Abstract
The pattern of collagen cross-linking is tissue specific primarily determined by the extent of hydroxylation and oxidation of specific lysine residues in the molecule. In this study, murine pre-myoblast cell line, C2C12 cells, were transdifferentiated into osteoblastic cells by bone morphogenetic protein (BMP)-2 treatment, and the gene expression of lysyl hydroxylases (LH1, 2a/b and 3) and lysyl oxidase (LOX)/lysyl oxidase-like proteins (LOXL1–4), and the extent of hydroxylysine were analyzed. After 24 hours of treatment, the expression of most isoforms were upregulated up to 96 hours whereas LH2a and LOXL2 decreased with time. In the treated cells, both hydroxyproline and hydroxylysine were detected at day 7 and increased at day 14. The ratio of hydroxylysine to hydroxyproline was significantly increased at day 14. The results indicate that LHs and LOX/LOXLs are differentially responsive to BMP-induced osteoblast differentiation that may eventually lead to the specific collagen cross-linking pattern seen in bone.
Keywords: collagen post-translational modifications, C2C12 cells, osteoblast, lysyl hydroxylase, lysyl oxidase, lysyl oxidase-like protein
Introduction
Fibrillar type I collagen is the most abundant protein in vertebrates providing the basis for form and connectivity of tissues and organs, but its post-translational modifications vary from tissue to tissue. A number of studies have demonstrated that the specific post-translational modifications are essential for the formation of functional fibrils in the respective tissues[1]. In bone, the specific pattern of lysine hydroxylation and covalent intermolecular cross-linking is important to regulate the process of biomineralization [2–5]. Lysyl hydroxylases (LHs) and lysyl oxidase (LOX)/LOX-like proteins (LOXLs) are two groups of enzymes that play critical roles in determining the pattern collagen cross-linking. At present, 3 genes encoding for LH (LH1, 2a/b, 3) and 5 for LOX/LOXL (LOX, LOXL1-4) have been identified and partially characterized. It has been reported that mutations in some of these genes are partly associated with inherited disorders, e.g. mutations in LH1 gene with the Ehlers-Danlos syndrome type VI [6] and LH2 with the Bruck syndrome [7]. Recently, we have demonstrated that most isoforms of LH [8, 9] and LOX/LOXL [10] are expressed in osteoblastic cell line, MC3T3-E1 cells, and that LH2b is critical in determining collagen cross-linking pattern [8]. However, the significance of the isoform expression and their regulatory mechanisms are still not well understood.
Bone morphogenetic protein (BMP)-2, a member of the transforming growth factor beta (TGF-β) superfamily, is one of the most potent osteogenic BMPs. It has been shown that BMP-2 is capable of driving the murine pre-myoblast cell line C2C12 cells into osteoblastic lineage (transdifferentiation) by suppressing the expression of myogenic genes and inducing that of osteogenic genes [11]. Thus, this model has been widely used to study the molecular events related to early osteoblast differentiation and BMP functions. Microarray analyses have revealed that genes of the extracellular matrix proteins such as type I collagen, biglycan and decorin were upregulated after BMP-2 treatment [12]. However, the response of collagen modifying enzyme genes or their products (i.e. modified amino acids) has not been investigated.
In this study, by employing this model, we examined the expression of genes for lysyl hydroxylase (LH1, 2a/b and 3) and lysyl oxidase (LOX, LOXL1–4), and the extent of hydroxylysine to obtain an insight into the significance of collagen post-translational modifications in early osteoblast differentiation.
Materials and methods
Cell culture
C2C12 cells were purchased from American Type Culture Collection (CRL-1772) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Carlsbad. CA, USA) containing 15 % Fetal Bovine Serum (FBS, Sigma, St. Louis, MO, USA), 100 U/ml penicillin and 100 μg/ml streptomycin in a 5 % CO2 atmosphere at 37 °C. The cells were plated onto 35 mm dishes at a density of 8 × 104 cells/dish and cultured for 24 hrs. The culture medium was then replaced with DMEM containing 5 % FBS with various concentrations (0, 50, 100, 150 ng/ml) of recombinant human BMP-2 (rhBMP-2, 355-BM/CF, R&D systems, Minneapolis, MN, USA). Cell morphology was observed under an inverted light microscopy (ECLIPSE TE300, Nikon, Tokyo, Japan) and subjected to the following analyses.
