Abstract
Background
We have shown recently that α2β1 integrin-mediated type I collagen adhesion promotes a more malignant phenotype in pancreatic cancer cell lines than other extracellular matrix (ECM) proteins. MiaPaCa-2 cells, by contrast, do not express collagen-binding integrins, but are metastatic in our orthotopic mouse model and migrate maximally on laminin-1 (Ln-1). It has also been shown that CXCR4 and IL-8 expression correlates directly with metastasis in pancreatic cancer in vivo. We therefore examined the potential of the ECM to regulate CXCR4 and IL-8 expression in pancreatic cancer cells.
Methods
We cultured 8 pancreatic cancer cell lines on fibronectin (Fn), types I and IV collagen, Ln-1 and vitronectin (Vn), and examined cell lysates for CXCR4 by immunoblotting and media for IL-8 by ELISA. We also conducted cell migration assays with stromal-derived factor-1 (SDF-1) as the chemoattractant to examine integrin-binding specificity and CXCR4 function.
Results
All cell lines expressed CXCR4 protein. MiaPaCa-2 cell growth on Ln-1 increased significantly CXCR4 and IL-8 expression relative to other ECM proteins. Migration inhibition studies showed that both the α6β1 and α3β1 integrins mediate MiaPaCa-2 migration on Ln-1.Growth studies showed further that CXCR4 expression on Ln-1 was mediated by the α6β1 integrin whereas IL-8 expression was mediated by both the α6β1 and α3β1 integrins. The expression of functional CXCR4 was also shown in migration assays, where SDF-1 significantly increased pancreatic cancer cell chemotaxis on Ln-1.
Conclusions
These data indicate that integrin-mediated Ln-1 adhesion upregulates CXCR4 and IL-8 expression and may play a mechanistic role in pancreatic cancer metastases.
Pancreatic adenocarcinoma is characterized by a hallmark desmoplastic response that includes extensive proliferation of stromal fibroblasts and deposition of extracellular matrix (ECM).1 The ECM, interacting with cells through cell adhesion receptors, in particular the integrins, has been shown to be a critical regulator of important cell processes such as angiogenesis, mitogenesis, migration, and differentiation.2 We have shown recently in two-and three-dimensional in vitro models that α2β1 integrin-mediated adhesion of pancreatic cancer cell lines to type I collagen, shown to be highly upregulated in pancreatic adenocarcinoma,3-10 up-regulates cell proliferation and haptokinetic migration, a measure of an ECM protein’s inherent ability to promote cell movement in the absence of a chemotactic gradient.11-15 These results support the involvement of α2β1 integrin-mediated adhesion to type I collagen in the generation of the malignant phenotype in pancreatic cancer. Similarly, several recent independent studies have also shown in vitro and in vivo that pancreatic cancer cells stimulate increased expression of type I collagen in pancreatic stellate cells and that this increased type I collagen expression promotes the malignant phenotype in cancer cells as defined by increased proliferation, resistance to chemically-induced apoptosis, and tumorigenesis.16,17
It has also been reported, however, that the α6β1 integrin is overexpressed in pancreatic cancer in vivo,3-6,8 and that α6β1 integrin binding to Ln-1 plays an important role in pancreatic cancer metastasis formation both in vitro and in vivo.18 As part of our recent studies examining the role of the ECM in the promotion of the malignant phenotype in pancreatic cancer, we identified 2 distinct subsets of pancreatic cancer cell lines; those more undifferentiated, including MiaPaCa-2, AsPC-1 and Panc-1, that exhibit maximal haptokinesis on Ln-1; and those more differentiated, including FG, Colo-357, CFPAC, Capan-1, and BxPC-3, that migrate maximally on type I collagen.11 The mechanisms regulating these 2 distinct pancreatic cancer cell phenotypes and their relevance in vivo are unclear.
Chemokines are members of the small molecule chemoattractant cytokine family that interact with target cells through G-protein-coupled receptors, designated CXCR or CCR, corresponding to their chemokine ligands.19 CXCR4 in particular, whose ligand is SDF-1, has been shown to be upregulated in several types of cancer, including oral squamous cell carcinoma, breast, ovary, prostate, kidney, brain, lung, thyroid, and pancreas.20-23 This up-regulated CXCR4 chemokine receptor expression in pancreatic cancer is associated with increased cell survival, proliferation, migration, invasion, and metastasis.
IL-8 belongs to the superfamily of CXC chemokines with known proinflammatory effects.24 IL-8 has been shown to be upregulated in response to TGFβ1 signaling25 and to be expressed in pancreatic cancer, also correlating with increased tumorigenic and metastatic potential.24,26 We have shown recently in multiple pancreatic cancer cell lines that adhesion to Fn upregulates whereas adhesion to type I collagen downregulates IL-8 expression in vitro.12,14
In the present study, we examined the potential of the ECM to regulate the expression of CXCR4 and IL-8 in 8 pancreatic cancer cell lines derived from all 3 tumor histological grades and from both primary and metastatic lesions. Our results indicate that CXCR4 protein is expressed to varying degrees by all cell lines tested. The highly undifferentiated MiaPaCa-2 cell line, expressing the lowest levels of CXCR4 and showing maximal α6β1 and α3β1 integrin-mediated migration on Ln-1, could be induced to upregulate CXCR4 expression significantly after growth on Ln-1. CXCR4 expression seemed to be regulated, at least in part, by the α6β1 integrin. By contrast, IL-8 cytokine expression was also upregulated after growth of MiaPaCa-2 cells on Ln-1, but both the α3β1 and α3β1 integrins seem to play regulatory roles in the expression of this cytokine. These data indicate that integrin-mediated upregulation of CXCR4 and IL-8 expression on Ln-1 in pancreatic cancer cells occurs through distinct mechanisms and may play a role in pancreatic cancer metastasis.
MATERIALS AND METHODS
Cell culture
Pancreatic adenocarcinoma cells lines, Capan-1, CFPAC, Colo-357, AsPC-1, BxPC-3, MiaPaCa-2, and Panc-1 were obtained from the American Type Culture Collection (Rockville, MD). FG cells13,14 are a fast-growing, metastatic variant of the Colo-357 cell line. All cell lines were cultured in DMEM supplemented with 10% FBS in a humidified atmosphere containing 5% CO2 at 37°C.
