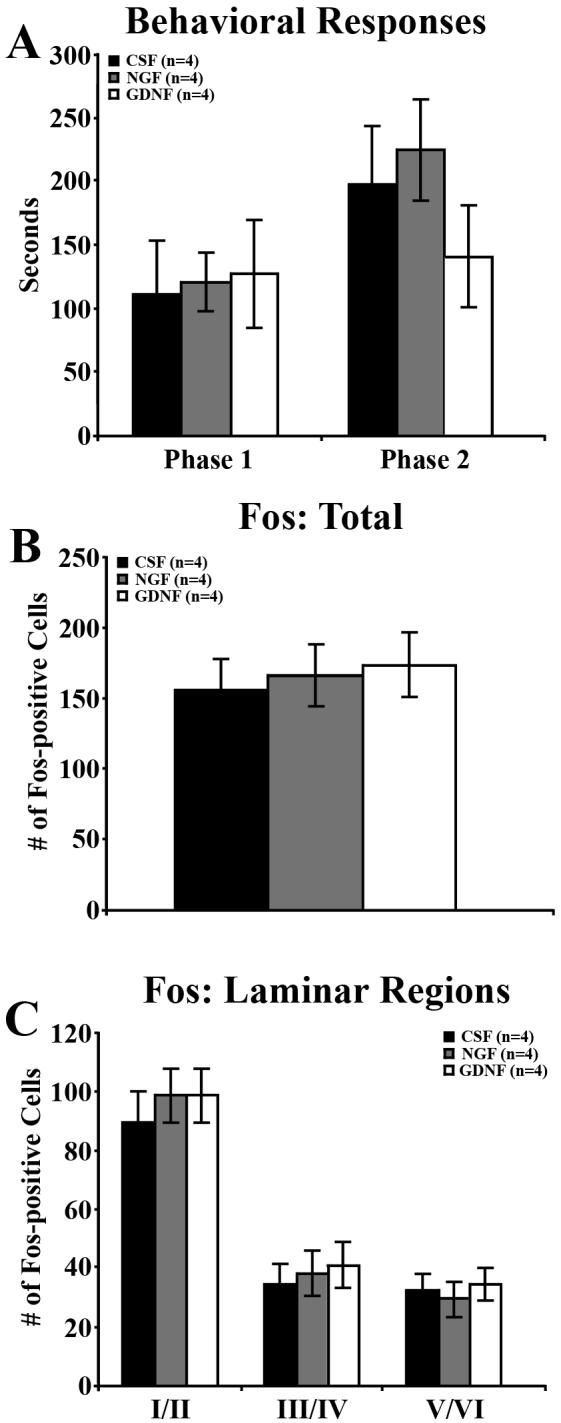
The behavioral responses (A) and Fos expression (B, C) of nondiabetic mice receiving 2 weeks of daily intrathecal injections of vehicle (CSF), NGF, or GDNF. A) At 5 weeks post-STZ, statistical analysis did not show an effect of treatment group on the Phase 1 or 2 behavioral responses over time. B) Neither NGF nor GDNF treatment altered the overall number of Fos-positive neurons per section compared to CSF-treated mice. C) NGF and GDNF treatment did not change the number of Fos-positive neurons within any laminar region. Data plotted as means +/− standard error of mean.
DISCUSSION
STZ-treated C57BL/6 mice progressively develop reduced sensitivity to mechanical and noxious chemogenic stimuli,13 and these sensory deficits resemble the insensate symptoms experienced by most human diabetic neuropathy patients. In addition to suppressed pain responses, these diabetic mice have significant reductions in hindlimb cutaneous nerve fibers12 and abnormal spinal afferent terminations.3 Collectively, these deficits suggest that chronic hyperglycemia causes nerve damage in C57Bl/6 mice sufficient to reduce sensory input from the periphery. The present study extends these findings by delineating the relationship between behavioral responses to formalin injection and spinal activation as measured by Fos expression. Our results demonstrate the time course by which STZ-induced diabetic mice develop increasingly diminished sensitivity to chemogenic noxious stimuli. Moreover, diabetic mice display reduced Fos expression in response to formalin, and these deficits parallel the progression of the behavioral abnormalities and are consistent with known diabetes-induced changes in neural anatomy and animal behavior.12,13 These results strengthen the view that neuropathy induced in STZ-treated C57BL/6 mice can be used to explore sensory loss as a complication of diabetes.