Alkaline Phosphatase (ALP) activity
After 4 days of culture, cell/matrix layer was washed with phosphate buffered saline (PBS) and lysed with 300 μl of tris buffer saline (TBS) containing 0.1 % Triton-X. ALP activity was measured using Alkaline Phosphatase Yellow (pNPP; p-nitrophenylphosphate) Liquid Substrate System for ELISA (Sigma, St. Louis, MO, USA) according to the manufacturer’s protocol. The reaction was terminated with 3 N NaOH to a final concentration of 0.5 N NaOH and the pNP production was measured by absorbance at 405 nm using a 96-well plate reader (PowerWave X 340, Bio Tek instruments, Winooski, VT, USA). The protein concentration was determined by a DC Protein Assay kit (Bio-Rad, Hercules, CA, USA) and ALP activity was calculated as mol of pNP/min/total protein [13].
Quantitative real-time Polymerase Chain Reaction (PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) after 0, 16, 24, 48, 72 and 96 hours of rhBMP-2 treatment and the first-strand cDNA was synthesized using an Omniscript Reverse Transcriptase Kit (Qiagen, Valencia, CA, USA). The mRNA expression levels of myogenic genes, i.e. myogenin and MyoD, osteogenic genes, i.e. alkaline phosphatase 2 (Akp2), core binding factor alpha 1 (Cbfa1), osterix (OSX), and type-1 collagen α1 chain (Col1a1), and isoforms of collagen modifying enzyme genes, i.e. LH1, LH2, LH3, LOX, LOXL1, LOXL2, LOXL3 and LOXL4, were quantitatively analyzed by real-time PCR. It was performed by the ABI Prism 7000 Sequence detection system (Applied Biosystems, Foster City, CA, USA) using the sequence specific primers. Primers used are as follows; myogenin (ABI assay No. Mm 00446194_m1), MyoD (Mm 00440387_m1), Akp2 (Mm 00475831_m1), Cbfa1 (Mm 00501578_m1), OSX (Mm 00504574_m1), Col1a1 (Mm 00801666_g1), LH1 (Mm 00599925_m1), LH2 (Mm 00478767_m1), LH3 (Mm 00478798_m1), LOX (Mm 00495386_m1), LOXL1 (174595), LOXL2 (Mm 00804740_m1), LOXL3 (Mm 00442953_m1), LOXL4 (Mm 00446385_m1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (4308313). The samples were analyzed in triplicate and two independent experiments were performed to confirm reproducibility of the results. The mRNA expression levels relative to GAPDH were determined and the fold changes were calculated using the values obtained before rhBMP-2 addition (0 hour) as a calibrator by means of 2−ΔΔCT method on each time point [14]. For OSX and Akp2, since the expression were not detected without the addition of rhBMP-2, the value obtained at after 16 hours of rhBMP-2 treatment was used as a calibrator.
Reverse Transcription (RT)-PCR
Specific primers for LH2 were designed to determine the two known splicing variants; forward primer, 5′GAAAGGAACTATTTTGTCCGTGATA3′ (position1440–1464); and reverse primer, 5′GGGATCTATAAATGACACTGCAAT3′ (position1575–1599). This set of primers amplifies both LH2a (without an insert of 63 bp exon 13A) and LH2b (with the splice insert) identifying the former as a 158 bp fragment and the latter a 221 bp fragment [6]. A set of GAPDH primers; forward, 5′ACCACAGTCCATGCCATCAC3′, reverse, 5′TCCACCACCCTGTTGCTGTA3′ were also designed to use as an internal control. RT-PCR amplifications were performed by HotStarTaq DNA polytmerase (Qiagen, Valencia, CA, USA) according to the manufacturer’s instruction. The optimal number of PCR cycles was selected for each amplification; 35 cycles for LH2a and GAPDH, and 22 cycles for LH2b. The PCR products were then resolved by 12% polyacrylamide gel electrophoresis (PAGE) and analyzed.