ECM proteins and antibodies
Human Fn and vitronectin (Vn), bovine type I collagen, and mouse Ln-1 were purchased from Chemicon International (Temecula, CA). The function-blocking monoclonal antibodies, GoH3, directed against the α6 integrin subunit, P1B5, directed against the α3 integrin subunit, P5D2, directed against the β1 integrin subunit, LM609, directed against αvβ3, P1F6, directed against αvβ5 ASC-3, directed against β4 integrin subunit, and P1D6, directed against the α5 integrin subunit were also purchased from Chemicon, and they have been described.27-33
Cell culture assays
Pancreatic cancer cell lines were serum-starved 24 hours before assay in DMEM supplemented with 1 mg/ml BSA (Sigma, St. Louis, MO). Culture plates (6-well) (Becton Dickinson, Franklin Lanes, NJ), not treated for tissue culture, were coated with ECM proteins at the following concentrations: MiaPaCa-2 cells, Ln-1 at 15 μg/ml, Fn at 25 μg/ml, type I collagen at 5 μg/ml, and Vn at 10 μg/ml; FG cells, Fn at 25 μg/ml, type I collagen at 3 μg/ml, Ln-1 at 10 μg/ml, and Vn at 10 μg/ml; BxPC-3 cells, Fn at 15 μg/ml, type I collagen at 5 μg/ml, Ln-1 at 15 μg/ml, and Vn at 15 μg/ml; AsPC-1 cells, Fn at 25 μg/ml, type I collagen at 5 μg/ml, Ln-1 at 15 μg/ml, and Vn at 5 μg/ml; and CFPAC cells, Fn at 25 μg/ml, type I collagen at 5 μg/ml, Ln-1 at 25 μg/ml, and Vn at 10 μg/ml. These ECM coating concentrations have been shown previously to promote optimal cell adhesion and proliferation for each cell line on each specified ECM protein.11 The ECM-coated wells were then washed twice with PBS and blocked with 1 mg/ml BSA in PBS for 1 hour at 37°C. Cells were trypsinized, treated with 1 mg/ml soybean trypsin inhibitor (Sigma, St. Louis, MO), and washed 3 times with PBS. Cells were resuspended in serum-free medium and seeded at 2.5 ×105/ml, 2 ml/well. Cultures were then incubated at 37°C and harvested after 96 hours, or daily over a 4-day time course.
Immunoblotting
At the indicated time points, cultures were harvested. Cell extracts were prepared by adding 1 ml lysis buffer (250 mM Tris, pH 7.4, 0.25% Nonidet P-40, 2 mM EDTA)/well of a 6-well plate, gentle pipetting to collect the lysate, centrifugation for 15 minutes at 16,000 × g, and transfer of the supernatant to a fresh tube. Total cell protein was then measured using a modified Bradford protein assay with BSA as the standard (Bio-Rad Laboratories, Hercules, CA). Three micrograms of total protein was separated for each lysate on each ECM substrate and transferred to nitrocellulose using standard electrophoretic techniques. Membranes were exposed subsequently to goat or rabbit polyclonal antibodies (Clones G-19, C-20, or H-118) (Santa Cruz Biotechnology, Santa Cruz, CA) raised against CXCR4, each at 1:500 for 2 hours at RT. After washing in PBS/0.1% Tween, the membranes were incubated subsequently with the appropriate HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) at 1:1,000 dilution for 1 hour at RT. Peroxidase activity was detected with chemiluminescence (Amersham Biosciences, Buckinghamshire, England).
Densitometry
Immunoblotting results were analyzed quantitatively with a digital imaging system (Alpha Innotech, San Leandro, CA). The intensities of the bands were assigned integrated density values, which represent the sum of all pixels in the box. All bands were quantified in equal area boxes and compared with β-actin controls to generate a ratio. Relative expression values for Ln-1, Fn, and Vn were generated by comparing these ratios to those obtained for type I collagen, which was arbitrarily assigned a value of 1. For antibody inhibition studies, the control lysate, grown in the absence of antibody for 96 hours, was arbitrarily assigned a value of 1. For time course studies, T = 0 cell lysates were arbitrarily assigned a value of 1.
Cytokine ELISA
To assess IL-8 levels, media from the cell culture experiments described above were assayed using an IL-8 ELISA according to manufacturer’s recommendations as described previously (BioSource, Camarillo, CA).14 The minimal detection limit was 3 pg/ml, and cytokine levels were normalized to cellular protein.
Migration assays
Migration assays were conducted using the modified Boyden chamber as described previously.34 Thirty microliters of serum-free DMEM supplemented with 1 mg/ml BSA were added to each lower chamber well. Pore polycarbonate membrane filters (8-μm) (Neuro Probe, Gaithersburg, MD) that were coated previously with Ln-1 at 5 μg/ml were placed on top of the lower chambers, and the upper chambers were secured in place. Upper chambers were filled with 5 × 104 MiaPaCa-2, FG, BxPC-3, CFPAC, or AsPC-1 cells that were serum-starved 24 hours before assay in the same serum-free media described above. Lower chamber final volumes were 30 μl and the upper chambers were 50 μl. The entire apparatus was then incubated for 24 hours at 37°C. Migratory cells on the underside of the filters were quantitated by counting 4 high-powered fields (100×magnification) per well using an inverted light microscope (Olympus BH 2). For inhibition of haptokinesis on Ln-1, function-blocking monoclonal antibodies directed against specific integrin subunits were added to the upper chamber at the same time the cells were added at a final concentration of 25 μg/ml. For chemotaxis experiments, SDF-1 (R&D Systems, Minneapolis, MN) was added to the lower chambers at the indicated concentrations.
Statistics
Statistical significance was determined using one- or two-tailed Student t tests.
RESULTS
Pancreatic cancer cell lines express the CXCR4 chemokine receptor
Eight pancreatic cancer cell lines derived from all 3 histologic tumor grades were examined for CXCR4 chemokine receptor expression by immunoblotting after growth on tissue culture plastic in complete DMEM. Cell lines included Capan-1, CFPAC, Colo-357, FG, AsPC-1, BxPC-3, MiaPaCa-2, and Panc-1. Figure 1A shows that all cell lines expressed CXCR4 at the protein level. These results were confirmed with 2 other polyclonal antisera raised against CXCR4 (data not shown).