Chemogenic Hypoalgesia in Diabetic C57Bl/6 Mice
Formalin injection into the hindpaw is a well-established model of chemogenic pain that causes an acute and a continuous secondary pain stimulus.33,36 Thought to arise from direct activation of primary afferent fibers, the acute phase elicits robust nocifensive responses toward the injected foot. During the second phase, these responses are induced by activation of sensory fibers as a secondary response to inflammation, although central sensitization of spinal neurons has also been posited as an additive mechanism.2,40 We have previously reported reductions in behavioral responses of C57BL/6 diabetic mice during both phases of the formalin test 9 weeks post-STZ.13 It is important to note that the presence of hypoalgesia versus hyperalgesia is likely dependent on the strain and species, as studies in diabetic rats have reported chemogenic hyperalgesia during both the quiescent and inflammatory phases.7,8,9 Similarly, studies in outbred strains of diabetic mice have reported chemogenic hyperalgesia in response to formalin.23,24,25,46
Here, we demonstrate that as the neuropathy progresses, reduced behavioral responses do not emerge in Phase 2 until 3 weeks post-STZ. In comparison, deficits during Phase 1 were not apparent until 5 weeks post-STZ. The precedent loss of the Phase 2 response suggests diabetes might initially affect inflammatory-associated aspects of pain. Such aspects may include the release or function of inflammatory mediators, the delivery of those mediators to the injury site (implicating microvascular occlusion), or the response of neurons to those mediators. The Phase 1 response appeared more resistant to diabetes and may reflect the degree of cutaneous innervation. This view is supported by recent studies in a Type 2 diabetes model (db/db mice) in which epidermal and dermal fiber density is normal in long-term hyperglycemic mice. These mice have reduced responses to formalin in Phase 2, but Phase 1 is normal.51
Diabetes-Induced Suppression of Spinal Fos Expression in Response to Formalin
Formalin injection induces spinal Fos expression in a temporal and spatial pattern consistent with the magnitude of nociceptive input from the hindpaw.1,39 Here, formalin injection induced Fos expression in both nondiabetic and diabetic mice within the superficial (laminae I/II), middle (III/IV) and neck (V/VI) regions of the ipsilateral dorsal horn. Neurons in laminae I/II are the terminal targets of primary nociceptive afferents and project to deeper laminae and higher brain regions. Formalin-induced Fos expression in these laminae is driven monosynaptically by small nociceptive primary afferents activated in the injected paw.39 In contrast, neurons in laminae III/IV respond to input from innocuous stimuli.4,20 Neurons in lamina V/VI also receive direct input from primary nociceptive afferents; however, a recent report suggested laminae V/VI neurons also receive projections from lamina II neurons, project to pain-related brain regions and may be an important convergence point of sensory information.5 Collectively, the overall laminar distribution of Fos expression appears to code the nature of the stimulus as well as the nociceptive intensity perceived by the animal.4,20
Here, decreased spinal Fos expression in STZ-injected diabetic mice paralleled the progression of deficits in nocifensive responses to formalin injection. Significant reductions in Fos expression were evident in lamina I/II 3 weeks post-STZ, concomitant with reduced behavioral responses during Phase 2 of the formalin test. By weeks 5 and 7, the suppressed responses of Phase 1 and 2 were manifested in reduced Fos expression in all laminae. It is plausible that chronic hyperglycemia may directly attenuate Fos expression in the cord. Indeed, several studies have reported that diabetes reduces the expression of a wide range of genes in neural tissues.41 However, recent studies in rats report increased Fos expression in diabetic rats with pain, suggesting that Fos expression likely remains a reliable indicator of peripheral nerve input.35
As mentioned above, STZ-induced diabetic rats display increased sensitivity to thermal, tactile, and chemical stimuli. It has been proposed that the occurrence of hyperalgesia and allodynia in the seemingly incongruous presence of reduced peripheral input (evidenced by reductions in intraepidermal nerve fiber density and diminished substance P release centrally) is the result of aberrant information processing at higher levels.6,8,29 Calcutt (2004) has suggested that studies showing an upregulation of spinal nociceptive mediators14,15,16,48 and increased electrophysiologic activity10,37 in spinal neurons of diabetic rats support this hypothesis and imply an enhancement of spinal nociceptive processing.6 However, our results demonstrating decreased Fos expression in the dorsal horn show this not to be the case in diabetic C57Bl/6 mice. Rather, our evidence suggests the reduced peripheral input in STZ-treated C57BL/6 mice results in decreased activation of the spinal cord by peripheral afferents and thus diminished pain perception.