Amino acid analysis
C2C12 cells were cultured with or without the addition of 100 ng/ml of rhBMP-2 in the presence of 50 μg/ml of ascorbic acid and, at day 7 and 14, cell/matrix layer was subjected to amino acid analysis as described[15]. Briefly, cultured cells/matrix layer was scraped, washed with PBS and distilled water, lyophilized, hydrolyzed with 6N HCl in vacuo at 105°C for 22 hrs and dried. The hydrolysates were dissolved in distilled water and subjected to amino acid analysis by HPLC (Varian 9050/9012; Varian, Walnut Creek, CA, USA). The content of hydroxyproline (Hyp) and hydroxylysine (Hyl) were expressed as res/1,000 amino acids, and the ratio of Hyl to Hyp was calculated and expressed as a percentage. The analysis was done in duplicate for two sets of experiments.
Results
Transdifferentiation from Myoblasts to Osteoblasts
The myotubes were observed in C2C12 cells after reaching the confluence at day 4 (Fig. 1a). When treated with rhBMP-2, however, the cell morphology was significantly changed into an osteoblast-like round shape in 4 days (Fig. 1c) as previously reported [9]. At day 14, the formation of myotubes became more prominent in the untreated cells (Fig. 1b) in contrast to the rhBMP-2 treated cells which formed cell/matrices layer (Fig. 1d). In addition, ALP activity at day 4 was increased with rhBMP-2 treatment in a dose dependent manner indicating the cell lineage was converted from myoblasts to osteoblasts (Fig. 1e).
Fig. 1.
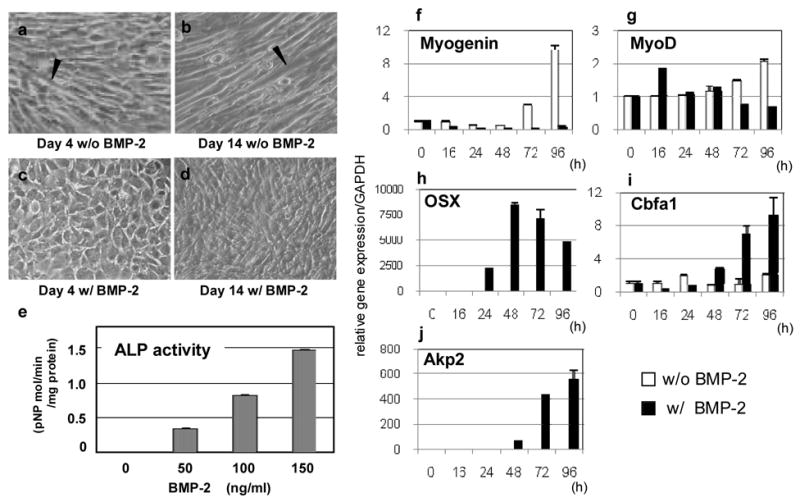
Transdifferentiation of C2C12 cells from myoblasts to osteoblasts induced by rhBMP-2. The cells were cultured for 4 (a, c) or 14 days (b, d), without (a, b) or with 100 ng/ml of rhBMP-2 (c, d) and observed (X 40). Arrowheads indicate the presence of myotubes. At 4 days of treatment, cell shape changed to osteoblast-like round shape (c). At day 14, typical myotubes were well formed without rhBMP-2 treatment (b) whereas cell/matrices layer was clearly formed with the treatment (d). At day 4, ALP activity was increased with rhBMP-2 treatment in a dose dependent manner (e). mRNA expression of myogenic and osteogenic genes with or without rhBMP-2 treatment at various time points analyzed by real-time PCR (f–j). Note that osteogenic genes were upregulated (h–j) whereas myogenic genes suppressed (f,g) with time.