Fig 1.
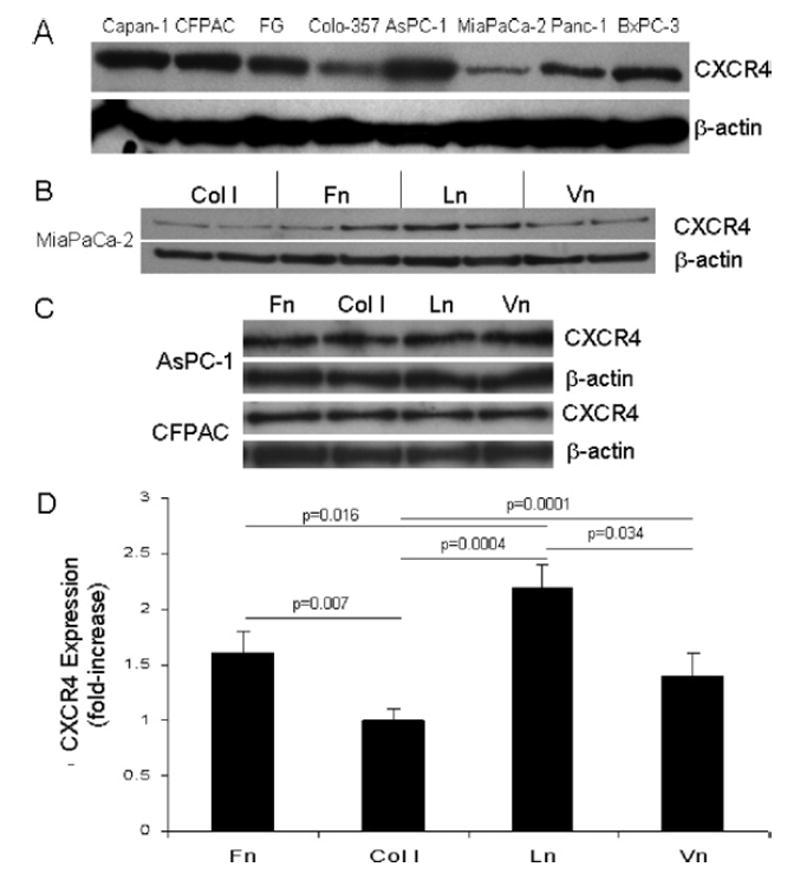
Pancreatic cancer cell lines express the CXCR4 chemokine receptor, and the ECM differentially regulates CXCR4 expression in MiaPaCa-2 pancreatic cancer cells. A, The indicated pancreatic cancer cell lines were grown on tissue culture plastic in DMEM supplemented with 10% FBS, lysates were prepared, and total protein determined as described in Materials and Methods. Ten micrograms of total protein were separated subsequently on 10% NuPage gels under reducing conditions and transferred to nitrocellulose using standard electrophoretic techniques. Membranes were exposed to polyclonal antisera (Clone G19; Santa Cruz Biotechnology) raised against CXCR4, followed by horseradish peroxidase-conjugated secondary antibody. The peroxidase was then detected using chemiluminescence according to manufacturer’s instructions (ECL kit; Amersham), and autoradiography. Membranes were also exposed to a monoclonal anti-β-actin antibody (Clone AC-15; Sigma), followed by horseradish peroxidase-conjugated secondary (Jackson ImmunoResearch Labs), and developed as described above. B, MiaPaCa-2 cells were cultured for 96 hours on Fn, type I collagen, Ln-1, or Vn, and total cell lysates were analyzed subsequently by immunoblotting for CXCR4 expression as described in Materials and Methods. β-actin immunoblotting results are also shown as a control for protein loading and for subsequent densitometry analyses. C, AsPC-1 and CFPAC cells were cultured for 96 hours on the indicated ECM proteins and analyzed by immunoblotting for CXCR4 expression as described. D, Immunoblotting results described in (B) above for CXCR4 expression in MiaPaCa-2 cells from 2 to 5 independent experiments conducted in triplicate for each substrate were analyzed by densitometry, and are expressed as fold-increase over type I collagen, which was arbitrarily assigned a value of 1. Significant differences in CXCR4 expression were observed between all substrates as indicated except in comparisons between Fn and Vn.
ECM differentially regulates CXCR4 expression in MiaPaCa-2 pancreatic cancer cells
We next examined the regulatory role of the ECM on CXCR4 expression. Ninety-six hour growth assays were conducted under serum-free conditions with MiaPaCa-2, AsPC-1, CFPAC, BxPC-3, and FG cells on type I collagen, Ln-1, Fn, and Vn using those concentrations of each ECM protein previously shown to promote maximal adhesion and proliferation for each cell line.11 Cell lysates were analyzed subsequently by immunoblotting for CXCR4 expression. Figure 1B shows that in MiaPaCa-2 cells, growth on the ECM regulated the expression of CXCR4 differentially. Relative to each other, growth of MiaPaCa-2 cells on Ln-1 resulted in the highest, type I collagen the lowest, and Fn and Vn were intermediate in the expression of CXCR4. By contrast, Fig 1C shows that the ECM had no effect on the expression of CXCR4 in AsPC-1 or CFPAC cells. Similar results were also observed for BxPC-3 and FG cells (not shown). Densitometric analyses confirmed that growth of MiaPaCa-2 cells on Ln-1 resulted in a 2-fold increase in CXCR4 expression compared with control type I collagen substrates (Fig 1D), where no adhesion, proliferation, or migration occur with MiaPaCa-2 cells because they express no collagen-binding integrins.11,12,35,36 Statistically significant differences in CXCR4 expression in MiaPaCa-2 cells were noted for all ECM substrates except in direct comparisons between Fn and Vn.