Restoration of Stimulus-Induced Behavior and Fos Expression by Insulin
The Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications follow-up study emphasized the importance of early interventions aimed at maintaining proper glycemic control for preventing neuropathy in human patients.17 In diabetic mice, insulin treatment has been shown to effectively restore mechanical and chemogenic behavioral responses.13 Here, we administered insulin to diabetic mice to test whether the diabetes-induced deficits in formalin responses and Fos expression were reversible. Diabetic mice given insulin resumed weight gain and improved blood glucose levels. Although insulin-treated diabetic mice did not reach euglycemia, tail vein blood was collected without prior removal of mouse feed, and the high metabolic rate and frequent feeding behaviors of mice may have increased the variability of measurements and resulted in higher levels. Despite the lack of euglycemia at sacrifice, insulin-treated diabetic mice responded normally during Phase 2 of the formalin test, significantly improving over untreated diabetic mice. This improvement was also reflected in spinal Fos expression. Insulin administration increased the total number of dorsal horn Fos-positive cells, specifically in laminae I/II, which suggests insulin prevented the diabetes-induced reduction in Fos expression in response to formalin. The ability of insulin to restore the sensory deficits in diabetic mice is supportive of the view that these deficits are specific to the chronic hyperglycemia, although direct actions of insulin cannot be ruled out.30
Neurotrophin Treatment Improves Stimulus-Induced Fos Expression
Neurotrophic support has long been thought to play a role in the progression of diabetic neuropathy, and as treatments neurotrophins have shown sporadic but encouraging effects on improving sensory deficits in diabetic neuropathy.31,52 Our previous studies have utilized both NGF and GDNF in a number of settings to test their actions on sensory deficits in diabetic C57Bl/6 mice.3,12,13 For example, intrathecal NGF treatment to diabetic mice increases behavioral responses to mechanical and chemogenic stimuli, but had no effect on increasing peripheral innervation of the hindpaw. The increased behavior in the absence of effects on peripheral innervation may be explained by NGF's ability to sensitize afferents, leading to increased central input. Here, NGF again had a non-significant but positive effect on chemogenic hypoalgesia and increased the Fos-positive neurons in all laminae. If NGF's actions are indeed via peripheral sensitization, the increase in Fos expression in diabetic mice suggests NGF can sensitize afferents sufficiently to increase central activation and pain behavior.
In contrast to NGF, we have reported that GDNF treatment of diabetic mice restores pain behaviors, increases axonal density in skin, and improves staining of terminals in the dorsal horn.3,12,13 Consistent with these actions, GDNF increased stimulus-induced Fos expression in the spinal cord, particularly in the deeper laminae. These effects are consistent with improved sensory innervation and function, leading to improved spinal activation and responses to painful stimuli. These results suggest both NGF and GDNF can influence spinal activation in response to peripheral stimulation. Accessing signaling pathways related to these neurotrophins may lead to increased sensation in patients with insensate symptoms.
Our results show that although there is a good correlation between behavior and Fos expression, there are subtle disconnects in the degree to which different measures of rodent somatosensation indicate pain sensitivity. Fos may be a more direct measure of afferent drive in response to a painful stimulus, whereas behavioral responses to an injured limb are a sum of peripheral and central processing. Our studies suggest multiple measures should be performed to acquire the best evaluation of pain sensation.