The expression of a myogenic gene, myogenin, was continuously downregulated with rhBMP-2 treatment but was markedly increased with the progression of culture in the untreated cells (Fig. 1f). MyoD, another myogenic gene, showed a transient increase at 16 hours then decreased thereafter in the treated cells but it was gradually increased with time in the untreated group (Fig. 1g) as previously reported [11]. Two genes encoding OSX and Cbfa1, transcriptional factors critical for osteoblast differentiation, were significantly upregulated with rhBMP-2 treatment. The expression of OSX was detected at 16 hours of treatment, increased at 48 hours and then gradually decreased (Fig. 1h). It was not detected in the untreated cells at any time points analyzed. Cbfa1 showed an increase at 48 hours and continued to increase thereafter (Fig. 1i). Akp2 was detected only in the treated cells at 16 hours and markedly increased thereafter (Fig. 1j). It was not detected at any time points in the untreated cells.
Expression of LH and LOX isoforms, and type I collagen
In the treated cells, the Col1a1 (Fig 2a) expression was significantly increased at 48 hours and continued to increase thereafter whereas such increases were not seen in the untreated cells. The expression of all LH and LOX isoforms changed with rhBMP-2 treatment (Fig. 2b–i). Of LH isoforms, LH1 showed the most and significant increase reaching to a 4.0 fold increase at 96 hours of treatment (Fig. 2b). The expression of LH2, after a transient decrease at 24 hours, showed a 1.5 fold increase at 96 hours (Fig 2c). LH3 expression decreased until 24 hours of treatment, then gradually increased and returned to the initial expression level (time 0) (Fig. 2d). The expression of LOX isoforms including LOX, LOXL1, LOXL3 and LOXL4 were all upregulated with rhBMP-2 treatment (Fig. 2e, f, h, i). In particular, LOX was highly responsive to the treatment reaching to a 6 fold increase at 96 hours of treatment. In the untreated cells, LOX expression was slightly suppressed with time. The expression of LOXL1 was upregulated in both untreated and treated cells after 24 hours of treatment but the latter showed slightly higher expression levels compared to the former. LOXL3 and 4 showed a similar pattern in the treated cells starting to increase after 24 hours of treatment and reached at a 2-fold increase at 96 hours whereas no significant changes were observed in the untreated cells. Of LOX isoforms, only LOXL2 expression was continuously diminished with the treatment while it increased in the untreated cells (Fig. 2g).
Fig. 2.
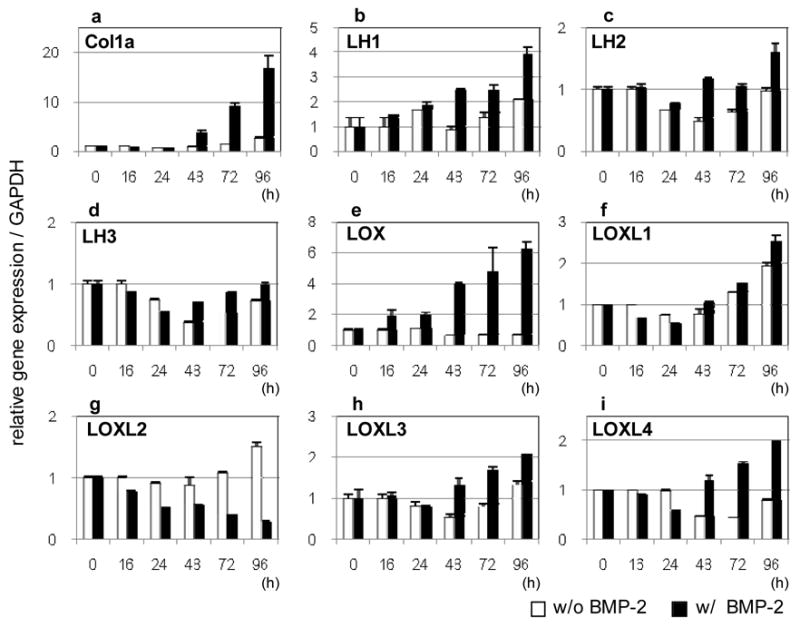
mRNA expression of type 1 collagen and collagen modifying enzymes with or without rhBMP-2 treatment at various time points analyzed by real-time PCR (ai). After 24 hours, only LOXL2 expression was decreased while the others were increased with time. Note that LH2 variants (LH2a and b) were not distinguished by this assay (see Fig 3). See text for the description of each abbreviation.