ECM differentially regulates IL-8 secretion in MiaPaCa-2 and AsPC-1 pancreatic cancer cells
We next examined MiaPaCa-2, AsPC-1, CFPAC, and BxPC-3 cells grown on Fn, type I collagen, Ln-1, and Vn for IL-8 secretion after 96 hours in culture under serum-free conditions. Figure 2 shows that the more undifferentiated cell lines, notably MiaPaCa-2 and AsPC-1, showed statistically significant Ln-1-mediated upregulation of IL-8, similar to that shown for CXCR4 expression in MiaPaCa-2 cells. The more differentiated cell lines, notably CFPAC and BxPC-3, were not affected with regard to IL-8 expression. Whereas CXCR4 expression in AsPC-1 cells was not affected by growth on Ln-1, IL-8 expression was upregulated compared with the other ECM proteins tested. These data are also consistent with our previously published results with these cell lines in direct comparisons of IL-8 expression between Fn and type I collagen.12,14
Fig 2.

ECM regulates the expression of IL-8 in pancreatic cancer cell lines. MiaPaCa-2, AsPC-1, CFPAC, and BxPC-3 cells were cultured for 96 hours on Fn, type I collagen, Ln-1, or Vn, under serum-free conditions as described in Materials and Methods, and conditioned media were subsequently analyzed by ELISA for IL-8 expression. Results presented were normalized to total cellular protein, expressed as % Max for each cell line, and represent the mean ± SEM from 2 to 5 experiments with duplicate determinations. Statistically significant differences were noted in MiaPaCa-2 and AsPC-1 cells in comparisons between all substrates except Fn and Vn as indicated. Significant differences were also noted in CFPAC cells as indicated between Fn and both Ln and Vn, and between type I collagen and Ln. No significant differences were noted in BxPC-3 cells.
α6β1 Integrin mediates MiaPaCa-2 haptokinesis on Ln-1 substrates
We have shown recently that MiaPaCa-2 cells exhibit maximal haptokinesis on Ln-1 compared with types I and IV collagen, Fn or Vn11; the integrins mediating this haptokinesis were not identified, however. Using function-blocking monoclonal antibodies directed against specific integrins and integrin subunits, we conducted migration inhibition assays on Ln-1 using the modified Boyden chamber. Figure 3 shows that antibodies directed against the α6 and β1 integrin subunits inhibited greater than 90% of MiaPaCa-2 cell migration on Ln-1, and that antibodies directed against the α3 integrin subunit also inhibited haptokinesis on Ln-1, though to a lesser extent (about 40%). These data collectively indicate that the α6β1and α3β1 integrins mediate MiaPaCa-2 migration on Ln-1.
Fig 3.

The α6β1 and α3β1 integrins mediate MiaPaCa-2 haptokinesis on Ln-1 substrates. Filters (8-μm pores) were coated with Ln-1 at 5 μg/ml and 5 × 104 MiaPaCa-2 cells/well were examined for haptokinetic migration under serum-free conditions using the modified Boyden chamber in the presence or absence of 25 μg/ml anti-integrin function-blocking monoclonal antibodies as described. Data presented are the mean ± SEM from 3 experiments, with 6 replicates per treatment per experiment. Significant differences compared with untreated control wells are indicated.
α6β1 Integrin mediates upregulated expression of CXCR4 on Ln-1 substrates in MiaPaCa-2 cells
Because both α6β1 and α3β1 integrins are involved in MiaPaCa-2 haptokinesis on Ln-1, we were interested to know which of these integrins mediated the upregulated expression of CXCR4 and IL-8. We therefore conducted 96-hour growth assays with MiaPaCa-2 cells on Ln-1 in the presence of function-blocking monoclonal antibodies directed against the α6, α3 and α2 integrin subunits and examined cell lysates for CXCR4 expression by immunoblotting and conditioned medium for IL-8 secretion by ELISA. The densitometry results shown in Fig 4A show that blocking the α6 integrin subunit at antibody concentrations sufficient to inhibit greater than 90% of cell migration (Fig 3) reduced significantly the expression of CXCR4 by approximately 25% compared with the α3 and α2 integrin subunits, which had no effect on CXCR4 expression. The time course results shown in Fig 4B show further that CXCR4 expression is upregulated significantly by 72 hours after seeding cells on Ln-1 substrates compared with tissue culture plastic.
Fig 4.
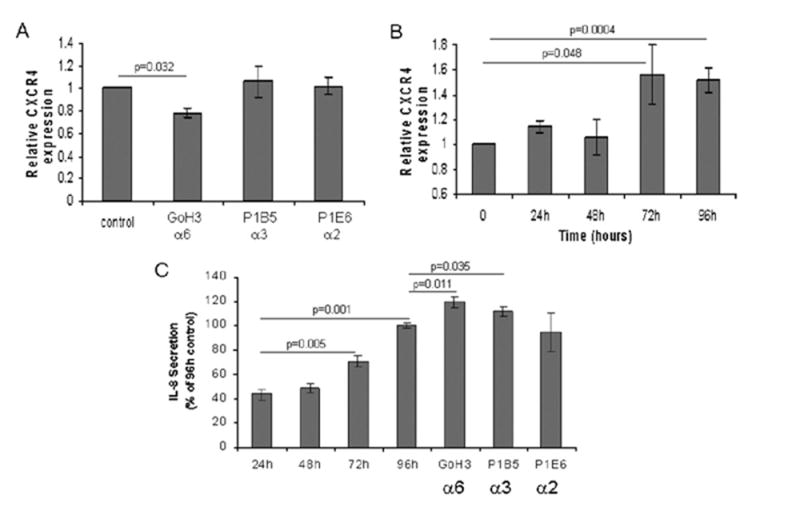
The α6β1 integrin regulates CXCR4 and both the α6β1 and α3β1 integrins regulate IL-8 expression on Ln-1 substrates in MiaPaCa-2 cells. A, MiaPaCa-2 cells were cultured on Ln-1 substrates for 96 hours under serum-free conditions in the presence or absence of anti-integrin function blocking monoclonal antibodies directed against the α6, α3, and α2 subunits at 25 μg/ml as described in Materials and Methods. Lysates were then prepared, protein was determined, and 3 μg total protein per treatment group were examined for CXCR4 expression by immunoblotting and densitometry as described in Fig 1 and Materials and Methods. Densitometry data presented represent the mean ± SEM from two independent experiments conducted in duplicate for each treatment group. The antibody clones and the integrin subunits they are directed against are shown on the x-axis. Significant differences from the 96-hour untreated control cultures are indicated. B, MiaPaCa-2 cells were grown on Ln-1 substrates as described in Materials and Methods over a 96-hour time course, and harvested at the indicated time points. Cell lysates were prepared and analyzed by immunoblotting and subsequent densitometry for CXCR4 expression. Densitometry results represent the mean ± SEM from 2 independent experiments with duplicate wells per time point per experiment. Significant differences from the tissue culture plastic controls (T = 0) harvested at the time of initial cell seeding are indicated. C, Conditioned culture media from MiaPaCa-2 cells grown on Ln-1 over a 96-hour time course as described above were analyzed by ELISA for IL-8 expression. Results presented represent the mean ± SEM from 2 independent experiments conducted in duplicate. Statistically significant differences in comparisons to 24-hour IL-8 expression levels are indicated, as are significant differences in cells treated with monoclonal antibodies compared with 96-hour untreated control cultures.