ACKNOWLEDGMENTS
Supported by NIH grant R01NS43314 (DEW). The authors would like to thank Dr. Kenneth McCarson, Dr. Michael Werle and Karra Jones for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbadie C, Lombard MC, Morain F, Besson J-M. Fos-like immunoreactivity in the rat superficial dorsal horn induced by formalin injection in the forepaw: effects of dorsal rhizotomies. Brain Res. 1992;578:17–25. doi: 10.1016/0006-8993(92)90224-w. [DOI] [PubMed] [Google Scholar]
- 2.Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–10. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- 3.Akkina SK, Patterson CL, Wright DE. GDNF rescues nonpeptidergic unmyelinated primary afferents in streptozotocin-treated diabetic mice. Exp Neurol. 2001;167:173–182. doi: 10.1006/exnr.2000.7547. [DOI] [PubMed] [Google Scholar]
- 4.Bon K, Wilson SG, Mogil JS, Roberts WJ. Genetic evidence for the correlation of deep dorsal horn Fos protein immunoreactivity with tonic formalin pain behavior. J Pain. 2002;3:181–189. doi: 10.1054/jpai.2002.123710. [DOI] [PubMed] [Google Scholar]
- 5.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Calcutt NA. Experimental models of painful diabetic neuropathy. J Neurosurg Sci. 2004;220:137–139. doi: 10.1016/j.jns.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68:293–9. doi: 10.1016/s0304-3959(96)03201-0. [DOI] [PubMed] [Google Scholar]
- 8.Calcutt NA, Stiller C, Gustafsson H, Malmberg AB. Elevated substance-P-like immunoreactivity levels in spinal dialysates during the formalin test in normal and diabetic rats. Brain Res. 2000;856:20–7. doi: 10.1016/s0006-8993(99)02345-8. [DOI] [PubMed] [Google Scholar]
- 9.Cesena RM, Calcutt NA. Gabapentin prevents hyperalgesia during the formalin test in diabetic rats. Neurosci Lett. 1999;262:101–4. doi: 10.1016/s0304-3940(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol. 2002;87:2726–2733. doi: 10.1152/jn.2002.87.6.2726. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, Chung SS, Chung SK. Noninvasive monitoring of diabetes-induced cutaneous nerve fiber loss and hypoalgesia in thy1-YFP transgenic mice. Diabetes. 2005;54.11:3112–3118. doi: 10.2337/diabetes.54.11.3112. [DOI] [PubMed] [Google Scholar]
- 12.Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179:188–189. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 13.Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4:493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ciruela A, Dixon AK, Bramwell S, Gonzalez MI, Pinnock RD, Lee K. Identification of MEK1 as a novel target for the treatment of neuropathic pain. Br J Pharmacol. 2003;138:751–756. doi: 10.1038/sj.bjp.0705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dualhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal MAPKs activation in neurons and microglia via NMDA-dependent mechanisms. Mol Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- 16.Freshwater JD, Svensson CI, Malmberg AB, Calcutt NA. Elevated spinal cyclooxygenase and prostaglandin release during hyperalgesia in diabetic rats. Diabetes. 2002;51:2249–2255. doi: 10.2337/diabetes.51.7.2249. [DOI] [PubMed] [Google Scholar]
- 17.Genuth S. Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes. Endocrine Practice. 2006;12:34–31. doi: 10.4158/EP.12.S1.34. [DOI] [PubMed] [Google Scholar]
- 18.Hermann DN, Griffen JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 19.Hirai A, Yasuda H, Joko M, Maeda T, Kikkawa R. Evaluation of diabetic neuropathy through the quantitation of cutaneous nerves. J Neurol Sci. 2000;172:55–62. doi: 10.1016/s0022-510x(99)00290-7. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 21.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 22.Jaffey PB, Gelman BB. Increased vulnerability to demyelination in streptozotocin diabetic rats. J Comp Neurol. 1996;373:55–61. doi: 10.1002/(SICI)1096-9861(19960909)373:1<55::AID-CNE5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Kamei J, Ohhashi Y, Aoki T, Kasuya Y. Streptozotocin-induced diabetes in mice reduces the nociceptive threshold, as recognized after application of noxious mechanical stimuli but not of thermal stimuli. Pharmacol Biochem Behav. 1991;39:541–4. doi: 10.1016/0091-3057(91)90224-p. [DOI] [PubMed] [Google Scholar]
- 24.Kamei J, Hitosugi H, Kasuya Y. Formalin-induced nociceptive responses in diabetic mice. Neurosci Lett. 1993;149:161–4. doi: 10.1016/0304-3940(93)90761-9. [DOI] [PubMed] [Google Scholar]
- 25.Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol. 2001;422:83–6. doi: 10.1016/s0014-2999(01)01059-7. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy JM, Zochodne DW. The regenerative deficit of peripheral nerves in experimental diabetes: its extent, timing and possible mechanisms. 2000. pp. 2118–2129. [DOI] [PubMed]