Expression of LH2 alternative splicing variant
Both LH2 splicing variants, LH2a and b, were expressed in C2C12 cells without rhBMP-2 treatment and the levels did not significantly change up to 96 hours of culture. With the treatment, however, LH2b expression was significantly enhanced while LH2a markedly diminished (Fig. 3). These opposite effects may explain a moderate increase of LH2 expression where both isoforms were not distinguished (Fig 2c).
Fig. 3.
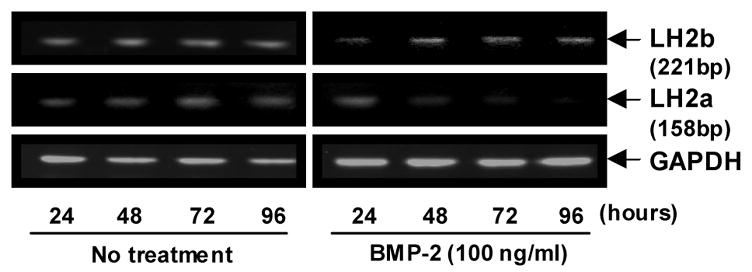
mRNA expression of LH2a and LH2b splicing variants in response to rhBMP-2 analyzed by RT-PCR. LH2a, identified as a 158 bp PCR product was decreased with rhBMP-2 treatment while LH2b, identified as a 221 bp fragment, increased with time.
Amino acid analysis
In the untreated cells, Hyp and Hyl were undetectable (<0.05 nmol) both at 7 and 14 days. However, with rhBMP-2 treatment, both amino acids became detectable at day 7 and significantly increased at day 14. The ratio of Hyl to Hyp was also significantly increased by ~2 fold at day 14 in comparison to that of day 7 (Table 1).
Table 1.
Hydroxyproline and hydroxylysine in the cell/matrix layer
| day | 7 | 7 | 14 | 14 |
|---|---|---|---|---|
| rhBMP-2 | − | + | − | + |
| Hyp* | nd | 1.72±0.28 | nd | 3.55±0.14 |
| Hyl * | nd | 0.14±0.01 | nd | 0.51±0.05 |
| Hyl/Hyp (%) | − | 8.4±0.8 | − | 14.2±0.1 |
Values of Hyp and Hyl are shown as res/1,000 of total amino acids.
nd; not detectable, (< 0.05 nmol)
Discussion
Pre-myoblast cell line, C2C12 cells, transdifferentiate from myoblasts to osteoblasts with rhBMP-2 treatment by changing the gene expression phenotype [11, 12, 16]. Balint et al [12] described the response of C2C12 cells to rhBMP-2 at four stages, 1. activation and repression of non-osteogenic developmental systems (1–4 h), 2. remodeling cell architecture (4–8 h), 3. commitment to osteogenesis (8–16 h) and 4. establishment of the bone phenotype (16–24 h). Myogenic genes were well expressed at stage 1, osteoblastic transcriptional factors, i.e. Cbfa1, OSX at stage 3, major extracellular matrix proteins, i.e. type I collagen, biglycan, decorin at stage 4. Though the genetic responses of myogenic and osteogenic genes were somewhat slower in our study than those reported by Balint et al (e.g. significant increases in Col1a1, Akp2, expression were observed only at 48 hours after treatment), the overall pattern and order of expression were similar. The delayed response may be due to the differences in culture conditions, e.g. rhBMP-2 concentrations (100 ng/ml vs. 300 ng/ml), means of assay (real-time PCR vs. microarray), etc.
It has now become clear that for a single post-translational modification of collagen such as lysine hydroxylation or oxidative deamination, several isoforms of the specific enzyme group can be involved [17]. The differential gene expression of various isoforms may lead to the well-documented tissue specific cross-linking pattern [1]. Though certain cytokines/growth factors have been demonstrated to regulate gene expression/protein synthesis of LH and LOX [18–21], the molecular events and regulatory mechanisms for their isoforms are still poorly understood. The present study represents, to the best to our knowledge, the first to demonstrate the response of the known LH and LOX isoforms to rhBMP-2 induced osteoblast differentiation.