Interestingly, Fig 4C shows that, whereas the α6 antibody inhibited CXCR4 expression, both the α6 and α3 antibodies upregulated significantly the expression of IL-8 after 96 hours in culture compared with the α2 antibody or no antibody-control cultures. Figure 4C also shows that, like CXCR4, IL-8 expression is upregulated on Ln-1 in a time-dependent manner, with significant increases observed after 72 hours.
Stromal-derived factor-1 increases MiaPaCa-2 cell migration on Ln-1 substrates
To determine whether CXCR4 was functional on MiaPaCa-2 cells and whether stromal-derived factor-1 (SDF-1), the ligand for CXCR4, stimulated chemotaxis in pancreatic cancer cell lines on Ln-1 substrates, we conducted chemotaxis assays with SDF-1 at 100 ng/ml. This dose has been shown previously to be effective in stimulating increased chemotaxis of pancreatic cancer cell lines on Fn.21,23 Figure 5A shows that SDF-1 increased migration significantly on Ln-1 substrates using FG, MiaPaCa-2, and CFPAC cells. BxPC-3 and AsPC-1 cells exhibited a similar trend, although statistical significance was not achieved. Figure 5B shows further that MiaPaCa-2 cell migration on Ln-1 substrates was responsive to SDF-1 in a dose-dependent manner, with maximal migration occurring at concentrations ranging between about 400 to 800 ng/ml. These data indicate that the CXCR4 receptor expressed in MiaPaCa-2 cells is functional and that SDF-1 promotes increased migration of pancreatic cancer cell lines on Ln-1 substrates.
Fig 5.

SDF-1 increases pancreatic cancer cell migration on Ln-1 substrates in a dose-dependent manner. A, Chemotactic migration assays were conducted on Ln-1 substrates under serum-free conditions using modified Boyden chambers in the presence or absence of SDF-1 (100 ng/ml) as the chemoattractant as described in Materials and Methods. MiaPaCa-2, FG, BxPC-3, CFPAC, or AsPC-1 cells were added to the upper chambers at 5 × 104/well and the entire apparatus was incubated at 37°C for 24 hours. Data presented are expressed as % Max, with BxPC-3 exhibiting maximal migration, and represent the mean ± SEM from 2 independent experiments with 4 replicates per treatment group. Black bars, with SDF-1; gray bars, without SDF-1. Significant differences are indicated. B, An SDF-1 dose-response curve was generated using chemotaxis assays as described above and in Materials and Methods with MiaPaCa-2 cells on Ln-1 substrates. The SDF-1 concentrations are indicated on the x-axis. Data presented are expressed as % Max, and represent the mean ± SEM from 2 independent experiments with 6 replicates per treatment group per experiment.
DISCUSSION
In the present study, we show that all 8 pancreatic cancer cell lines tested express CXCR4, including MiaPaCa-2. Compared with FG, AsPC-1, CFPAC and BxPC-3 cells, however, where no ECM-mediated differences were observed, growth of MiaPaCa-2 cells on Ln-1 resulted in significantly increased expression of CXCR4 relative to Fn, Vn, or type I collagen. IL-8 expression, by contrast, was increased in MiaPaCa-2 and AsPC-1 cells and decreased in BxPC-3 and CFPAC cells grown on Ln-1. Growth and migration inhibition studies using MiaPaCa-2 cells and function-blocking anti-integrin monoclonal antibodies showed further that increased CXCR4 expression on Ln-1 is mediated specifically by the α6β1 integrin, and increased IL-8 expression is mediated by both the α6β1 and α3β1 integrins. This upregulated CXCR4 and IL-8 expression correlated directly with maximal MiaPaCa-2 haptokinesis on Ln-1. The expression of functional CXCR4 in our pancreatic cancer cell lines, and MiaPaCa-2 cells in particular, was shown in migration assays on Ln-1, where stromal-derived factor-1 (SDF-1), the ligand for CXCR4, dose-dependently increased chemotaxis significantly.
Previous immunoblotting studies have shown substantial CXCR4 expression in vitro in CFPAC, Capan-2, AsPC-1, Panc-1, BxPC-3, and SUIT-2 pancreatic cancer cell lines.23 Another study showed expression of CXCR4 at the mRNA level in AsPC-1, BxPC-3, CFPAC, HPAC, and Panc-1 cells.21 In a recent study, MiaPaCa-2 cells were considered to be negative for CXCR4 at the mRNA level using reverse transcription-PCR, though their semi-quantitative real-time PCR data showed slight but elevated mRNA expression relative to the normal pancreatic ductal epithelial cell line, HPDE6.22 Protein expression for CXCR4 in MiaPaCa-2 cells was not shown, however. In the present study, we show that all 8 pancreatic cancer cell lines tested, including MiaPaCa-2, express CXCR4 at the protein level. These data are consistent with in vivo immunohistochemical analyses, where greater than 70% of pancreatic cancer tissues (37 of 52 samples) were positive for CXCR4 expression, with a trend toward increased expression in liver and lymph node metastases.21
We have shown recently that MiaPaCa-2 cells exhibit relatively weak haptokinesis on Fn and Vn, even though they adhere very strongly to those ECM substrates.11 In contrast, whereas MiaPaCa-2 cells adhere only moderately to Ln-1, they migrate maximally on the substrate. Our current studies indicate that migration on Ln-1 is predominantly α6β1 integrin-mediated, with modest activity observed with the α3β1 integrin as well. Our data also indicate that upregulated expression of CXCR4 on Ln-1 substrates in MiaPaCa-2 cells is mediated, at least in part, by the α6β1 integrin. Based on our present findings, it does not seem that the integrin subunits are involved in the regulation of CXCR4 expression in MiaPaCa-2 cells cultured on Ln-1 substrates. Because the anti-α6 antibody, GoH3, used in these studies essentially completely inhibited MiaPaCa-2 cell migration at 25 μg/ml after 24 hours but resulted in only a 25% decrease in CXCR4 expression after 96 hours, we cannot rule out the possibility that other integrin-ECM interactions or other factors not related to the integrin-ECM axis may be upregulated over the longer 96-hour time course, and they may also be involved in the regulation of CXCR4 expression in MiaPaCa-2 pancreatic cancer cells cultured on Ln-1.