- 28.Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE. The potential mechanism of the diabetogenic action of streptozotocin: inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase. Biochem J. 2001;356:31–41. doi: 10.1042/0264-6021:3560031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- 30.Leinninger GM, Feldman EL. Insulin-like growth factors in the treatment of neurological disease. Endocr Dev. 2005;9:135–59. doi: 10.1159/000085763. [DOI] [PubMed] [Google Scholar]
- 31.Leinninger GM, Vincent AM, Feldman EL. The role of growth factors in diabetic peripheral neuropathy. J Peripher Nerv Syst. 2004;9:26–53. doi: 10.1111/j.1085-9489.2004.09105.x. [DOI] [PubMed] [Google Scholar]
- 32.Low PA, Dotson RM. Symptomatic treatment of painful neuropathy. JAMA. 1998;280:1863–1864. doi: 10.1001/jama.280.21.1863. [DOI] [PubMed] [Google Scholar]
- 33.McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996;208:45–8. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffen JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 35.Morgado C, Tavares IF. Baseline c-fos expression in the spinal dorsal horn neurons of streptozotocin diabetic rats. Soc. Neurosci Abst. 2006;43.10 [Google Scholar]
- 36.Murray CW, Porreca F, Cowan A. Methodological refinements to the mouse paw formalin test: an animal model of tonic pain. J Pharmacol Methods. 1988;20:175–186. doi: 10.1016/0160-5402(88)90078-2. [DOI] [PubMed] [Google Scholar]
- 37.Pertovaara A, Wei H, Kalmari J, Ruotsalainen M. Pain behavior and response properties of spinal dorsal horn neurons following experimental diabetic neuropathy in the rat: modulation by nitecapone, a COMT inhibitor with antioxidant properties. Exp Neurol. 2001;167:425–434. doi: 10.1006/exnr.2000.7574. [DOI] [PubMed] [Google Scholar]
- 38.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fiber regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 39.Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–333. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–55. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 41.Purves TD, Tomlinson DR. Diminished transcription factor survival signals in dorsal root ganglia in rats with streptozotocin-induced diabetes. Ann N Y Acad Sci. 2002;973:472–6. doi: 10.1111/j.1749-6632.2002.tb04686.x. [DOI] [PubMed] [Google Scholar]
- 42.Quattrini C, Tesfaye S. Understanding the impact of painful diabetic neuropathy. Diabetes Metab Res Rev. 2003;19(Suppl 1):S2–8. doi: 10.1002/dmrr.360. [DOI] [PubMed] [Google Scholar]
- 43.Schmader K. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Sinnreich M, Taylor BV, Dyck PJB. Diabetic neuropathies: classification, clinical features, and pathophysiological basis. Neurologist. 2005;11:63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen L, Molyneaux L, Yue DK. Insensate vs. painful diabetic neuropathy: the effects of height, gender, ethnicity and glycaemic control. Diabetes Res Clin Pract. 2002;57:45–51. doi: 10.1016/s0168-8227(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 46.Takeshita N, Yamaguchi I. Insulin attenuates formalin-induced nociceptive response in mice through a mechanism that is deranged by diabetes mellitus. J Pharmacol Exp Ther. 1997;281:315–21. [PubMed] [Google Scholar]
- 47.Thomas PK. Diabetic neuropathy: mechanisms and future treatment options. J Neurol Neurosurg Psychiatry. 1999;67:277–281. doi: 10.1136/jnnp.67.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res. 2005;136:275–281. doi: 10.1016/j.molbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Walwyn WM, Matsuka Y, Arai D, Bloom DC, Lam H, Tran C, Spigelman I, Maidment NT. HSV-1 mediated NGF delivery delays nociceptive deficits in a genetic model of diabetic neuropathy. Exp Neurol. 2006;198:260–270. doi: 10.1016/j.expneurol.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Wang ZC, Dohle JF, Friemann J, Green BS. Prevention of high- and low-dose STZ-induced diabetes with d-glucose and 5-thio-d-glucose. Diabetes. 1993;42:420–428. doi: 10.2337/diab.42.3.420. [DOI] [PubMed] [Google Scholar]
- 51.Wright DE, Ryals JM, Johnson MS, Smittkamp SE. Discrete neuropathic symptoms in the peripheral nervous system of a Type 2 model of diabetes: db/db mice. Soc. Neurosci Abstract. 2006;443.13 [Google Scholar]
- 52.Zochodne DW. Neurotrophins and other growth factors in diabetic neuropathy. Semin Neurol. 1996;16:153–61. doi: 10.1055/s-2008-1040971. [DOI] [PubMed] [Google Scholar]