After 24 hours of rhBMP-2 treatment, the expression of most isoforms of LH and LOX, except LH2a and LOXL2, were upregulated suggesting their positive involvement in the process of early osteoblast differentiation. It is of interest to note that the expression levels of both LH2a and LOXL2 are very low or undetectable in differentiating MC3T3-E1 osteoblast [8, 10]. The LOXL2 mRNA expression is also downregulated in bone marrow stromal cells during differentiation [22]. Though the functions of LH2a and LOXL2 in osteoblast are still not well understood, these particular forms of LH and LOXL may be associated with certain cell types or pathological states [23, 24] but likely not with osteoblast phenotypes. It is also of interest to note that both LH1 and LOX genes were upregulated by rhBMP-2 treatment as early as 16 hours of treatment. These are significantly earlier than that of type I collagen, i.e. 48 hours, suggesting that those collagen modifying enzymes may need to be synthesized before collagen itself. The properly modified collagen then forms the functional fibrils in the respective tissues, thus, underscoring the importance of collagen modifications.
In this study, we also analyzed the extent of Hyl and Hyp in the cell/matrix layer. Both amino acids were not detectable at day 7 and 14 in the untreated cells. However, in the treated cells, both were detected at day 7 and significantly increased at day 14. This increase was not detected in the absence of ascorbic acid (data not shown) indicating a critical role of ascorbic acid in collagen biosynthesis [25]. Interestingly, the ratio of Hyl to Hyp was significantly increased from day 7 (8.4%) to 14 (14.2%) indicating that the extent of Lys hydroxylation of collagen synthesized was increased during this period. The ratio at day 14 is comparable to that of bone collagen [26]. The data also indicate that it requires some time for transdifferentiating cells to deposit collagen matrix with appropriate post-translational modifications. Due to the limited quantity of collagen, collagen cross-links were not measured.
Taken together, the results of this study indicate that the genes of collagen modifying enzymes, groups of LH and LOX/LOXL, are differentially responsive to BMP-2 that may lead to specific cross-linking pattern of collagen important for bone formation.
Acknowledgments
This study was supported by NIH grants DE10489 and AR052824.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamauchi M. Collagen Biochemistry: An Overview. World Scientific Publishing; 2002. [Google Scholar]
- 2.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi M, Katz EP. The post-translational chemistry and molecular packing of mineralizing tendon collagens. Connect Tissue Res. 1993;29:81–98. doi: 10.3109/03008209309014236. [DOI] [PubMed] [Google Scholar]
- 4.Bank RA, Tekoppele JM, Janus GJ, Wassen MH, Pruijs HE, Van der Sluijs HA, Sakkers RJ. Pyridinium cross-links in bone of patients with osteogenesis imperfecta: evidence of a normal intrafibrillar collagen packing. J Bone Miner Res. 2000;15:1330–1336. doi: 10.1359/jbmr.2000.15.7.1330. [DOI] [PubMed] [Google Scholar]
- 5.Otsubo K, Katz EP, Mechanic GL, Yamauchi M. Cross-linking connectivity in bone collagen fibrils: the COOH-terminal locus of free aldehyde. Biochemistry. 1992;31:396–402. doi: 10.1021/bi00117a013. [DOI] [PubMed] [Google Scholar]
- 6.Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol Genet Metab. 2000;71:212–224. doi: 10.1006/mgme.2000.3076. [DOI] [PubMed] [Google Scholar]
- 7.van der Slot AJ, Zuurmond AM, Bardoel AF, Wijmenga C, Pruijs HE, Sillence DO, Brinckmann J, Abraham DJ, Black CM, Verzijl N, DeGroot J, Hanemaaijer R, TeKoppele JM, Huizinga TW, Bank RA. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 8.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J Bone Miner Res. 2004;19:1349–1355. doi: 10.1359/JBMR.040323. [DOI] [PubMed] [Google Scholar]