The present study also indicates that CXCR4 is functional in MiaPaCa-2 cells, as integrin-mediated migration on Ln-1 can be increased in a dose-dependent manner with the CXCR4 ligand, SDF-1. Optimal chemotaxis on Ln-1 occurred at SDF-1 concentrations between 400 and 800 ng/ml. SDF-1 has been shown previously to increase chemotaxis of AsPC-1, Panc-1, and SUIT-2 cells in vitro at 100 ng/ml, though the ECM substrate was not indicated.23 AsPC-1 cells were also shown to increase chemotaxis on Fn with 100 ng/ml SDF-1.21 Another study indicated that SDF-1 at 300 ng/ml stimulated increased chemotaxis on Fn substrates in CXCR4 positive Hs766T, AsPC-1, A8184, and CFPAC cells, but not in the CXCR4-negative cell lines PT45 and MiaPaCa-2.22 Importantly, our present findings are the first to show in MiaPaCa-2, as well as in other pancreatic cancer cell lines, that SDF-1 stimulates increased CXCR4-dependent chemotaxis on Ln-1 substrates significantly.
It has been shown previously in vivo that antibodies directed against the α6 or β1 integrins inhibited metastasis in an experimental model of pancreatic cancer in nude mice.18,36 It has also been shown that transfection of CXCR4 into human osteosarcoma cells (HOS) conferred a chemotactic response to SDF-1 in vitro and enhanced the growth of HOS transfectants after intradermal injection. Both the chemotactic response and tumor growth in vivo were related directly to the level of CXCR4 expression; antibodies directed against the α2β1 and α5β1 integrins that mediated SDF-1 stimulated migration on type I collagen, Ln-1, and Fn in vitro inhibited tumor growth in SCID mice.37 Another study using small cell lung cancer (SCLC) cells showed that SDF-1 induced integrin activation that resulted in the increased adhesion of SCLC cells to Fn and collagen.38 This increased adhesion was mediated by the α2, α4, α5, and β1 integrins along with CXCR4. The authors thus concluded that activation of β1 integrins and CXCR4 cooperate in mediating adhesion and survival signals from the tumor microenvironment to SCLC cells.38 Our present findings are consistent with these previous reports showing a relationship between CXCR4 and the integrin-ECM axis by showing that, at least in some cases of pancreatic cancer, α6β1 integrin-mediated Ln-1 adhesion upregulates CXCR4 expression.
Expression of IL-8 in pancreatic cancer cell lines, including Capan-1, CFPAC, FG, SG, Colo-357, L3.3, AsPC-1, BxPC-3, MiaPaCa-2, and Panc-1, has been shown recently in several studies.12,14,24,26 These studies showed a trend toward increasing IL-8 expression in cell lines derived from metastatic compared with primary tumor origin. MiaPaCa-2 cells, however, which are derived from a primary tumor, expressed relatively high levels of IL-8 compared with other primary tumor-derived cell lines and to other pancreatic cancer cell lines in general. Consistent with those previous studies, our current results show that adhesion to type I collagen decreases the expression of IL-8 compared with Fn in FG, Colo-357, AsPC-1, and MiaPaCa-2 cells.12 Our present results also extend those previous studies by showing that Ln-1 adhesion increases IL-8 expression in the more undifferentiated cell lines, MiaPaCa-2 and AsPC-1, but not in the more differentiated cell lines, CFPAC and BxPC-3.
Our results show further that function-blocking monoclonal antibodies GoH3 and P1B5 directed against the α6 and α3 integrin subunits, respectively, actually increase the expression of IL-8 in MiaPaCa-2 cells on Ln-1 substrates compared with controls. These results are not unprecedented. Besides their ability to block adhesion, both of these antibodies as well as other anti-integrin function-blocking monoclonal antibodies have been shown to be integrin-activating agonists that promote integrin clustering and result in high-affinity binding and downstream intracellular signaling that mimic ligand function.39 For example, the anti-α3 integrin subunit monoclonal antibody, P1B5, has been shown previously to promote fibroblast lamellipodia formation similar to the natural ligand Ln-540 and to induce tyrosine phosphorylation of focal adhesion kinase in melanoma cells.41 The anti-α6 integrin subunit monoclonal antibody, GoH3, has been shown to completely block IL-8-induced CD8+ and CD4+ lymphocyte migration.42 With regard to anti-integrin antibody-induced stimulation of IL-8 expression, it has been shown previously that ligation of β1- containing integrins with a function-blocking monoclonal antibody directed against the β1 integrin subunit resulted in a 2-fold increase in IL-8 production compared with natural ECM ligands, including Fn, Ln, and type IV collagen, in primary human endometrial stromal cells.43 Similar upregulated IL-8 secretion has also been shown to be induced in primary human monocytes by antibodies directed against β2 intergrins.44 Perhaps most relevant to the present study, upregulated IL-8 secretion of MiaPaCa-2 cells on Fn substrates in the presence of soluble function-blocking monoclonal antibodies directed against the β1, α5, and αv integrin subunits as well as the αvβ5 integrin has been observed previously.45 These results support our present observations, where antibodies directed against the α6 and α3 integrin subunits but not the α2 integrin subunit stimulate IL-8 secretion in MiaPaCa-2 pancreatic cancer cells, indicating that both the α6β1 and α3β1 integrins play functional roles in the upregulation of IL-8 secretion observed on Ln-1 substrates in MiaPaCa-2 cells.
MiaPaCa-2 cells are unusual relative to the other pancreatic cancer cell lines used in these studies in that they express no α1 or α2 integrin subunits and do not adhere to types I or IV collagen.11,36,46 In fact, it has been shown recently that attempts to grow MiaPaCa-2 cells on type I collagen substrates resulted in apoptosis.47 Additionally, MiaPaCa-2 cells do not express E-cadherin.12,48,49 Although this cell line is derived from a primary tumor and is often considered to be non-metastatic,35,36 we have shown in several studies that MiaPaCa-2 cells are metastatic in our orthotopic mouse model of pancreatic cancer.50-54 Specifically, Mia-PaCa-2 tumors produced extensive local disease and metastases to the retroperitoneum (100%), spleen (100%), intestinal and periportal lymph nodes (100%), liver (40%), and diaphragm (80%), and gave rise to malignant ascites and peritoneal carcinomatosis in 80% of cases.
In conclusion, the present studies indicate that integrin-mediated pancreatic cancer cell migration on Ln-1, which includes the upregulation of CXCR4 and IL-8 expression and responsiveness to SDF-1 stimulation, is part of the mechanism promoting the metastatic phenotype in pancreatic cancer, at least in some cases. Future studies will be aimed at inhibition of MiaPaCa-2 tumor growth, progression, and metastasis in our orthotopic mouse models of pancreatic cancer using a combination approach that includes function-blocking monoclonal antibodies directed against CXCR4, and the α6, α3, and β1 integrin subunits. Such combination therapies directed against integrins and other mediators of cell-stroma interactions have shown promising results previously; for example, dual blockade of P-selectin and β2 integrins has been effective in the inhibition of the liver inflammatory response after uncontrolled hemorrhagic shock.55
Acknowledgments
We thank Drs. E. Engvall and K. Vuori for helpful discussions and critical readings of the manuscript, and C. Truong for technical assistance. This work was funded by VA Merit grants from the Department of Veterans Affairs (M.B. and L.J.D.), National Institutes of Health grants DK60588 and AR47347 (L.J.D.), CA109949-01 (MB), American Cancer Society grant RSG-05-037-01-CCE (M.B.), and National Pancreas Foundation grant (M.B.).
Supported by the Department of Veterans Affairs, the National Institutes of Health under contract number CA109949-01, the American Cancer Society under contract number RSG-05-037-01-CCE, and the National Pancreas Foundation.
References
- 1.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 3.Weinel RJ, Rosendahl A, Neumann K, Chaloupka B, Erb D, Rothmund M, et al. Expression and function of VLA-alpha 2, -alpha 3, -alpha 5 and -alpha 6-integrin receptors in pancreatic carcinoma. Int J Cancer. 1992;52:827–33. doi: 10.1002/ijc.2910520526. [DOI] [PubMed] [Google Scholar]
- 4.Rosendahl A, Neumann K, Chaloupka B, Rothmund M, Weinel RJ. Expression and distribution of VLA receptors in the pancreas: an immunohistochemical study. Pancreas. 1993;8:711–8. doi: 10.1097/00006676-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Shimoyama S, Gansauge F, Gansauge S, Oohara T, Beger HG. Altered expression of extracellular matrix molecules and their receptors in chronic pancreatitis and pancreatic adenocarcinoma in comparison with normal pancreas. Int J Pancreatol. 1995;18:227–34. doi: 10.1007/BF02784946. [DOI] [PubMed] [Google Scholar]
- 6.Linder S, Castanos-Velez E, von Rosen A, Biberfeld P. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1321–7. [PubMed] [Google Scholar]
- 7.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–22. [PubMed] [Google Scholar]
- 8.Binkley CE, Zhang L, Greenson JK, Giordano TJ, Kuick R, Misek D, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–63. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Stein WD, Litman T, Fojo T, Bates SE. A serial analysis of gene expression (SAGE) database analysis of chemosensitivity: comparing solid tumors with cell lines and comparing solid tumors from different tissue origins. Cancer Res. 2004;64:2805–16. doi: 10.1158/0008-5472.can-03-3383. [DOI] [PubMed] [Google Scholar]
- 10.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Grzesiak JJ, Bouvet M. The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer. 2006;94:1311–9. doi: 10.1038/sj.bjc.6603088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grzesiak JJ, Smith KC, Chalberg C, Burton DW, Deftos LJ, Bouvet M. Type I collagen and divalent cation shifts disrupt cell-cell adhesion, increase migration, and decrease PTHrP, IL-6, and IL-8 expression in pancreatic cancer cells. Int J Gastrointest Cancer. 2005;36:131–46. doi: 10.1385/IJGC:36:3:131. [DOI] [PubMed] [Google Scholar]
- 13.Grzesiak JJ, Clopton P, Chalberg C, Smith K, Burton DW, Silletti S, et al. The extracellular matrix differentially regulates the expression of PTHrP and the PTH/PTHrP receptor in FG pancreatic cancer cells. Pancreas. 2004;29:85–92. doi: 10.1097/00006676-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ, Bouvet M. GSK3 and PKB/Akt are associated with integrin-mediated regulation of PTHrP, IL-6 and IL-8 expression in FG pancreatic cancer cells. Int J Cancer. 2005;114:522–30. doi: 10.1002/ijc.20748. [DOI] [PubMed] [Google Scholar]
- 15.Grzesiak JJ, Bouvet M. Determination of the ligand binding specificities of the a2b1 and a1b1 integrins in a novel three-dimensional in vitro model of pancreatic cancer. Pancreas. 2007;34:220–28. doi: 10.1097/01.mpa.0000250129.64650.f6. [DOI] [PubMed] [Google Scholar]
- 16.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–21. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–37. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 18.Vogelmann R, Kreuser ED, Adler G, Lutz MP. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer. 1999;80:791–5. doi: 10.1002/(sici)1097-0215(19990301)80:5<791::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 20.Uchida D, Begum NM, Tomizuka Y, Bando T, Almofti A, Yoshida H, et al. Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in human oral squamous cell carcinoma. Lab Invest. 2004;84:1538–46. doi: 10.1038/labinvest.3700190. [DOI] [PubMed] [Google Scholar]