- 9.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Overexpression of lysyl hydroxylase-2b leads to defective collagen fibrillogenesis and matrix mineralization. J Bone Miner Res. 2005;20:81–87. doi: 10.1359/JBMR.041026. [DOI] [PubMed] [Google Scholar]
- 10.Atsawasuwan P, Mochida Y, Parisuthiman D, Yamauchi M. Expression of lysyl oxidase isoforms in MC3T3-E1 osteoblastic cells. Biochem Biophys Res Commun. 2005;327:1042–1046. doi: 10.1016/j.bbrc.2004.12.119. [DOI] [PubMed] [Google Scholar]
- 11.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balint E, Lapointe D, Drissi H, van der Meijden C, Young DW, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Phenotype discovery by gene expression profiling: mapping of biological processes linked to BMP-2-mediated osteoblast differentiation. J Cell Biochem. 2003;89:401–426. doi: 10.1002/jcb.10515. [DOI] [PubMed] [Google Scholar]
- 13.Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi M, Shiiba M. Lysine hydroxylation and crosslinking of collagen. Methods Mol Biol. 2002;194:277–290. doi: 10.1385/1-59259-181-7:277. [DOI] [PubMed] [Google Scholar]
- 16.Korchynskyi O, Dechering KJ, Sijbers AM, Olijve W, ten Dijke P. Gene array analysis of bone morphogenetic protein type I receptor-induced osteoblast differentiation. J Bone Miner Res. 2003;18:1177–1185. doi: 10.1359/jbmr.2003.18.7.1177. [DOI] [PubMed] [Google Scholar]
- 17.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Koslowski R, Seidel D, Kuhlisch E, Knoch KP. Evidence for the involvement of TGF-beta and PDGF in the regulation of prolyl 4-hydroxylase and lysyloxidase in cultured rat lung fibroblasts. Exp Toxicol Pathol. 2003;55:257–264. doi: 10.1078/0940-2993-00323. [DOI] [PubMed] [Google Scholar]
- 19.Palamakumbura AH, Sommer P, Trackman PC. Autocrine growth factor regulation of lysyl oxidase expression in transformed fibroblasts. J Biol Chem. 2003;278:30781–30787. doi: 10.1074/jbc.M305238200. [DOI] [PubMed] [Google Scholar]
- 20.van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MM, Middelkoop E, Boers W, Karel Ronday H, DeGroot J, Huizinga TW, Bank RA. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23:251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 21.van der Slot AJ, van Dura EA, de Wit EC, De Groot J, Huizinga TW, Bank RA, Zuurmond AM. Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. Biochim Biophys Acta. 2005;1741:95–102. doi: 10.1016/j.bbadis.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Monticone M, Liu Y, Tonachini L, Mastrogiacomo M, Parodi S, Quarto R, Cancedda R, Castagnola P. Gene expression profile of human bone marrow stromal cells determined by restriction fragment differential display analysis. J Cell Biochem. 2004;92:733–744. doi: 10.1002/jcb.20120. [DOI] [PubMed] [Google Scholar]
- 23.Salo AM, Sipila L, Sormunen R, Ruotsalainen H, Vainio S, Myllyla R. The lysyl hydroxylase isoforms are widely expressed during mouse embryogenesis, but obtain tissue- and cell-specific patterns in the adult. Matrix Biol. 2006 doi: 10.1016/j.matbio.2006.08.260. [DOI] [PubMed] [Google Scholar]
- 24.Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB, Neufeld G. Abnormal deposition of collagen around hepatocytes in Wilson's disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol. 2005;43:499–507. doi: 10.1016/j.jhep.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78:2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiiba M, Arnaud SB, Tanzawa H, Kitamura E, Yamauchi M. Regional alterations of type I collagen in rat tibia induced by skeletal unloading. J Bone Miner Res. 2002;17:1639–1645. doi: 10.1359/jbmr.2002.17.9.1639. [DOI] [PubMed] [Google Scholar]