- 21.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–5. [PubMed] [Google Scholar]
- 22.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–7. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 23.Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 24.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711–21. [PubMed] [Google Scholar]
- 25.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Wigmore SJ, Fearon KC, Sangster K, Maingay JP, Garden OJ, Ross JA. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int J Oncol. 2002;21:881–6. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–9. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 28.Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987;105:1873–84. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988;107:1881–91. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wayner EA, Gil SG, Murphy GF, Wilke MS, Carter WG. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J Cell Biol. 1993;121:1141–52. doi: 10.1083/jcb.121.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattaramalai S, Skubitz KM, Skubitz AP. A novel recognition site on laminin for the alpha 3 beta 1 integrin. Exp Cell Res. 1996;222:281–90. doi: 10.1006/excr.1996.0036. [DOI] [PubMed] [Google Scholar]
- 32.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–11. [PubMed] [Google Scholar]
- 33.Hoffstrom BG, Wayner EA. Immunohistochemical techniques to study the extracellular matrix and its receptors. Methods Enzymol. 1994;245:316–46. doi: 10.1016/0076-6879(94)45018-8. [DOI] [PubMed] [Google Scholar]
- 34.Grzesiak JJ, Davis GE, Kirchhofer D, Pierschbacher MD. Regulation of alpha 2 beta 1-mediated fibroblast migration on type I collagen by shifts in the concentrations of extracellular Mg2+ and Ca2+ J Cell Biol. 1992;117:1109–17. doi: 10.1083/jcb.117.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Ma Z, Zhu J, Li K, Wang S, Bie P, Dong J. Laminin-5 promotes cell motility by regulating the function of the integrin alpha6beta1 in pancreatic cancer. Carcinogenesis. 2004;25:2283. doi: 10.1093/carcin/bgh319. [DOI] [PubMed] [Google Scholar]
- 36.Sawai H, Yamamoto M, Okada Y, Sato M, Akamo Y, Takeyama H, et al. Alteration of integrins by interleukin-1alpha in human pancreatic cancer cells. Pancreas. 2001;23:399–405. doi: 10.1097/00006676-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Miura K, Uniyal S, Leabu M, Oravecz T, Chakrabarti S, Morris VL, et al. Chemokine receptor CXCR4-beta1 integrin axis mediates tumorigenesis of osteosarcoma HOS cells. Biochem Cell Biol. 2005;83:36–48. doi: 10.1139/o04-106. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–71. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 39.Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J Immunol. 2000;164:2684–91. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- 40.Dogic D, Rousselle P, Aumailley M. Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. J Cell Sci. 1998;111:793–802. doi: 10.1242/jcs.111.6.793. [DOI] [PubMed] [Google Scholar]
- 41.Lauer JL, Gendron CM, Fields GB. Effect of ligand conformation on melanoma cell alpha3beta1 integrin-mediated signal transduction events: implications for a collagen structural modulation mechanism of tumor cell invasion. Biochemistry. 1998;37:5279–87. doi: 10.1021/bi972958l. [DOI] [PubMed] [Google Scholar]
- 42.Friedl P, Noble PB, Zanker KS. T lymphocyte locomotion in a three-dimensional collagen matrix. Expression and function of cell adhesion molecules. J Immunol. 1995;154:4973–85. [PubMed] [Google Scholar]
- 43.Garcia-Velasco JA, Arici A. Interleukin-8 expression in en-dometrial stromal cells is regulated by integrin-dependent cell adhesion. Mol Hum Reprod. 1999;5:1135–40. doi: 10.1093/molehr/5.12.1135. [DOI] [PubMed] [Google Scholar]
- 44.Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood. 2001;97:2932–40. doi: 10.1182/blood.v97.10.2932. [DOI] [PubMed] [Google Scholar]
- 45.Lowrie AG, Salter DM, Ross JA. Latent effects of fibronectin, alpha5beta1 integrin, alphaVbeta5 integrin and the cytoskeleton regulate pancreatic carcinoma cell IL-8 secretion. Br J Cancer. 2004;91:1327–34. doi: 10.1038/sj.bjc.6602132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188–202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]
- 48.Toyoda E, Doi R, Koizumi M, Kami K, Ito D, Mori T, et al. Analysis of E-, N-cadherin, alpha-, beta-, and gamma-catenin expression in human pancreatic carcinoma cell lines. Pancreas. 2005;30:168–73. doi: 10.1097/01.mpa.0000148514.69873.85. [DOI] [PubMed] [Google Scholar]
- 49.Weinel RJ, Neumann K, Kisker O, Rosendahl A. Expression and potential role of E-cadherin in pancreatic carcinoma. Int J Pancreatol. 1996;19:25–30. doi: 10.1007/BF02788372. [DOI] [PubMed] [Google Scholar]
- 50.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21:7–12. doi: 10.1023/b:clin.0000017160.93812.3b. [DOI] [PubMed] [Google Scholar]
- 51.Katz MH, Bouvet M, Takimoto S, Spivack D, Moossa AR, Hoffman RM. Survival efficacy of adjuvant cytosine-analogue CS-682 in a fluorescent orthotopic model of human pancreatic cancer. Cancer Res. 2004;64:1828–33. doi: 10.1158/0008-5472.can-03-3350. [DOI] [PubMed] [Google Scholar]
- 52.Katz MH, Bouvet M, Takimoto S, Spivack D, Moossa AR, Hoffman RM. Selective antimetastatic activity of cytosine analog CS-682 in a red fluorescent protein orthotopic model of pancreatic cancer. Cancer Res. 2003;63:5521–5. [PubMed] [Google Scholar]
- 53.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–60. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 54.Katz MH, Spivack DE, Takimoto S, Fang B, Burton DW, Moossa AR, et al. Gene therapy of pancreatic cancer with green fluorescent protein and tumor necrosis factor-related apoptosis-inducing ligand fusion gene expression driven by a human telomerase reverse transcriptase promoter. Ann Surg Oncol. 2003;10:762–72. doi: 10.1245/aso.2003.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Anaya-Prado R, Toledo-Pereyra LH, Collins JT, Smejkal R, McClaren J, Crouch LD, et al. Dual blockade of P-selectin and beta2-integrin in the liver inflammatory response after uncontrolled hemorrhagic shock. J Am Coll Surg. 1998;187:22–31. doi: 10.1016/s1072-7515(98)00125-2. [DOI] [PubMed] [Google Scholar]


